
Case Report
Austin J Reprod Med Infertil. 2023; 9(1): 1061.
Two Step Procedure; Radiofrequency Ablation and Second Look Hysteroscopy in a Patient with Diffuse Adenomyosis: An Interesting Case Report and Review of the Literature
Kolovos G*; Dedes I; Lanz S; Mueller M
Department of Obstetrics and Gynecology, University of Bern, Bern, Switzerland
*Corresponding author: Kolovos G Department of Obstetrics and Gynecology, University of Bern, Theodor-Kocher-Haus, Friedbühlstrasse 19, 3010 Bern, Switzerland Tel: +41 31 632 10 10 Email: georgios.kolovos@insel.ch
Received: July 11, 2023 Accepted: August 09, 2023 Published: August 16, 2023
Abstract
Background: Radiofrequency Ablation (RFA) is an FDA-approved minimally invasive technique widely used for the treatment of uterine fibroids. Recent studies have explored the potential of RFA for the management of adenomyosis, a benign disorder characterized by the infiltration of endometrial glands and stroma into the myometrium, resulting in dysmenorrhea and abnormal uterine bleeding.
Case Report: In this case report, we present the clinical findings of a patient who experienced persistent hypermenorrhea and dysmenorrhea ten months after undergoing RFA for symptomatic uterine fibroids and diffuse adenomyosis. Magnetic Resonance Imaging (MRI) revealed significant regression of necrotic tissue with associated cystic hemorrhages measuring approximately 4 cm in diameter. During therapeutic hysteroscopy, necrotic adenomyotic tissue was observed protruding from the posterior uterine wall and cautiously resected.
Conclusions: We summarize our experience with this case and provide conclusions based on a comprehensive review of the relevant literature. These findings highlight the challenges and potential considerations in the management of adenomyosis using RFA and emphasize the importance of further research in this area.
Keywords: Case report; Adenomyoma; Radiofrequency ablation; Uterus sparing
Introduction
Adenomyosis, a condition characterized by the invasion of endometrial glands and stroma into the myometrium, was first described by the German pathologist Carl von Rokitansky in 1860. However, it took more than a century to fully understand the origin and nature of this disease. In 1972, Bird provided a comprehensive definition of adenomyosis as a "benign invasion of endometrium into the myometrium, with ectopic non-neoplastic, endometrial glands and stroma surrounded by hypertrophic and hyperplastic myometrium." Initially, adenomyosis was predominantly diagnosed through histopathological examination of hysterectomy specimens in perimenopausal women, primarily presenting with bleeding disorders. The estimated prevalence among hysterectomy patients varies significantly, ranging from 8.8% to 61.5%. This wide range can be attributed to the challenges associated with histopathological diagnosis, as adenomyosis can manifest in different extents. It may appear as a solitary unifocal lesion on a normal-sized uterus or as diffuse, globular enlargement of the uterus, resembling multiple-week-sized masses due to a diffuse infiltration of the uterine wall. Another relatively rare subtype of adenomyosis is the "chimera" between uterine fibroids and adenomyosis, referred to as adenomyoma. Focal disease can easily be missed during histopathological examination if multiple sections of the hysterectomy specimen are not thoroughly examined.
Several theories regarding the pathogenesis of adenomyosis have been suggested, though its pathogenesis is not yet fully understood [1]. The clinical presentation of adenomyosis is highly heterogeneous, often coexisting with other benign gynecological conditions such as endometriosis and uterine fibroids. Onchee et al., in a population-based retrospective study, demonstrated a substantial healthcare burden associated with adenomyosis. Among women included in the study, 82.0% underwent hysterectomies, nearly 70% underwent imaging studies (e.g., MRI) suggestive of adenomyosis, and 37.6% relied on chronic pain medications [2]. Recent meta-analyses conducted by Nirgianakis et al. [3] revealed important implications for reproductive outcomes in women with adenomyosis. It was found that women with adenomyosis exhibit lower clinical pregnancy rates and higher miscarriage rates compared to those without the condition. Additionally, the severity of adenomyosis was found to correlate with reproductive outcomes, further underscoring the importance of disease severity in determining fertility outcomes. Similar trends were observed in Assisted Reproductive Technology (ART) settings, with significantly lower rates of implantation, clinical pregnancy per cycle, clinical pregnancy per embryo transfer, ongoing pregnancy, and live birth among women with adenomyosis compared to those without adenomyosis [4].
