
Research Article
Austin J Reprod Med Infertil. 2019; 6(1): 1052.
Hysteroscopy in Patients with Normal Transvaginal Ultrasound Prior to IVF: A Randomized Controlled Trial
Sanz C*, Armijo O, Lobo S, Iniesta S, Silva P, Sanchez MJ, Fernandez S, Hernández A and Bartha JL
Department of Obstetrics and Gynecology, University Hospital La Paz, Spain
*Corresponding author: Sanz C, Department of Obstetrics and Gynecology, University Hospital La Paz, Madrid, Spain
Received: February 25, 2019; Accepted: April 02, 2019; Published: April 09, 2019
Abstract
Objectives: The hypothesis of the study is that outpatient hysteroscopy improves the results of IVF cycle. The main objective is to evaluate the percentage of biochemical, clinical, on-going pregnancy and livebirths of IVF treatment in patients with no abnormality detected in transvaginal ultrasound examination, who underwent hysteroscopy compared with patients treated with direct cycle treatment.
Methods: This is a prospective randomized open-label trial. Women scheduled for their first or second IVF/ICSI cycle and with no abnormality detected in transvaginal ultrasound examination, were randomized in a 1:1 ratio to underwent office hysteroscopy before IVF or immediate IVF.
Results: Between July 2014 and December 2015 we randomly assigned 75 patients to receive hysteroscopy (n=35) or immediate IVF (n=40). Abnormal hysteroscopic findings were observed in 19, 4% of patients. There were not statistically significant differences in biochemical pregnancy rate (58, 1% vs 54, 1%), clinical pregnancy rates (54, 8% vs 48, 6%), ongoing pregnancy rates (48, 4% vs 35, 1%) and livebirth rates (48, 4% vs 35, 1%).
Conclusions: Office hysteroscopy is not recommended for routine evaluation in patients with normal transvaginal ultrasound prior to IVF.
Keywords: Assisted reproductive technology; Infertility; Pregnancy; Hysteroscopy
Abbreviations
IVF: In Vitro Fertilization; ICSI: Intracytoplasmic Sperm Injection; IF: Implantation Failure; FSH: Follicle Stimulating Hormone; HMG: Human Menopausal Gonadotropin; HCG: Human Chorionic Gonadotropin; IU: International Units; US: Ultrasound
Introduction
In Vitro Fertilization (IVF) treatment is used widely for women who have had difficulty conceiving. Nowadays more than 700000 treatment cycles were given in the USA and Europe alone and the number is expanding steadily worldwide [1,2].
Despite numerous improvements in assisted reproductive techniques, only 30% of cycles of IVF lead to the birth of a child [2]. IVF is an expensive treatment and implantation failure could be due to a variety of reasons, including embryo quality and uterine receptivity, but remains unexplained in many cases [3-6]. According to several studies the most frequent cause of Implantation Failure (IF) is the presence of chromosomal abnormalities in the embryo [7]. However, abnormalities of the uterine cavity such as myomas, polyps and adhesions are also related with impaired implantation and reduced chance of pregnancy [5,8]. Approximately 25% of infertile patients have defects in uterine cavity and abnormal uterine findings are reported in about 50% of women with recurrent implantation failure [9,10]. Hysteroscopy is considered the reference standard to detect these uterine disorders [11,12]. It has been suggested that these abnormalities should be diagnosed and treated to optimize the condition of the uterine environment and the outcome of IVF treatment. Moreover, hysteroscopy could improve pregnancy rates through dilatation of the cervical canal to favour embryo transfer as well as through the induction of an inflammatory reaction of the endometrium with the procedure.
Two meta-analysis have concluded that routine hysteroscopy is an essential step for infertility workup before IVF even in patients with normal vaginal ultrasound [10,13]. However, in a recent randomised trial, routine hysteroscopy didn’t improve live birth rate in infertile women with a normal transvaginal ultrasound previous a first IVF treatment [14]. Giving these controversial results we have designed a randomized controlled trial to evaluate if routine hysteroscopy previous the first IVF treatment cycle could improve the outcomes of IVF cycle.
