
Research Article
Austin J Reprod Med Infertil. 2022; 8(1): 1060.
PD-1, PD-L1, and DNA Mismatch Repair Molecules in Endometriosis and Endometrial Carcinoma: What are the Shared Molecular Characteristics?
Maryam Zare1, Behrouz Gharesi-Fard1,2, Mojgan Akbarzadeh Jahromi3, Fatemeh Hesampour1, Maryam Valibeigi1, Tahereh Poordast4,2, Zahra Hoseini4, Zahra Shiravani4, Fatemeh Sadat Najib4,2, Zahra Anvar4,2,5*, Fatemeh Nasri6,7*
1Department of Immunology, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran
2Infertility Research Center, Shiraz University of Medical Sciences, Shiraz, Iran
3Fetal and Maternal Research Center, Department of Pathology, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran
4Department of Obstetrics and Gynecology, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran
5Department of Obstetrics and Gynecology, Baylor College of Medicine, Texas, United States
6Department of Laboratory Sciences, School of Paramedical Sciences, Shiraz University of Medical Sciences, Shiraz, Iran
7Diagnostic Laboratory Sciences and Technology Research Center, School of Paramedical Sciences, Shiraz University of Medical Sciences, Shiraz, Iran
*Corresponding author: Fatemeh Nasri, Department of Immunology, Meshkin-Fam St. School of Paramedical Sciences, Shiraz University of Medical Sciences, Shiraz, Iran
Zahra Anvar, Department of Obstetrics and Gynecology and Infertility Research Center, Shiraz University of Medical Sciences, Shiraz, Iran
Received: May 21, 2022; Accepted: June 23, 2022; Published: June 30, 2022
Abstract
Aim and Background: Lack of immunological surveillance and stressinduced DNA damage are important in the context of endometriosis (EM) and endometrial carcinoma (EC). The purpose of this study was to shed light on the shared immunological and biological mechanisms between endometriosis compared to endometrial cancer by assessing the PD-1/PD-L1 pathway and DNA mismatch repair system.
Material and Method: Thirty tissue samples from EM patients, 22 samples from EC patients, and 19 sections from healthy women were included. The expression of PD-1, PD-L1, and DNA mismatch repair molecules (MLH1 and PMS2) was assessed using the immunohistochemistry technique.
Results: Only 26% of normal endometrial samples were PD-1pos, while 80% of EM tissues and 82% of EC sections were PD-1pos (P<0.001). Similarly, higher proportions of PD-L1pos immune or stromal cells existed in the EM and EC sections compared to the control ones; however, the differences were not significant (Ps>0.05). Concerning MLH1 and PMS2, all control or endometriosis sections expressed these proteins and was MLH1/PMS2-proficient. However, 2 out of 22 EC patients lacked these proteins concomitantly and were MLH1/ PMS2-deficient. Finally, there were no associations between PD-1 or PD-L1 expression and patients’ clinicopathological characteristics such as metastasis, recurrence, invasion, and disease score.
Conclusion: The expression of PD-1 and PD-L1 molecules is a shared immunologic mechanism between EM and EC, suggesting the possibility for the use of similar immunotherapeutics in these contexts.
Keywords: B7-H1 Antigen; DNA Mismatch Repair; Endometriosis; Endometrial Carcinoma; Immune Checkpoint Inhibitors; Programmed Cell Death 1 Receptor
Abbreviations
EM: Endometriosis; EC: Endometrial Carcinoma; PD-1: Programmed Cell Death-1; MMR: DNA Mismatch Repair; MLH1: mutL Homologue 1; PMS2: Post Meiotic Segregation Increased 2; MSH2 mutS Homologue 2; MSH6: mutS 6; OS: Overall Survival; DFS: Disease-Free Survival; Mantel-Cox: Log-rank; IHC: Immunohistochemistry.
Introduction
Endometriosis (EM) is a common disease affecting 5% to 10% of women of reproductive age [1]. Endometrial carcinoma (EC) is another reproductive disorder that is the second most prevalent gynecological cancer, accounting for 6% of all newly diagnosed malignancies in Europe and the United States [2-4]. There is a putative association between these two disorders and they share common etiological mechanisms, including estrogen stimulation and chronic inflammation [5-7]. In addition, patients diagnosed with endometriosis may harbor an increased risk of developing endometrial cancer later in their lives [6]. Recently, the importance of the immune system in the induction of endometriosis and endometrial carcinoma is highlighted. The lack of immunological surveillance is considered a hallmark for endometrial cancer and endometriosis development, allowing cancer cells and endometrial cells to evade the human immune system during the formation of tumor and endometriosis lesions [8-10]. Programmed cell death-1 (PD-1) receptor, along with its ligands, PD-L1 and PD-L2, plays crucial roles in regulating immune system responses and tissuehomeostasis [11,12]; therefore, this pathway is of particular importance in several disorders such as gynecological malignancies [13]. The expression level of PD-1 and its legends is associated with clinicopathological characteristics of EC patients [14,15], and there are several lines of evidence introducing PD-1/PD-L1 pathway blockade as an effective immunotherapeutic strategy for several gynecological cancers and endometrial carcinoma [16,17]. In the case of endometriosis, Walankiewicz et al. showed that endometriosis patients with advanced disease had higher frequencies of PD-1pos T and B cells in their peripheral blood samples [18]. This finding reveals that the PD-1/PD-L1 pathway may contribute to the pathogenesis of endometriosis; however, there is limited data about tissue expression of PD-1 and PD-L1 molecules and the importance of this axis in endometriosis.