Advancements in imaging techniques, specifically transvaginal ultrasound and Magnetic Resonance Imaging (MRI), have significantly improved the ability to diagnose adenomyosis in a non-invasive manner. These imaging modalities have facilitated the establishment of a diagnosis "in vivo," reducing the reliance on histopathological examination alone. Currently, the prevalence of adenomyosis in symptomatic women, as determined by imaging studies, is reported to be around 20% to 30% [5]. These improved imaging techniques have contributed to a shift in the age profile of patients diagnosed with adenomyosis, with a greater number of women of childbearing age being identified with the condition.
Adenomyosis, a prevalent gynecological disorder, necessitates an expanded treatment approach encompassing conservative surgical interventions and medical therapies. Unlike endometriosis, specific medications approved for the treatment of adenomyosis are currently unavailable, and standardized management guidelines remain elusive. However, hormonal treatments have demonstrated efficacy in alleviating adenomyosis-related symptoms. Notably, Dienogest, although effective for managing endometriosis, may not adequately address Abnormal Uterine Bleeding (AUB) associated with adenomyosis. Consequently, there is a pressing need to explore alternative therapeutic strategies that specifically target the unique challenges posed by adenomyosis, particularly in terms of AUB management. Further research is imperative to develop targeted therapies and establish evidence-based guidelines for the optimal management of adenomyosis [6].
Levonogestrel IUDs, which are effective in improving both dysmenorrhea and abnormal uterine bleeding, are limited to small uteri due to a higher expulsion rate reported in larger adenomyotic uteri of up to 150mL [6,7]. Regarding the surgical strategies, the standard of care for the patients with severe symptomatology, impaired quality of life and no desire to have children is hysterectomy [8].
Uterus-sparing surgical approaches, such as metroplasty, are employed in the management of adenomyosis but demand exceptional surgical proficiency and entail heightened perioperative risks and morbidity, including the potential for uterine rupture during pregnancy [9,10]. Alternative methods encompass uterine artery embolization, endometrial ablation (suitable when adenomyosis does not extensively infiltrate the myometrium), High-Intensity Focused Ultrasound (HIFU), and the recently introduced minimally invasive option of Radiofrequency Ablation (RFA). Among these, ultrasound-guided Radiofrequency Ablation (RFA) exhibits promising potential, a treatment which has already been approved from the FDA for the treatment of uterine fibroids. Ning Hai et al. recently reported a significant decrease on VAS Scores of Dysmenorrhea and in the mean volume of focal adenomyosis change at 1, 6 and 12 months after RFA application [11].
Case Presentation
We present a case study of a 40-year-old premenopausal patient who experienced secondary infertility and progressive dysmenorrhea (Visual Analog Scale [VAS] score: 7/10), necessitating the use of analgesics such as NSAIDs and paracetamol. The patient had previously undergone laparoscopic myomectomy in 2013, followed by a vaginal delivery one year later, and subsequently experienced a miscarriage in 2017. Given the secondary infertility, persistent fibroids observed on ultrasound, and diffuse adenomyosis affecting the posterior wall of the uterus, an MRI scan was conducted, confirming the presence of diffuse adenomyosis throughout the fundus (Figure 1) and posterior wall. Additionally, at least six intramural fibroids were identified, with some demonstrating slight progression in size compared to an MRI performed two years earlier, wherein no intracavitary fibroids were detected. A three-month course of Lucrin therapy was prescribed, successfully inducing secondary amenorrhea. Subsequently, the patient was admitted to our clinic for diagnostic hysteroscopy and transcervical sonographically guided radiofrequency ablation using the Sonata system.
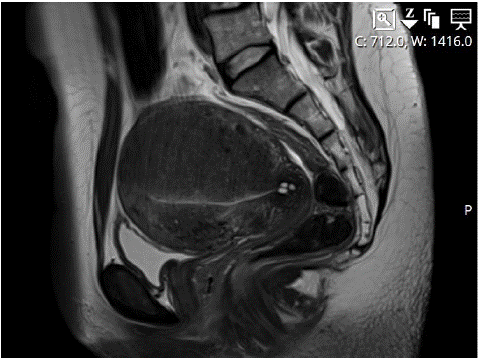
Figure 1:
The diagnostic hysteroscopy revealed no submucosal fibroids, and following cervical canal dilatation to Hegar Size 9, Radiofrequency Ablation (RFA) was performed using the Sonata® system (Gynesonics, Inc., Redwood City, CA, United States). The Sonata system comprises an integrated intrauterine sonography probe and radiofrequency ablation handpiece. The system was inserted, and the massively thickened posterior wall, affected by diffuse adenomyosis, was subjected to ablation using five slightly overlapping zones measuring 26x19 mm, 33x24 mm, 30x22 mm, 34x26 mm and in depth 37x30 mm. Strict protection of a safety zone up to 1 cm from the endometrium was maintained during the ablation. Additionally, a zone measuring 26x19 mm was ablated in the anterior wall.
