
Short Communication
Austin J Reprod Med Infertil. 2015;2(3): 1018.
The Effect of Estrogen Receptor Modulator (Raloxifene) on Endometrioma Cells in Culture
Laura Spector DO, Lydia Kaufman BS and Badawy SZA*
Department of Obstetrics and Gynecology, Division of Reproductive Endocrinology and Infertility, State University of New York, Upstate Medical University, Syracuse, New York, USA
*Corresponding author: Shawky ZA Badawy, Department of Obstetrics and Gynecology, Division of Reproductive Endocrinology and Infertility, State University of New York Upstate Medical University, Syracuse, Syracuse, NY 13224, New York, USA
Received: June 01, 2015; Accepted: June 21, 2015; Published: June 29, 2015
Abstract
Objective: To study the effect of raloxifene on endometrioma cells in culture.
Methods: Three cell lines were developed from endometriomas removed surgically. These were treated with Raloxifene in a concentration of 200 micrograms/milliliter of culture medium. Control cell lines received culture medium only.
Results: Raloxifene caused significant suppression of endometrioma cells in culture as compared to controls.
Conclusion: The results of this research suggest that Raloxifene acts directly on endometrioma cells, an estrogen antagonist, thus inhibiting growth of these cells.
Keywords: Endometrioma Cells; Raloxifene; Estrogen antagonist; Raloxifene
Introduction
Raloxifene is a Selective Estrogen Receptor Modulator (SERM). SERMs are competitive inhibitors of estrogen binding to estrogen receptors [1]. They act by attaching themselves to the estrogen receptors, and their effect could be either an antagonist or agonist effect, depending on the tissue. Raloxifene has been approved by the FDA for the treatment of osteopenia and osteoporosis [2,3,4,5]. It is known to have an estrogen antagonist effect on the breast tissue and the endometrial tissue and an estrogen agonist effect on the bone, lipids, brain and liver [2].
Raloxifene is a polyhydroxylated nonsteroidal compound with a benzothiophene core that binds with high affinity to both estrogen receptor α and estrogen receptor β. It is absorbed rapidly after oral administration (bioavailability ~2%). The half-life of Raloxifene is approximately 27 hours and it is eliminated primarily in the colon [6].
Conflicting data exists regarding the effect of Raloxifene on the endometrium. While studies performed in humans in vivo indicate a neutral or non-agonist effect on the endometrium, an experimental study conducted on ovariectomized mice demonstrated an agonist effect on the endometrium. The conflicting data may be due to the estrogen status of the subjects. It has been implicated that a mild agonist effect is present with significantly nonestrogenized females [7,8].
Raloxifene has been shown to have an estrogen antagonist effect on the endometrium. Sonogram and endometrial biopsy studies in postmenopausal women with osteoporosis treated with Raloxifene have shown absence of any stimulatory effect, and the endometrium remained atrophic. Studies that compared Raloxifene and Tamoxifene on the endometrium demonstrated occurrence of endometrial cancer in the latter [9].
The atrophic changes in the endometrium after Raloxifene use encouraged us to evaluate its effects on endometriosis tissue. The present study attempts to demonstrate the inhibitory effect of Raloxifene on endometrioma cells in culture.
Materials and Methods
This study was approved by the Institutional Review Board of SUNY Upstate Medical University and Crouse Hospitals. Patients entered in the study signed a consent form. During the surgery, the endometrioma was removed and an approximately 1cm piece of the endometrioma tissue was placed in a culture medium tube on ice and sent to our research laboratory. The rest of the tissue was sent to surgical pathology for evaluation.
Endometrioma tissue, from IRB consented patients, were then grown in Medium 199 (Mediatech) with 10% Fetal Bovine serum (FBS, Hyclone) and an antibiotic/antimycotic solution (Hyclone). The cell lines developed were used in our study. All cells were used at passage #1 or #2 and grown in sterile 12-well cell culture plates (Corning, Costar) coated with fibronectin-like protein polymer plus (Sigma-Aldrich) [10]. Three cell lines were used in this study (1507, 4655A, MH1129).
The Experimental media containing 200ug/mL of Raloxifene (EVISTA, Lilly) was placed on the wells at the beginning of the cells exponential growth phase (when the cells were most metabolically active), to test the effect of Raloxifene on endometrioma cells in culture. Cells were lifted with trypsin-EDTA (Mediatech) and viable cells were counted by hemocytometer in 50% Trypan blue exclusion dye (Mediatech).
Control wells and Raloxifene treated wells were counted at 0, 72 and 96 hours. The number of cells was then compared for analysis.
Statistical analysis comparing effects of Raloxifene as compared to controls used the Student T-test where significance is less than 0.05.