Another shared mechanism between EM and EC is DNA damage caused by prolonged estrogen stimulation and chronic inflammation [6,7,19]. In fact, evidence suggests that oxidative stress can cause inflammatory conditions in endometriosis, and in the long term, it can induce genetic damage, such as mutations to DNA single base pairs [20,21]. The DNA mismatch repair (MMR) system is a highly conserved repair mechanism that serves crucial roles in fixing DNA damages [22-24]. This system relies on four critical proteins: mutL homologue 1 (MLH1), postmeiotic segregation increased 2 (PMS2), mutS homologue 2 (MSH2) and mutS 6 (MSH6) [25]. There is some evidence suggesting that MLH1 can be important in the context of endometrial cancer [26,27]. For example, a study showed that epigenetic silencing of MLH1 is associated with poorer outcomes in endometrial cancers, such as increased tumor burden, increased rate of lymph node positivity, and decreased recurrence-free survival (26). In the case of endometriosis, a study demonstrated increased expression of MSH2 and a positive correlation between MSH2 and Ki-67 levels in endometriotic lesions [28]. Increased expression of MSH2 in endometriotic cells appeared to be linked to their increased proliferative capacity, proposing a new pathophysiological mechanism underlying cell proliferation and scar formation in endometriosis [28]. However, there are a few studies investigating the importance of MMR deficiency in endometriosis and endometrial cancer.
Therefore, we aimed to assess the role of the PD-1/PD-L1 pathway in endometriosis compared to endometrial cancer. Also, we investigated the possible relationship between the PD-1/PD-L1 pathway and the MMR system (MLH1 and PMS2 proteins) in the context of endometriosis and endometrial carcinoma.
Materials and Methods
Subjects
Endometriosis was suspected in women who were referred to the infertility research center of Shiraz Ghadir Mother and Child Hospital based on the following clinical complaints: severe or incapacitating dysmenorrhea, deep dyspareunia, chronic pelvic pain, infertility, and urinary abnormalities (pain and/or bleeding) or cyclic bowel abnormalities (pain and/or bleeding). Consequently, all women were subjected to video laparoscopy and30 women suffering from stages III or IV of endometriosis were enrolled as the endometriosis group. The endometriosis stage was determined according to the revised American Society for Reproductive Medicine (rASRM) classification. Collected endometrial lesions were kept in formalin until the time of block preparation. We also considered 22 patients who were referred to Faqihi Hospital and suffered from endometrial cancer in this study. Clinicopathological data and paraffin-embedded tissue blocks of these patients were collected. Moreover, 19 normal endometrial tissues were collected as the control group.
None of the participants had any history of immunological disorders such as autoimmunity, immune deficiency, and active infection. Before entering the study, all participants read and signed a written informed consent approved by the Ethics Committee of Shiraz University of Medical Sciences.
Immunohistochemistry (IHC)
Four serial sections of 3 μm thickness were prepared from each formalin-fixed and paraffin-embedded (FFPE) tissue blocks and mounted on positively charged glass slides. Sections were heated at 61°C for 15 min, deparaffinized in xylene (Merck Millipore, Burlington, USA) for 30 min, and rehydrated in decreasing graded ethanol solutions (100% and 96%) (Merck Millipore, Burlington, USA). To retrieve PD-1 and PD-L1 proteins, the slides were boiled in the retrieval solution (Tris-EDTA, pH=9) in a pressure cooker for 15 minutes. This time was extended to 20 minutes for MLH1 and PMS2 molecules. Non-specific hydrophobic interactions and endogenous peroxides activity were blocked by 10% goat serum and 10% H2O2, respectively. Primary anti-PD-1, anti-PD-L1, anti- MLH1, and anti-PMS2 antibodies (all from Sinabiotech, Tehran, Iran) were added to the sections in appropriate concentrations and incubated for one hour at room temperature. Visualization was done by Master Polymer plus Detection System (Peroxidase) kit (Master Diagnostic, Granada, Spain) according to the manufacturer’s instructions. Finally, tissues were counterstained using hematoxylin solution (Neutronpharmachemical, Tehran, Iran), dehydrated in an increasing graded ethanol series, cleared in xylene, and mounted with a mounting medium. In order to obtain reliable results,human tonsil sectionsand a section on which no primary antibody was applied were used asthe positive and negative controls, respectively.