During the postoperative follow-up, the patient initially reported the absence of dysmenorrhea and reduced bleeding, which gradually increased over time. Subsequent sonographic examinations raised suspicion of a necrotized adenomyoma causing uterine cavity impairment. A postoperative MRI performed six months later confirmed the presence of a demarcated necrotic area measuring 40x39x31 mm on the posterior wall (Figure 2). Consequently, the patient underwent a second-look hysteroscopy to perform resection of the necrotic adenomyotic tissue.
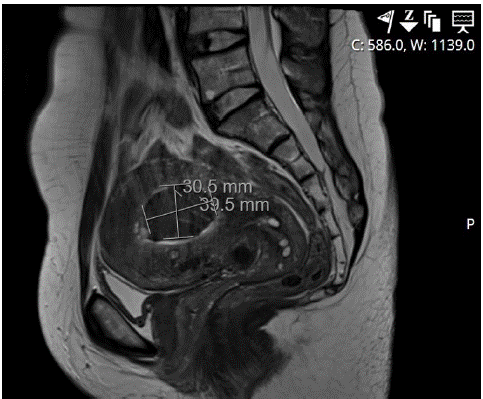
Figure 2:
During the second-look hysteroscopy, a yellowish necrotic adenomyotic tissue was observed protruding from the posterior wall into the uterine cavity, just above the inner cervix (Figure 3). In the areas of the anterior wall, lateral walls, and fundus, large regions of unremarkable endometrium were noted, with both tubal ostia being visible. The necrotic tissue was meticulously removed using a cold snare technique, ensuring that it was excised up to the level of the surrounding viable wall. Approximately 3x3 cm of tissue was extracted. As a prophylactic measure against adhesions, Mate Regen gel was inserted at the end of the operation. The procedure was successfully completed without any complications, and the patient tolerated it well. She was discharged on the same day in a symptom-free condition. Since the surgery, the patient has not experienced any significant recurrence of pain or encountered any postoperative medical issues.
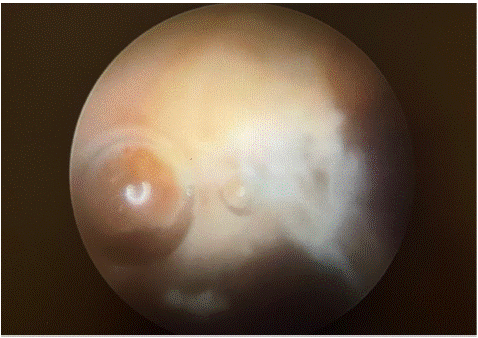
Figure 3:
Discussion
The availability of robust evidence supporting the effectiveness of surgical and medical therapeutic options for women with adenomyosis remains limited to date. Hysterectomy remains the gold standard option for patients experiencing severe symptomatology, even though some of them desire to preserve their uterus. However, this can lead to emotional trauma for certain women who wish to retain their uterus, particularly premenopausal women who seek to preserve their fertility potential. Consequently, in recent years, there has been an increasing emphasis on the development of new and less radical therapeutic options to address this unmet need.
Radiofrequency Ablation (RFA) is a well-established technique for the treatment of various tumors, including hepatocellular carcinoma, renal, adrenal, bone, lung, and breast tumors [12]. The procedure involves the application of radiofrequency waves to generate heat within the tumor tissue surrounding the electrode, effectively destroying the tumor while preserving the surrounding healthy tissue. Over time, the dead tumor cells are replaced by scar tissue, resulting in tumor shrinkage. RFA has emerged as an effective method for treating uterine fibroids, delivering excellent outcomes in symptom reduction [13]. While initially considered an off-label approach for adenomyosis, RFA has gained recognition in Asian countries like South Korea. In a retrospective study conducted by Nam JH et al. [14] involving 81 patients, significant symptom relief was observed in individuals with adenomyosis who underwent ultrasound-guided RFA. Moreover, among those who attempted to conceive after the procedure, 29 patients (35.8%) achieved a total of 39 pregnancies. Excluding the 23 patients who did not actively pursue conception or discontinued pregnancy attempts, the overall success rate reached 50%.
In a separate retrospective study conducted in China, significant improvements were observed in both the Visual Analog Scale (VAS) score and Symptom Severity Score following Radiofrequency Ablation (RFA) treatment for adenomyosis. These improvements were statistically significant at the end of the first year of follow-up. Additionally, the study found that the mean uterine volume reduction rate was 35.8% at 1 month, 40.8% at 6 months, and 41.2% at 12 months post-ablation. Importantly, no significant complications or adverse events were reported during the study [15]. For cases involving large adenomyomas with a high likelihood of demarcation and prolapsing within the uterine cavity, it is recommended to plan a second-look hysteroscopy. This additional procedure can aid in the successful treatment of adenomyosis by addressing any residual or recurrent adenomyotic tissue and ensuring optimal outcomes.