Material and Methods
The study was approved by the medical ethical review committee of La Paz Hospital and all patients provided written informed consent before enrolment. The study was conducted in accordance with the International Conference on Harmonisation Good Clinical Practice guidelines and the provisions of the Declaration of Helsinki.
Patients were randomized to underwent office hysteroscopy before IVF cycles or immediate IVF. Before inclusion in the study, all the couples were evaluated prior to IVF by recent hormonal profile, transvaginal ultrasound and semen analysis.
Patients younger than 40 years old with normal uterine cavity appearance on vaginal ultrasonography programmed to receive their first or second IVF cycle were included. Exclusion criteria were recurrent miscarriage, recurrent embryo transfer failure, body mass index >30 and previous hysteroscopy.
Women assigned to the intervention group were scheduled for hysteroscopy by a rigid hysteroscope with continuous flow, 30 degree view and 4.3mm diameter diagnostic sheath, in the early to midfollicular phase of the menstrual cycle in an outpatient setting without anaesthesia, one month before the start of IVF treatment. In case of patient intolerance, hysteroscopy was deferred to a further procedure with anaesthesia. Normal saline 0.9% was used as a distension media. Vaginoscopic approach was adopted and nor tentaculum nor speculum were applied. The surgeon inspected the endocervical canal, the endometrial lining of the uterine cavity and the tubal ostia, and recorded all findings on a standardised form.
Intrauterine abnormalities were defined as the presence of polyps, myomas, adhesions or uterine malformations. Therapeutics interventions were done in the same procedure if intrauterine abnormalities were detected. After hysteroscopy, women were observed in a recovery area before being discharged.
Long or short protocols of ovarian stimulation were used for the IVF treatment cycles. Briefly recombinant Follicle Stimulating Hormone (FSH) or Human Menopausal Gonadotropin (HMG) injections for multifollicular ovarian stimulation were started at a dose of 150-300 IU daily. Final oocyte maduration was induced using 6500 IU of recombinant Human Chorionic Gonadotropin (HCG) when at least three 18mm follicles were seen on ultrasound scan. Ultrasound guided oocyte retrieval was done 36h after HCG administration. Embryo transfer was performed on day 2 or 3 with a soft catheter. Vaginal progesterone supplementation was used for luteal phase support and continued for up to 8 week of gestation if pregnancy had occurred. Pregnancy test was done 2 weeks after embryo transfer and positive test confirmed by a transvaginal ultrasound at 7 weeks and 12 weeks of gestation if the first ultrasound showed an intrauterine pregnancy. We contacted the participating women directly to record live births.
Objectives
The primary objective was to determinate the live birth rate defined as proportion of women in the population who had at least one live baby beyond 24 weeks of gestation after one cycle of IVF.
Secondary objectives were rates of pregnancy (defined as proportion of women with positive HCG test), clinical pregnancy (proportion of women with fetal heartbeat on Ultrasound Scan (US) at 7 weeks of gestation), on-going pregnancy (proportion of women with fetal heartbeat on US at 12 weeks of gestation), and miscarriage (defined as the proportion of women with pregnancy loss before 24 weeks of gestation). We also recorded abnormal hysteroscopy findings, hysteroscopy tolerance and hysteroscopy related adverse events.
Statistical analysis
Sample size was calculated considering the results of a pilot study showing a difference in the livebirth rate of 40% between the two groups (30% vs 70%). A sample of 72 patients (36 in each group) was needed to account for an alpha value of 0.05 and a power (1-beta) of 80%.
Analyses were done using SAS 9.3 (SAS Institute, Cary, NC, USA). The values of measurement data are expressed as mean +/- SD when applicable. Χ2 test was used to calculate p values for categorical variables and t test for continuous variables. A p value of ‹0.05 was considered statistically significant.