Microscopic Analysis and Positive Cell Scoring
An expert pathologist examined all slides using an optical microscope. The IHC results were classified and reported based on these criteria: any sample was defined as PD-1- or PD-L1-positiveif the positive cell proportion was ≤10%; otherwise, if the positive cell proportion was ≤10%, it was defined as PD-1- or PD-L1-negative. Regarding MLH1 and PMS2, if these proteins were not expressed, the status was called deficient; otherwise, the status was considered proficient.
Statistical analyses
Statistical analyses were conducted by SPSS (version 18) or GraphPad Prism (version 9.0.0) software. The Levene’s and Kolmogorov-Smirnov tests were used to assess the homogeneity of variances and normality of data, respectively. Based on the normality and homogeneity of variances, the one-way ANOVA test or its nonparametric counterpart, the Kruskal-Wallis test, was used to analyze continuous variables. The comparison of clinicopathological and the proportion of PD-1+ and PD-L1+ cells in endometriosis, endometrial carcinoma, and control groups was performed using the Chi-square (or Fisher’s) test and the Z-test for independent proportions. . In order to assess the association between individual variables and overall survival (OS) or disease-free survival (DFS), log-rank (Mantel-Cox) test was used. P values < 0.05 were considered statistically significant.
Results
Clinicopathologic Characteristics of Endometriosis and Endometrial Cancer Patients
As PD-1 and PD-L1 expression can be age-dependent (15), we matched the age variable between the three studied groups. The means ±SD of age in the studied groups were as followed: 45.47 ± 2.50 in healthy groups, 44.46 ± 3.45 in endometriosis patients, and 47.27 ± 6.29 in endometrial cancer patients (P>0.05). Table 1 summarizes other clinicopathologic characteristics of endometriosis and endometrial cancer patients.
Group
Characteristics
Number or range
Endometriosis patients
Stage:
III
n= 11
IV
n= 19
Score:
III
range: 20-86
IV
range: 104-196
Endometrial cancer
Histologic Type:
patients
Endometrioid
n=21
Serous papillary
n=1
Stage:
I
n=19
II
n=2
III
n= 1
Grade:
I
n=13
II
n=6
III
n= 3
Invasion:
Yes
n=15
Myometrium
n=15
Serosal
n=1
Cervical
n=1
No
n=7
Metastasis:
Yes
n=1
No
n=19
Missing
n=2
Recurrence:
Yes
n=3
No
n=19
Table 1: Clinicopathologic characteristics of endometriosis and endometrial cancer patients.