Results
The control endometrioma cells continued their growth for the duration of the experimental study. The cells treated with Raloxifene showed inhibition of their growth with statistical significance at 96 hours (Figure 1, 2, 3, 4).
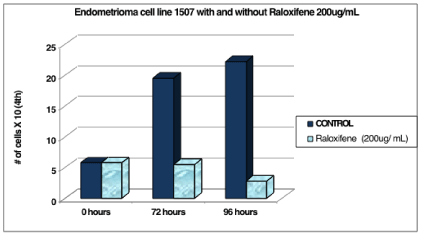
Figure 1: Endometrioma cell line 1507 with and without raloxifene 200 ug/
mL.
Cell line 1507 (P.2) was counted at 0 hours and was 5.8 x 104 cells. After the addition of the 200ug/mL Raloxifene media, the experimental cells decreased in number to 5.5 x 104 cells at 72 hours and 2.8 x 104 cells at 96 hours. The control cells continued to increase in number to 19.5 x 104 cells at 72 hours and 22.2 x 104 cells at 96 hours (Table 1 and Figure 1).
1507
CONTROL
Raloxifene (200ug/ mL)
0 hours
5.8
5.8
72 hours
19.5
5.5
96 hours
22.2
2.8
Table 1: The control cells continued to increase in number to 19.5 x 104cells at 72 hours and 22.2 x 104 cells at 96 hours.
Cell line 4655A (P.1) was counted at 0 hours and was 11.5 x 104 cells. After the addition of the 200ug/mL Raloxifene media, the experimental cells decreased in number to 3 x 104 cells at 72 hours and 0.18 x 104 cells at 96 hours. The control cells continued to increase in number to 29 x 104 cells at 72 hours and began to plateau at 28.3 x 104 cells at 96 hours (Table 2 and Figure 2).
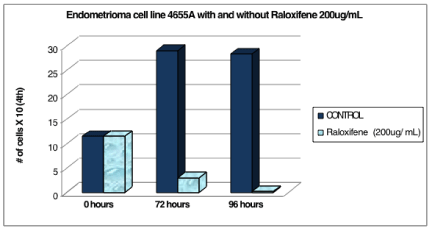
Figure 2: Endometrioma cell line 4655A with and without raloxifene 200 ug/
mL.
4655A
CONTROL
Raloxifene (200ug/ mL)
0 hours
11.5
11.5
72 hours
29
3
96 hours
28.3
0.18
Table 2: The control cells continued to increase in number to 29 x 104 cells at 72 hours and began to plateau at 28.3 x 104 cells at 96 hours.
Cell line MH1129 (P.2) was counted at 0 hours and was 23 x 104 cells. After the addition of the 200ug/mL Raloxifene media, the experimental cells decreased in number to 20 x 104 cells at 72 hours and 16 x 104 cells at 96 hours. The control cells continued to increase in number to 35 x 104 cells at 72 hours and began to plateau at 34 x 104 cells at 96 hours (Table 3 and Figure 3).
MH1129
CONTROL
Raloxifene (200ug/ mL)
0 hours
23
23
72 hours
35
20
96 hours
34
16
Table 3: The control cells continued to increase in number to 35 x 104 cells at 72 hours and began to plateau at 34 x 104 cells at 96 hours.
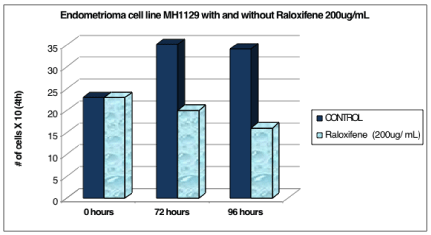
Figure 3: Endometrioma cell line MH1129 with and without raloxifene 200
ug/ mL.
The three cells lines described above demonstrate that growth of endometrioma cells in culture at 96 hours was significantly suppressed with the addition of 200ug/mL of Raloxifene for the three cell lines tested (Table 4, Figure 4).
Average of 3 lines
Control
200ug/mL Raloxifene
0 hour
13.4
13.4
72 hours
27.8
9.5
96 hours
28.2
6.3
Table 4: The three cells lines demonstrate that growth of endometrioma cells in culture at 96 hours was significantly suppressed with the addition of 200ug/mL of Raloxifene for the three cell lines tested.
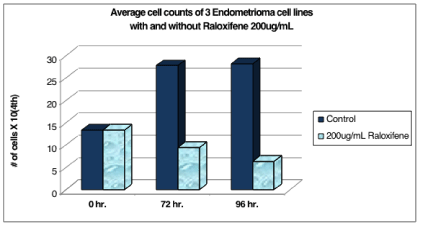
Figure 4: The p-value for 72 hours is 0.058 (borders on significance). The
p-value for 96 hours is 0.022 (statistically significant).