Conclusion
Based on current data, Radiofrequency Ablation (RFA) demonstrates potential efficacy in preserving the uterus while treating adenomyosis, accompanied by a safe risk profile and minimal adverse events. This is exemplified in our case study involving the persistent necrotized adenomyoma. In cases where residual or recurrent adenomyotic tissue is a concern, as is often the case with other hysteroscopic procedures such as large submucosal fibroids or persistent placental remnants, a second-look hysteroscopy is recommended. In conclusion, the promising results from observational studies highlight the need for further evaluation of RFA for adenomyosis in prospective and large randomized clinical trials. Such studies would help expand the range of treatment options available for this complex condition, thereby providing more effective management strategies for patients.
Author Statements
Author Contributions
All authors contributed to the study conception and design. The first draft of the manuscript was written by G.K. and all authors commented on previous versions of the manuscript. Supervision was carried out by M.M. All authors have read and agreed to the published version of the manuscript.
Funding
No funding from an external source was sought or secured in relation to this case report.
Patient Consent
Written informed consent was obtained from the patient for publication of this case report.
Declaration of Competing Interest
The authors declare that they have no conflict of interest regarding the publication of this case report.
References
- Antero MF, Ayhan A, Segars J, Shih IM. Pathology and pathogenesis of adenomyosis. Semin Reprod Med. 2020; 38: 108-18.
- Ms OY, Schulze-Rath R, Bs JG. Bs KH, Scholes D, Mph SDR. Adenomyosis incidence, prevalence and treatment: United States population-based study. 2006: 1–10.
- Nirgianakis K, Kalaitzopoulos DR, Schwartz ASK, Spaanderman M, Kramer BW, Mueller MD MM. Fertility, pregnancy and neonatal outcomes of patients with adenomyosis: a systematic review and meta-analysis. Reprod Biomed Online. 2020; 42: 185-206.
- Younes G, Tulandi T. Effects of adenomyosis on in vitro fertilization treatment outcomes: a. Fertil Steril. 2016; 108: 483-490.
- Upson K, Missmer SA. Epidemiology of adenomyosis. Semin Reprod Med. 2020; 38: 89-107.
- Vannuccini S, Luisi S, Tosti C, Sorbi F, Petraglia F. Role of medical therapy in the management of uterine adenomyosis. Fertil Steril. 2018; 109: 398-405.
- Lee KH, Kim JK, Lee MA, Ko YB, Yang JB, Kang BH, et al. Relationship between uterine volume and discontinuation of treatment with levonorgestrel-releasing intrauterine devices in patients with adenomyosis. Arch Gynecol Obstet. 2016; 294: 561-6.
- Pontis A, Alterio MND, Pirarba S, et al. Adenomyosis: a systematic review of medical treatment adenomyosis: a systematic review of medical treatment. 2017; 3590: 2017.
- Osada H. PhD. uterine adenomyosis and adenomyoma: the surgical approach. Fertil Steril. 2018; 109: 406-17.
- Nikolaou M, Kourea HP, Antonopoulos K, Geronatsiou K, Adonakis G, Decavalas G. Spontaneous uterine rupture in a primigravid woman in the early third trimester attributed to adenomyosis: A case report and review of the literature. J Obstet Gynaecol Res. 2013; 39: 727-32.
- Hai N, Hou Q, Xiaoping Ding XD, MJ, Jin M. Ultrasound-guided transcervical radiofrequency ablation for symptomatic uterine adenomyosis. Br J Radiol. 2017; 90: 20160119.
- Friedman M, Mikityansky I, Kam A, Libutti SK, Walther MM, Neeman Z, et al. Radiofrequency ablation of cancer. Cardiovasc Intervent Radiol. 2004; 27: 427-34.
- Lukes A, Green MA. Three-year results of the Sonata pivotal trial of transcervical fibroid ablation for symptomatic uterine myomata. J Gynecol Surg. 2020; 36: 228-33.
- Nam JH. Pregnancy and symptomatic relief following ultrasound-guided transvaginal radiofrequency ablation in patients with adenomyosis. J Obstet Gynaecol Res. 2020; 46: 124-32.
- Hai N, Hou Q, Ding X, Dong X, Jin M. Ultrasound-guided transcervical radiofrequency ablation for symptomatic uterine adenomyosis. Br J Radiol. 2017; 90: 20160119.