Expression of PD-1, PD-L1, MLH1, and PMS2 in Normal Endometrium, Endometriosis, and Endometrial Cancer
IHC staining for PD-1, PD-L1, MLH1, and PMS2 was performed using 19 normal endometrium tissues, 30 endometriosis tissues (11 patients were in stage III, and 19patients were in stage IV), and 22 EC tissues. Representative IHC staining pictures are shown in (Figure 1).
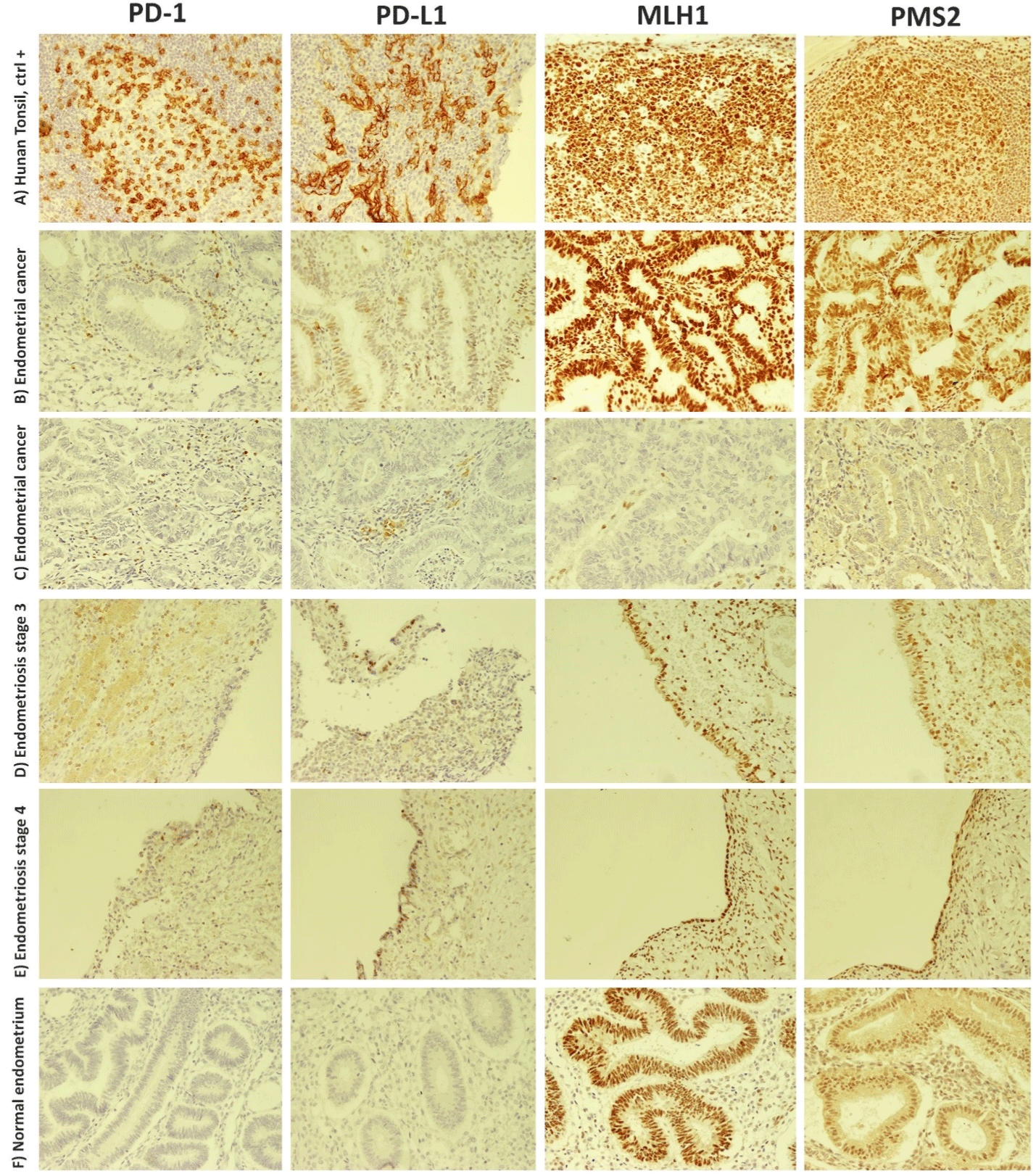
Figure 1: Immunohistochemistry staining of PD-1, PD-L1, MLH1, and PMS2 molecules in endometriosis, endometrial cancer, and healthy control
subjects. Images illustrate the staining patterns of the aforementioned molecules in A) human tonsil sections as the positive control, B and C) endometrial cancer
patients. MLH1/PMS2 deficiency is seen in the third row. D and E) show the staining pattern of studied molecules in endometriosis patients in stages 3 or 4,
respectively. F) The last row depicts the lack of PD-1 or PD-L1 expression in a healthy endometrium. Brown spots represent positively stained cells. MLH1: mutL
homologue 1, PD-1: programmed death-1 receptor; PD-L1: programmed death ligand-1; PMS2: postmeiotic segregation increased 2.
It was found that only 26% of the normal endometrial samples were positive for PD-1 expression, whereas 80% of endometriosis tissues (73% of stage III and 84% of stage IV) and 82% of EC tissues were positive for PD-1 staining (Table 2, P<0.001).
PD-1 on immune cells
PD-L1 on immune cells
PD-L1 on stromal or glandular cells
Group (n)
Positive n (%)
P value
Positive n (%)
P value
Positive n (%)
P value
Control (19)
5 (26%)a
P<0.001a
4 (21%)
P>0.05
2 (10%)
P>0.05
Endometriosis stage III (11)
8 (73%)
4 (40%)
3 (30%)
Endometriosis stage IV (19)
16 (84%)a
6 (32%)
5 (26%)
Endometrial cancer (22)
18 (82%)a
9 (41%)
4 (18%)
P values are calculated using the Chi-Square or Fisher's test. Z-test for independent proportions was used to determine the differences between groups. aThe differences were between the control group and two other groups. PD-1: Programmed death-1 receptor, PD-L1: Programmed death ligand-1.
Table 2: Expression of PD-1 and PD-L1 molecules in normal endometrium, endometriosis, and endometrial cancer.
Regarding the expression of PD-L1 molecule on immune cells and stromal cells, although endometriosis and the EC sections had higher proportions of PD-L1pos cells compared to the control sections (Table 2), the differences between studied groups were not statistically significant (P values> 0.05).