Discussion
Endometriosis is a common medical condition, affecting 5-10% of reproductive age women. Symptoms, including pain, infertility and gastrointestinal abnormalities, can significantly interfere with women’s lives. Several treatments are available for endometriosis, including combined OCPs, progestins, Danocrine, GnRH agonists, and surgery. Some of these medications have side effects, such as hypertension with OCPs, irregular vaginal bleeding with progestins, acne with Danocrine, hot flushes and decreased bone mineralization with GnRH agonists, and complications of surgery. Despite the multiple options for treatment, many women’s endometriosis recurs. The cumulative recurrence rate is 13.5% and 40.3% in 35 years [11]. Investigators must work diligently to identify other medical treatments to treat this disease.
Our study shows that Raloxifene is successful in suppressing endometrioma cell growth at 72 and 96 hours in culture. This suggests that Raloxifene acts as an antagonist on endometrioma tissue, and that there may be other uses for Raloxifene besides treatment of osteopenia and osteoporosis. Lack of the above mentioned side effects and favorable effects on bone metabolism and lipids make this a promising treatment for endometriosis.
Our findings are supported by studies performed in rats showing a suppression of endometriosis volume and glandular composition by Raloxifene when tissue was examined surgically [12].
Implications for these findings are significant. They suggest a possible role for Raloxifene for the treatment of endometriosis. With the results obtained, Raloxifene may suppress growth of endometriosis, which could lead to relief of symptoms and/or treatment of endometriosis related infertility. It is our hope that this study will lead to further investigation into the effects and response for women with endometriosis in vivo.
Our study shows the direct effect of Raloxifene on endometrioma cells in culture. Future in vivo studies are needed to prove clinical significance for the findings outlined here.
References
- Raloxifene for postmenopausal osteoporosis. The Medical Letter on Drugs and Therapeutics (serial online). March 13, 1998; 40: 29-30. Available from: MEDLINE with Full Text, Ipswich, MA. Accessed February 17, 2015.
- Walsh BW, Kuller LH, Wild RA, Paul S, Farmer M, Lawrence JB, et al. Effects of raloxifene on serum lipids and coagulation factors in healthy postmenopausal women. JAMA. 1998; 279: 1445-1451.
- Cohen FJ, Watts S, Shah A, Akers R, Plouffe L Jr. Uterine effects of 3-year raloxifene therapy in postmenopausal women younger than age 60. Obstet Gynecol. 2000; 95: 104-110.
- Delmas PD, Bjarnason NH, Mitlak BH, Ravoux AC, Shah AS, Huster WJ, et al. Effects of raloxifene on bone mineral density, serum cholesterol concentrations, and uterine endometrium in postmenopausal women. N Engl J Med. 1997; 337: 1641-1647.
- Ettinger B, Black DM, Mitlak BH, Knickerbocker RK, Nickelsen T, Genant HK, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA. 1999; 282: 637-645.
- Goldstein SR, Siddhanti S, Ciaccia AV, Plouffe L Jr. A pharmacological review of selective oestrogen receptor modulators. Hum Reprod Update. 2000; 6: 212-224.
- Sato M, Rippy MK, Bryant HU. Raloxifene, tamoxifen, nafoxidine, or estrogen effects on reproductive and nonreproductive tissues in ovariectomized rats. FASEB J. 1996; 10: 905-912.
- García-Pérez MA, Noguera R, del Val R, Noguera I, Hermenegildo C, Cano A. Comparative effects of estradiol, raloxifene, and genistein on the uterus of ovariectomized mice. Fertil Steril. 2006; 86: 1003-1005.
- DeMichele A, Troxel AB, Berlin JA, Weber AL, Bunin GR, Turzo E, et al. Impact of raloxifene or tamoxifen use on endometrial cancer risk: a population-based case-control study. J Clin Oncol. 2008; 26: 4151-4159.
- Badawy SZ, Holland J, Landas S, Frankel L, Cuenca V, Khan S. The role of estradiol, progesterone, and transforming growth factor on human endometrioma cell culture. Am J Reprod Immunol. 1996; 36: 58-63.
- Wheeler JM, Malinak LR. Recurrent endometriosis: incidence, management, and prognosis. Am J Obstet Gynecol. 1983; 146: 247-253.
- Altintas D, Kokcu A, Kandemir B, Tosun M, Cetinkaya MB . Comparison of the effects of raloxifene and anastrozole on experimental endometriosis. Eur J Obstet Gynecol Reprod Biol. 2010; 150: 84-87.