Results
We enrolled 75 women between July 2014 and December 2015. 35 and 40 patients were assigned to hysteroscopy group and immediate start of IVF respectively. 7 subjects were excluded: 4 in the intervention group (1 spontaneous pregnancy, 1 withdrawal of consent, 1 cycle cancelled without response after treatment, 1 cycle converted to artificial insemination due to a unique follicle growth) and 3 in the control group (1 patient was cancelled for ovarian hyper stimulation syndrome, 1 for absence of embryos after IVF and 1 due to body mass index over the threshold). 31 patients were included in the analysis in the hysteroscopy group and 37 in the immediate IVF group (Figure 1).
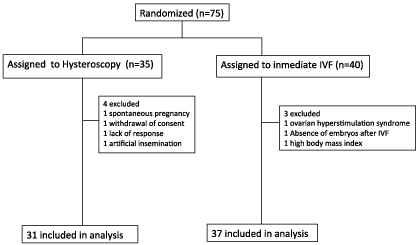
Figure 1: Trial Profile.
There were no differences in baseline characteristics, including mean age (years), body mass index, seminogram parameters, level of FSH and anti-mullerian hormone between the two groups (Table 1).
*Media
Hysteroscopy
Immediate IVF
n=31
n=37
Age *
36 years
35.2 years
Previous Miscarriage
16%
18.90%
Body mass index*
24.8
24
n° cycle
1st: 74.2%
1st: 81.1%
2nd: 25.8%
2nd: 18.9%
Antral follicles
5-10: 54.8%
5-10: 43.1%
11-18: 45.2%
11-18: 56.9%
AMH*
2.5 ng/dl
3.2 ng/dl
FSH*
7.5 UI
6.4 UI
Seminogram
Normal 58.1%
Normal 48.6%
Pathologic 41.9%
Pathologic 51.4%
Table 1: Basal Characteristics.
In patients assigned to hysteroscopy, the procedure was not completed in 2 women (6.7%) due to pain and a difficult passage of the endocervical canal. These women were offered a second hysteroscopy under general anaesthesia, and the hysteroscopy was successfully performed.
No abnormalities were detected in 80.6% of them, while 19.4% showed abnormal hysteroscopy findings. The most common pathologic findings on hysteroscopic examination were endometrial polyps (9.7%), followed by cervical stenosis (6.5%) and endocervical polyps (3.2%).
Five French scissors were used to resect endometrial and endocervival polyps. For cervical stenosis, cervical dilatation under anaesthesia was performed to make easier embryo transfer. Only one hysteroscopy related complication was reported (false route in a patient with cervical stenosis).
IVF was performed in all subjects of the study with no statistically significant differences regarding the number of metaphase II oocytes retrieved, fertilization rate and number of embryo transferred, as shown in Table 2.
Hysteroscopy
Immediate IVF
N° of metaphase II oocytes*
7.29
7.7
Fertilization rate
86.40%
84.40%
N° of embryos transferred
1: 6.5%
1: 16.2%
2: 90.5%
2: 81.1%
3: 3.2%
3: 2.7%
Table 2: Number of metaphase II oocytes, fertilization rate and number of embryos transferred.
Pregnancy rate in patients of hysteroscopy group was 58.1% vs 54.1% in the immediate IVF group (OR 1.2, 95% IC 0.4-3.1; p=0.80). Clinical pregnancy rate in hysteroscopy group was 54.8% compared with 48.6% in the immediate IVF group (OR 1.3, 95% IC 0.5-3.3; p=0.63). Ongoing pregnancy rate was 48.4% in hysteroscopy group vs 35.1% in immediate IVF group (OR 1.7, 95% IC 0.6-4.6; p=0.32). There were no differences in live birth rate 48.4% vs 35.1%; (OR 1.7,95% IC 0.6-4.6, p=0.32) between hysteroscopy and immediate IVF group respectively (Table 3).