In the case of MLH1 and PMS2, all sections derived from control or endometriosis samples expressed these two molecules and were MLH1/PMS2-proficient. However, two patients suffering from endometriosis carcinoma concomitantly lacked these proteins and were MLH1/PMS2-deficient (Figure 1C). Descriptive analyses revealed that both patients had tumor sizes greater than 2cm and experienced myometrial invasion. None of these MLH1/PMS2- deficient patients experienced metastasis.
Association between PD-1 or PD-L1 Expression and Clinicopathological Characteristics of Patients with Endometriosis and Endometrial Cancer
In EC patients, descriptive analyses showed that only three patients had DFS and OS less than five years; therefore, we did not observe any significant association between the frequency of PD-1pos immune cells, PD-L1pos immune cells, or PD-L1pos tumoral cells and survival times (Figure 2). Besides, we did not find any significant associations between PD-1 or PD-L1 expression and experiencing invasion, metastasis, or recurrence.
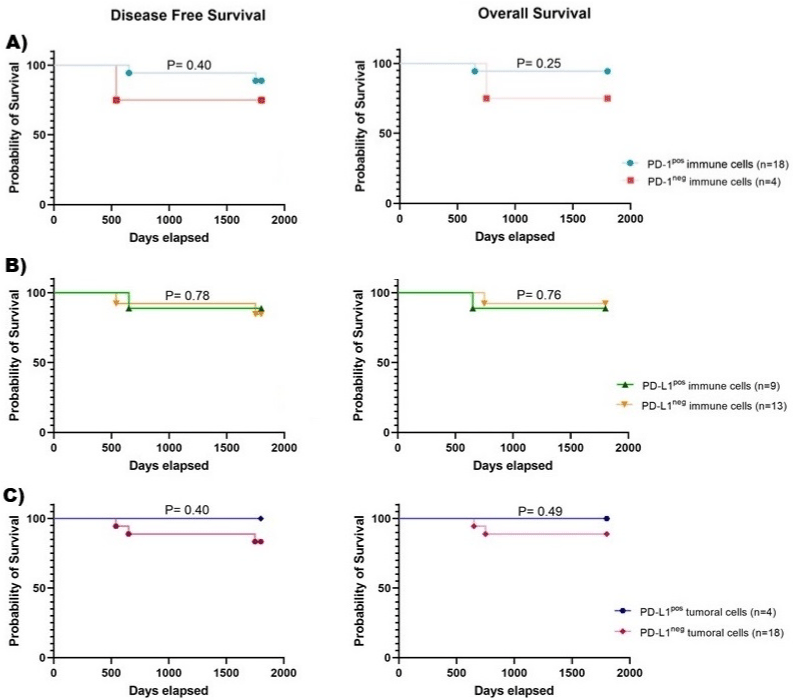
Figure 2: Kaplan-Meier curves of disease-free survival or overall survival in endometrial cancer patients. Kaplan-Meier curves of DFS and OS according to
A) PD-1 molecule expression on immune cells, B) PD-L1 expression on immune cells, or C) PD-L1 expression on tumoral cells. DFS: disease-free survival; OS:
overall survival; PD-1: programmed death-1 receptor; PD-L1: programmed death ligand-1.
Concerning EM patients, our analyses revealed no association between PD-1 or PD-L1 expression and endometriosis severity score.
Discussion
Endometriosis is a common gynecological disorder linked with cancers such as ovarian and endometrial cancer [29-31]. Painter et al. revealed that endometriosis and endometrial cancer share some genetic similarities [32], suggesting that there might be other similarities between these two disorders including impaired functionality of the immune system or DNA repair. Therefore, this study aimed to assess the association between endometriosis and endometrial cancer by analyzing the expression of PD-1, PD-L1, MLH1, and PMS2 molecules, which are involved in the regulation of immune and DNA-damage responses.
We found that almost 80% of individuals who had endometriosis expressed PD-1 molecules on the surface of their immune cells, which was four times higher than the proportion of PD-1pos samples in the control group. This finding was in line with those of other researchers who showed that the expression of PD-1 molecule is increased both in endometrial tissue and on the peripheral blood lymphocytes [18,33,34]. Interestingly, the proportion of PD-1pos immune cells was similar between endometriosis patients and those patients who had endometrial cancer. In line with other studies, our results showed that EC patients had a higher proportion of PD-1pos immune cells compared to control subjects (15). Considering the expression of PD-L1 on immune, stromal, or tumoral cells, in line with other studies, we found that both endometriosis patients and endometrial cancer patients had a higher proportion of PD-L1pos cells compared to control subjects [18,33,34]; however, it did not reach the level of significance. Consequently, our results suggest that endometriosis and endometrial cancer have similarities regarding PD1/PD-L1 axis. As checkpoint inhibitors have shown promising results in the context of endometrial cancer [35,36], we believe that our results can open up new ways to target this axis and use immunotherapy in the context of endometriosis [37].
Besides, evidence suggests that oxidative stress and reactive oxygen species seen in the context of endometriosis and endometrial cancer can induce genetic damage, such as mutations to DNA single base pairs [38]. Therefore, it would be essential to see whether the MMR system is proficient in these contexts or not. In our study, we evaluated the expression of MMR molecules in endometriosis patients, endometrial cancer patients, and healthy individuals. We found that all endometriosis patients and healthy controls were MLH1/PMS2 proficient. However, in the case of endometrial cancer, two patients who had tumor sizes greater than 2 cm were MLH1/ PMS2 deficient, which is the most frequent type of MMR deficiency among endometrial cancer patients [39,40]. In this regard, other most recently published studies have also revealed that MMR deficiency is seen in patients suffering from endometrial cancer, and it might be associated with some clinicopathologic characteristics of patients [41,42]. Overall, our results revealed that although MMR deficiency is important in the context of endometrial cancer, this system is intact in the context of endometriosis, and these patients are MLH1/PMS2 proficient.
In conclusion, based on the result of the present study, it seems that both endometriosis and endometrial cancer are associated in terms of expressing immune-inhibitory molecules such as PD-1 and PD-L1, suggesting that there would be this possibility to use similar immunotherapies in the context of these disorders. However, MMR deficiency is most prevalent in the context of endometrial cancer and doesn’t seem to be a shared feature between these two disorders.
Acknowledgments
This study was supported by the Vice-Chancellor for Research, Shiraz University of Medical Sciences, Shiraz, Iran [grant numbers: 16688 and 17920]. The authors would like to thank Mrs. Simin Ahmadvand for her sincere help through the steps of IHC test. Also, we are grateful to all staffs of the clinical and pathological laboratories of Ghadir Mother and Child, and Faqihi hospitals for their sincere help in collecting paraffin-embedded tissue samples.
References
- Song M, Karabina SA, Kavtaradze N, Murphy AA, Parthasarathy S. Presence of endometrial epithelial cells in the peritoneal cavity and the mesothelial inflammatory response. Fertility and sterility. 2003; 79: 789-794. doi:10.1016/ S0015-0282(02)04836-7.
- Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA: a cancer journal for clinicians. 2011; 61(4): 212-36.
- Münstedt K, Grant P, Woenckhaus J, Roth G, Tinneberg H. Cancer of the endometrium: current aspects of diagnostics and treatment. World Journal of Surgical Oncology. 2004; 2(1): 24. doi:10.1186/1477-7819-2-24.
- Murali R, Soslow RA, Weigelt B. Classification of endometrial carcinoma: more than two types. The Lancet. Oncology. 2014; 15(7): e268-e278. doi:10.1016/S1470-2045(13)70591-6.
- Rueda-Martínez A, Garitazelaia A, Cilleros-Portet A, Marí S, Arauzo R, Miguel JD, et al. Genetic Contribution of Endometriosis to the Risk of Developing Hormone-Related Cancers. International Journal of Molecular Sciences. 2021; 22(11): 6083. doi:10.3390/ijms22116083.
- Yu H, Lin C, Chang W, Shen B, Chang W, Chuang C. Increased Association Between Endometriosis and Endometrial Cancer. International Journal of Gynecological Cancer. 2014; 25(3): 447-452. doi:10.1097/ IGC.0000000000000384.
- Kokcu A. Relationship between endometriosis and cancer from current perspective. Archives of Gynecology and Obstetrics. 2011; 284(6): 1473- 1479. doi:10.1007/s00404-011-2047-y.
- Liu Y, Liu Y, Hu W, Tang L, Sheng Y, Wei C, et al. Elevated heme impairs macrophage phagocytosis in endometriosis. Reproduction. 2019; 158(3): 257-266. doi:10.1530/REP-19-0028.
- Guo S, Du Y, Liu X. Platelet-derived TGF-β1 mediates the down-modulation of NKG2D expression and may be responsible for impaired natural killer (NK) cytotoxicity in women with endometriosis. Human reproduction. 2016; 31(7): 1462-1474. doi:10.1093/humrep/dew057.
- Bruno V, Corrado G, Baci D, Chiofalo B, Carosi MA, Ronchetti L, et al. Endometrial Cancer Immune Escape Mechanisms: Let Us Learn From the Fetal–Maternal Interface. Frontiers in Oncology. 2020; 10. doi:10.3389/ fonc.2020.00156.
- Dai S, Jia R, Zhang X, Fang Q, Huang L. The PD-1/PD-Ls pathway and autoimmune diseases. Cellular immunology. 2014; 290(1): 72-79. doi:10.1016/j.cellimm.2014.05.006.
- Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annual review of immunology. 2008; 26(1): 677-704. doi:10.1146/annurev.immunol.26.021607.090331.