Hysteroscopy
IVF
OR (95% CI)
p value
n= 31
n= 37
Pregnancy rate
18 (58.1%)
20 (54.1%)
1.2 (0.4-3.1)
0.8
Clinical pregnancy rate
17(54.8%)
18 (48.6%)
1.3 (0.5-3-3)
0.63
Ongoing pregnancy
15 (48.4%)
13 (35.1%)
1.7 (0.6-4.6)
0.32
Livebirth rate
15 (48.4%)
13 (35.1%)
1.7 (0.6-4.6)
0.32
Table 3: Pregnancy and live birth rates.
Multiple pregnancy rates were similar in the 2 groups (approximately 15%). The pregnancy rate, clinical pregnancy rate, ongoing pregnancy rate and live birth rate did not differ between women with normal or abnormal hysteroscopy.
The miscarriage rate was 11.8% in the hysteroscopy group vs 27.8% in the control group (p>0.05).
Discussion
Hysteroscopy has been proposed to improve IVF outcomes through detection and treatment of intrauterine abnormalities. The reported sensitivity and specificity of transvaginal ultrasound compared with hysteroscopy varies between 0.75-0.93 and 0.60- 0.97 respectively [15,16]. Other techniques such as saline-infusion sonohysterography and 3D ultrasonography could possibly have a diagnostic accuracy comparable to hysteroscopy [17].
Office hysteroscopy is a safe procedure associated with a low rate of complications. It can be performed in an office-based gynecological practice with no need for hospitalization or anaesthesia. Complications of hysteroscopy can be reduced by entry into the uterine cavity under vision, avoiding volume overload and reducing operation time. In our trial, the procedure was well tolerated and only one mild complication was reported. Vaginoscopic approach without using speculum and tenaculum is helpful in improving patient’s tolerance, and the procedure has a very low technical failure rates [18]. Minor abnormalities have successfully been treated during the same procedure.
Intracavitary pathology could have a negative impact in implantation rates. Several studies have evaluated the role of office hysteroscopy before IVF cycles. Karayalcin et al analysed 2500 patients who underwent office base diagnostic hysteroscopy before IVF [19]. All of them had normal ultrasound examination before the procedure. They reported that 23% of women had endometrial pathology. This result is similar to 19% of uterine abnormalities we have found in our study. However other studies have shown lower rates. In a study that included 678 unselected infertile women, Fatemi et al observed 11% abnormal hysteroscopic findings before IVF cycles [20], and in a randomised controlled trial of 750 women, Smit et al found 12% abnormal hysteroscopy findings [14]. Treatment of intracavitary pathology has been associated with a higher pregnancy rate [21]. For example, uterine septum is related with recurrent implantation failure and hysteroscopic resection is usually recommended for correction of uterine cavity and enhancing pregnancy-outcomes [22]. Moreover the procedure causes stimulation of the endometrium that might increase the implantation competency [23].
Different studies showed the positive effect of hysteroscopy examination on the outcome of in vitro fertilization. Bahadur et al showed that 21.1% of patients had confirmed abnormalities that required to be treated before performing IVF and concluded that routine diagnostic hysteroscopy becomes mandatory before expensive procedures of assisted reproduction [24]. However, Fadhlaoui et al concluded that the benefit of routine hysteroscopy is significant only in women 40 years and older [25]. In 2014, a meta-analysis from Pundir et al showed increased live birth rates after hysteroscopy in women scheduled for a first IVF cycle [10]. The findings of this meta-analysis should be questioned because the authors included one small-randomized trial and four non-randomized studies. A recent randomized controlled trial in women scheduled for a first IVF treatment cycle, showed improved pregnancy rates of up to 70% after hysteroscopy [26]. Alleyasin et al in a prospective randomized study showed that the pregnancy rate in the hysteroscopy group was significantly higher that in the control group [27]. The TROPHY trial didn’t find an improvement in live birth rates after hysteroscopy in women with 2 to 4 failed IVF cycles [28]. In another randomized trial that enrolled 750 patients scheduled for their first IVF cycle, hysteroscopy did not improve live birth rates in women with a normal transvaginal ultrasound [14]. Cumulative rates of pregnancy leading to a live birth and the time to this pregnancy also did not differ between groups. In our study, clinical pregnancy, ongoing pregnancy and live birth rates were not significantly higher in women screened by hysteroscopy, neither in patients with detected and treated pathology nor in patients with normal hysteroscopy.