- Marinelli O, Annibali D, Aguzzi C, Tuyaerts S, Amant F, Morelli MB, et al. The Controversial Role of PD-1 and Its Ligands in Gynecological Malignancies. Frontiers in Oncology. 2019; 9. doi:10.3389/fonc.2019.01073.
- Mo Z, Liu J, Zhang Q, Chen Z, Mei J, Liu L, et al. Expression of PD-1, PDL1 and PD-L2 is associated with differentiation status and histological type of endometrial cancer. Oncology Letters. 2016; 12(2): 944-950. doi:10.3892/ ol.2016.4744.
- Sungu N, Yildirim M, Desdicioglu R, Aydogdu B, Kiliçarslan A, Dogan HT, et al. Expression of Immunomodulatory Molecules PD-1, PD-L1, and PD-L2, and their Relationship With Clinicopathologic Characteristics in Endometrial Cancer. International Journal of Gynecological Pathology. 2018; 38(5): 404- 413. doi:10.1097/PGP.0000000000000543.
- Gadducci A, Guerrieri ME. Immune Checkpoint Inhibitors in Gynecological Cancers: Update of Literature and Perspectives of Clinical Research. Anticancer research. 2017; 37(11): 5955-5965. doi:10.21873/ ANTICANRES.12042.
- Mittica G, Ghisoni E, Giannone G, Aglietta M, Genta S, Valabrega G. Checkpoint inhibitors in endometrial cancer: preclinical rationale and clinical activity. Oncotarget. 2017; 8(52): 90532-90544. doi:10.18632/ oncotarget.20042.
- Walankiewicz M, Grywalska E, Polak G, Korona-Glowniak I, Witt E, Surdacka A, et al. The Increase of Circulating PD-1- and PD-L1-Expressing Lymphocytes in Endometriosis: Correlation with Clinical and Laboratory Parameters. Mediators of Inflammation. 2018; 2018: 1-12. doi:10.1155/2018/7041342.
- Varma R, Rollason T, Gupta JK, Maher ER. Endometriosis and the neoplastic process. Reproduction. 2004; 127(3): 293-304. doi:10.1530/REP.1.00020.
- Kobayashi H, Kajiwara H, Kanayama S, Yamada Y, Furukawa N, Noguchi T, et al. Molecular pathogenesis of endometriosis-associated clear cell carcinoma of the ovary (review). Oncology reports. 2009; 22(2): 233-40. doi:10.3892/OR_00000429.
- Fuseya C, Horiuchi A, Hayashi A, Suzuki A, Miyamoto T, Hayashi T, et al. Involvement of pelvic inflammation-related mismatch repair abnormalities and microsatellite instability in the malignant transformation of ovarian endometriosis. Human pathology. 2012; 43(11): 1964-1972. doi:10.1016/j. humpath.2012.02.005.
- Takamochi K, Takahashi F, Suehara Y, Sato E, Kohsaka S, Hayashi T, et al. DNA mismatch repair deficiency in surgically resected lung adenocarcinoma: Microsatellite instability analysis using the Promega panel. Lung cancer. 2017; 110: 26-31. doi:10.1016/j.lungcan.2017.05.016.
- Ionov Y, Peinado MA, Malkhosyan S, Shibata D, Perucho M. Ubiquitous somatic mutations in simple repeated sequences reveal a new mechanism for colonic carcinogenesis. Nature. 1993; 363(6429): 558-561. doi:10.1038/363558A0.
- Peltomäki P, Aaltonen LA, Sistonen P, Pylkkänen L, Mecklin JP, Järvinen H, et al. Genetic mapping of a locus predisposing to human colorectal cancer. Science. 1993; 260(5109): 810-812. doi:10.1126/SCIENCE.8484120.
- Zhao P, Li L, Jiang X, Li Q. Mismatch repair deficiency/microsatellite instability-high as a predictor for anti-PD-1/PD-L1 immunotherapy efficacy. Journal of Hematology & Oncology. 2019; 12(1). doi:10.1186/s13045-019- 0738-1.
- Cosgrove CM, Cohn DE, Hampel H, Frankel WL, Jones D, McElroy JP, et al. Epigenetic silencing of MLH1 in endometrial cancers is associated with larger tumor volume, increased rate of lymph node positivity and reduced recurrence-free survival. Gynecologic oncology. 2017; 146(3): 588-595. doi:10.1016/j.ygyno.2017.07.003.
- Kim J, Kong JK, Yang W, Cho H, Chay DB, Lee BH, et al. DNA Mismatch Repair Protein Immunohistochemistry and MLH1 Promotor Methylation Testing for Practical Molecular Classification and the Prediction of Prognosis in Endometrial Cancer. Cancers. 2018; 10(9): 279. doi:10.3390/ cancers10090279.