These findings suggest that is neither useful nor cost-effective to use hysteroscopy to screen for intrauterine pathology in women with a normal ultrasound. Currently, the European Society for Human Reproduction and Embryology guidelines recommend hysteroscopy only for the evaluation and treatment of suspicion uterine cavity pathology. The underlying reasons for this recommendation are that hysteroscopy is an invasive procedure and the unknown significance of the observed intrauterine pathology on fertility [29].
Conclusion
In conclusion, the results of this randomized controlled trial show that routine hysteroscopy before the first or second IVF treatment cycle does not improve live birth in infertile woman with a normal transvaginal ultrasound of the uterine cavity.
Abstract
References
- Sunderam S, Kissin DM, Crawford SB, Folger SG, Jamieson DJ, Warner L. Assisted Reproductive Technology Surveillance - United States, 2014. Morb Mortal Wkly Rep Surveill Summ Wash DC 2002. 2017; 66: 1-24.
- European IVF-Monitoring Consortium (EIM), European Society of Human Reproduction and Embryology (ESHRE), Kupka MS, D’Hooghe T, Ferraretti AP, de Mouzon J, et al. Assisted reproductive technology in Europe, 2011: results generated from European registers by ESHRE. Hum Reprod Oxf Engl. 2016; 31: 233-248.
- Margalioth EJ, Ben-Chetrit A, Gal M, Eldar-Geva T. Investigation and treatment of repeated implantation failure following IVF-ET. Hum Reprod Oxf Engl. 2006; 21: 3036-3043.
- Simón C, Martín JC, Pellicer A. Paracrine regulators of implantation. Baillieres Best Pract Res Clin Obstet Gynaecol. 2000; 14: 815-826.
- Taylor E, Gomel V. The uterus and fertility. Fertil Steril. 2008; 89: 1-16.
- Singh M, Chaudhry P, Asselin E. Bridging endometrial receptivity and implantation: network of hormones, cytokines, and growth factors. J Endocrinol. 2011; 210: 5-14.
- Macklon NS, Geraedts JPM, Fauser BCJM. Conception to ongoing pregnancy: the “black box” of early pregnancy loss. Hum Reprod Update. 2002; 8: 333-343.
- Pérez-Medina T, Bajo-Arenas J, Salazar F, Redondo T, Sanfrutos L, Alvarez P, et al. Endometrial polyps and their implication in the pregnancy rates of patients undergoing intrauterine insemination: a prospective, randomized study. Hum Reprod Oxf Engl. 2005; 20: 1632-1635.
- Levi Setti PE, Colombo GV, Savasi V, Bulletti C, Albani E, Ferrazzi E. Implantation failure in assisted reproduction technology and a critical approach to treatment. Ann N Y Acad Sci. 2004; 1034: 184-199.
- Pundir J, Pundir V, Omanwa K, Khalaf Y, El-Toukhy T. Hysteroscopy prior to the first IVF cycle: a systematic review and meta-analysis. Reprod Biomed Online. 2014; 28: 151-161.
- Ceci O, Bettocchi S, Pellegrino A, Impedovo L, Di Venere R, Pansini N. Comparison of hysteroscopic and hysterectomy findings for assessing the diagnostic accuracy of office hysteroscopy. Fertil Steril. 2002; 78: 628-631.
- Bettocchi S, Nappi L, Ceci O, Selvaggi L. Office hysteroscopy. Obstet Gynecol Clin North Am. 2004; 31: 641-654.
- zPotdar N, Gelbaya T, Nardo LG. Endometrial injury to overcome recurrent embryo implantation failure: a systematic review and meta-analysis. Reprod Biomed Online. 2012; 25: 561-571.
- Smit JG, Kasius JC, Eijkemans MJC, Koks CAM, van Golde R, Nap AW, et al. Hysteroscopy before in-vitro fertilisation (inSIGHT): a multicentre, randomised controlled trial. Lancet Lond Engl. 2016; 387: 2622-2629.
- Bingol B, Gunenc Z, Gedikbasi A, Guner H, Tasdemir S, Tiras B. Comparison of diagnostic accuracy of saline infusion sonohysterography, transvaginal sonography and hysteroscopy. J Inst Obstet Gynaecol. 2011; 31: 54-58.
- Soares SR, Barbosa dos Reis MM, Camargos AF. Diagnostic accuracy of sonohysterography, transvaginal sonography, and hysterosalpingography in patients with uterine cavity diseases. Fertil Steril. 2000; 73: 406-411.
- El-Sherbiny W, Nasr AS. Value of 3-dimensional sonohysterography in infertility work-up. J Minim Invasive Gynecol. 2011; 18: 54-58.
- El-Toukhy T, Campo R, Sunkara SK, Khalaf Y, Coomarasamy A. A multicentre randomised controlled study of pre-IVF outpatient hysteroscopy in women with recurrent IVF implantation failure: Trial of Outpatient Hysteroscopy - [TROPHY] in IVF. Reprod Health. 2009; 6: 20.
- Karayalcin R, Ozcan S, Moraloglu O, Ozyer S, Mollamahmutoglu L, Batioglu S. Results of 2500 office-based diagnostic hysteroscopies before IVF. Reprod Biomed Online. 2010; 20: 689-693.
- Fatemi HM, Kasius JC, Timmermans A, van Disseldorp J, Fauser BC, Devroey P, et al. Prevalence of unsuspected uterine cavity abnormalities diagnosed by office hysteroscopy prior to in vitro fertilization. Hum Reprod Oxf Engl. 2010; 25: 1959-1965.
- Gera PS, Allemand MC, Tatpati LL, Galanits TM, Morbeck D, Coddington CC. Role of saline infusion sonography in uterine evaluation before frozen embryo transfer cycle. Fertil Steril. 2008; 89: 562-566.
- Bakas P, Gregoriou O, Hassiakos D, Liapis A, Creatsas M, Konidaris S. Hysteroscopic resection of uterine septum and reproductive outcome in women with unexplained infertility. Gynecol Obstet Invest. 2012; 73: 321-325.
- Li R, Hao G. Local injury to the endometrium: its effect on implantation. Curr Opin Obstet Gynecol. 2009; 21: 236-239.
- Bahadur A, Malhotra N, Singh N, Gurunath S, Mittal S. Comparative study on the role of diagnostic hysteroscopy in evaluation of the uterine cavity prior to in vitro fertilization in a developing country. Arch Gynecol Obstet. 2013; 288: 1137-1143.
- Fadhlaoui A, Khediri Z, Khrouf M, Chaker A, Zhioua F. Diagnostic hysteroscopy before the first in vitro fertilization. For whom?. Tunis Med. 2013; 91: 310-316.
- Elsetohy KAAA, Askalany AH, Hassan M, Dawood Z. Routine office hysteroscopy prior to ICSI vs. ICSI alone in patients with normal transvaginal ultrasound: a randomized controlled trial. Arch Gynecol Obstet. 2015; 291: 193-199.
- Alleyassin A, Abiri A, Agha-Hosseini M, Sarvi F. The Value of Routine Hysteroscopy before the First Intracytoplasmic Sperm Injection Treatment Cycle. Gynecol Obstet Invest. 2017; 82: 125-130.
- El-Toukhy T, Campo R, Khalaf Y, Tabanelli C, Gianaroli L, Gordts SS, et al. Hysteroscopy in recurrent in-vitro fertilisation failure (TROPHY): a multicentre, randomised controlled trial. Lancet Lond Engl. 2016; 387: 2614-2621.
- Crosignani PG, Rubin BL. Optimal use of infertility diagnostic tests and treatments. The ESHRE Capri Workshop Group. Hum Reprod Oxf Engl. 2000; 15: 723-732.