- Grassi T, Calcagno A, Marzinotto S, Londero AP, Orsaria M, Canciani GN, et al. Mismatch repair system in endometriotic tissue and eutopic endometrium of unaffected women. International journal of clinical and experimental pathology. 2015; 8(2): 1867-77.
- Capmas P, Suarthana E, Tulandi T. Further evidence that endometriosis is related to tubal and ovarian cancers: A study of 271,444 inpatient women. European journal of obstetrics, gynecology, and reproductive biology. 2021; 260: 105-109. doi:10.1016/j.ejogrb.2021.02.022.
- Hermens M, Altena AMV, Velthuis I, Laar DCMVD, Bulten J, Vliet HAAMV, et al. Endometrial Cancer Incidence in Endometriosis and Adenomyosis. Cancers. 2021; 13(18): 4592. doi:10.3390/cancers13184592.
- Kavoussi SK, Odenwald KC, As-Sanie S, Lebovic DI. Incidence of ovarian endometrioma among women with peritoneal endometriosis with and without a history of hormonal contraceptive use. European journal of obstetrics, gynecology, and reproductive biology. 2017; 215: 220-223. doi:10.1016/j. ejogrb.2017.06.028.
- Painter JN, O’Mara TA, Morris AP, Cheng THT, Gorman M, Martin L, et al. Genetic overlap between endometriosis and endometrial cancer: evidence from cross-disease genetic correlation and GWAS meta-analyses. Cancer Medicine. 2018; 7(5): 1978-1987. doi:10.1002/cam4.1445.
- Wu L, Lv C, Su Y, Li C, Zhang H, Zhao X, et al. Expression of programmed death-1 (PD-1) and its ligand PD-L1 is upregulated in endometriosis and promoted by 17beta-estradiol. Gynecological Endocrinology. 2019; 35(3): 251-256. doi:10.1080/09513590.2018.1519787.
- Walankiewicz M, Grywalska E, Krasowska E, Hymos A, Polak G, Kotarski J. PD-1/PDL-1 pathway in patients with endometriosis. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2016; 206: e122.
- Green AK, Feinberg J, Makker V. A Review of Immune Checkpoint Blockade Therapy in Endometrial Cancer. American Society of Clinical Oncology educational book. American Society of Clinical Oncology. Annual Meeting. 2020; 40(40): 238-244. doi:10.1200/EDBK_280503.
- Musacchio L, Boccia SM, Caruso G, Santangelo G, Fischetti M, Tomao F, et al. Immune Checkpoint Inhibitors: A Promising Choice for Endometrial Cancer Patients?. Journal of Clinical Medicine. 2020; 9(6): 1721. doi:10.3390/ jcm9061721.
- Agostinis C, Balduit A, Mangogna A, Zito G, Romano F, Ricci G, et al. Immunological Basis of the Endometriosis: The Complement System as a Potential Therapeutic Target. Frontiers in Immunology. 2020; 11. doi:10.3389/ fimmu.2020.599117.
- Kim S, Hwang K, Choi K. Potential roles of reactive oxygen species derived from chemical substances involved in cancer development in the female reproductive system. BMB Reports. 2018; 51(11): 557-562. doi:10.5483/ BMBRep.2018.51.11.056.
- Segura SE, Nobre SP, Hussein YR, Abu-Rustum NR, Weigelt B, Soslow RA, et al. DNA Mismatch Repair–deficient Endometrial Carcinosarcomas Portend Distinct Clinical, Morphologic, and Molecular Features Compared With Traditional Carcinosarcomas. The American Journal of Surgical Pathology. 2020; 44(11): 1573-1579. doi:10.1097/PAS.0000000000001561.
- Kurpiel B, Thomas MS, Mubeen M, Ring KL, Modesitt SC, Moskaluk CA, et al. MLH1/PMS2-deficient Endometrial Carcinomas in a Universally Screened Population: MLH1 Hypermethylation and Germline Mutation Status. International journal of gynecological pathology: official journal of the International Society of Gynecological Pathologists. 2021; 41(1): 1-11. doi:10.1097/PGP.0000000000000767.
- Kumar P, Gupta P, Gupta N, Rajwanshi A, Rai B, Shalini G. Evaluation of DNA Mismatch Repair Protein Deficiency in Primary Endometrial Carcinoma. Journal of Gynecologic Surgery. 2019; 35(3): 177-83.
- Yamamoto A, Yamaguchi T, Suzuki O, Ito T, Chika N, Kamae N, et al. Prevalence and molecular characteristics of DNA mismatch repair deficient endometrial cancer in a Japanese hospital-based population. Japanese journal of clinical oncology. 2020; 51(1): 60-69. doi:10.1093/jjco/hyaa142.