
Research Article
Sarcoma Res Int. 2022; 7(1): 1050.
Clinical Activity of Sirolimus in Classic Kaposi’s Sarcoma Patients: Long-Term Remission of Lesions
Zhimin Su1#, Fang Li1#, Hexia X Gan1, Jingmei Zheng2, Zhiming Wang1,3* and Yuhong Zhou3
1Department of Medical Oncology, Xiamen Branch, Zhongshan Hospital, Fudan University, Xiamen, 361015, China
2Department of Pathology, Xiamen Branch, Zhongshan Hospital, Fudan University, Xiamen, 361015, China
3Department of Medical Oncology, Zhongshan Hospital, Fudan University, Shanghai, 200032, China
#These authors have contributed equally to this work
*Corresponding author: ZhimingWang, Department of Medical Oncology, Xiamen Branch, Zhongshan Hospital, Fudan University, Xiamen, 361015, China, 668 Jinhu Road, Huli District, Xiamen, 361015, China
Received: May 17, 2022; Accepted: June 20, 2022; Published: June 27, 2022
Abstract
Introduction: Kaposi’s sarcoma (KS) is a multicentric angiomatous neoplasm of lymphatic endothelium-derived cells that leads to angioproliferative diseases and affects the skin and internal organs. Currently, the main treatment options include radiotherapy, chemotherapy and targeted treatment. However, due to the low incidence rate and high malignancy, prognosis remains dismal and thus novel strategies are warranted.
Methods: We treated 3 cases of KS with sirolimus, and evaluated the effectiveness and related side effects. Two were not suitable for chemotherapy, andone refused chemotherapy.
Results: In this study, three patients all achieved symptom relief within three days. One patient died of pulmonary infection, and the overall survival time was 12.0 months. The other two patients had not reached disease progression, and the progression free survival time (PFS) of one patient was more than 18 months. The main adverse reaction was grade 1-2 oral mucositis, which was well controlled and well tolerated.
Conclusion: This study shows the long-term remission of lesions and satisfactory safety of oral sirolimus in KS patients who are unsuitable for chemotherapy or refuse chemotherapy.
Keywords: Classic Kaposi’s Sarcoma; Sirolimus; mTOR Inhibitors; Clinical Activity; Long-Term Remission
Introduction
Kaposi’s sarcoma (KS) is a multicentric angiomatous neoplasm of lymphatic endothelium-derived cells [1,2]. The level of Immunosuppression and HHV-8 infection are the most commonly implicated etiological factors affecting the development and progression of KS [2]. It is generally categorized into four subtypes: classic (sporadic initially described by Kaposi), endemic (observed in African), iatrogenic (immunosuppressive related) and epidemic (AIDS related) [2,3]. KS usually presents with purple, red or dark brown macules, patches, papules or nodules, involving the lower limbs, and may be bleeding, ulcer, verrucous and hyperkeratosis [2,4]. Lymphoedema and oral involvement are commonly seen and can precede maculopapular lesions [4]. Besides, visceral involvement, for example, lung, gastrointestinal tract, bone and liver, most frequently occurs in epidemic type. It is usually manifested as slow progression and indolent biologic behavior, whereas epidemic and iatrogenic KS may be extensive and life threatening. According to the research of the Italian cancer registry, the 5-year and 10-year survival rates of KS are 69% and 46% respectively with a median survival of 9. 4 years [5]. One hundred and thirty-nine were men (median age 70 years) and 65 were women (median age 72). One, 5 and 10 year survival rates were 0. 92, 0. 69 and 0. 46 respectively. Survival did not vary according to sex and tumor sites. Owing to its slow progression, KS aims to control disease progression and improve quality of life.
At present, based on the European consensus-based interdisciplinary guideline, the treatments of KS mainly include local therapies, radiotherapy, surgical excision, cryosurgery and laser, interferon and chemotherapy [6]. Radiotherapy is feasible for early localized lesions, and surgical resection is another option, but it is only limited to small superficial lesions, and the recurrence rate is high. In addition, most chemotherapeutic drugs have potential serious side effects, and lack sufficient evidence. Due to the low incidence rate and comorbidities, there is no standard therapy for KS patients who do not tolerate chemotherapy or refuse chemotherapy.
The mTOR is a serine threonine protein kinase, which plays a central regulatory role in gene transcription, protein synthesis, apoptosis and other important processes [7]. Sirolimus is a typical inhibitor of mammalian target of rapamycin (mTOR) signaling pathway. Recently, it has become a new treatment for KS because it blocks the tumor formation of G protein coupled receptor (vgpcr) encoded by KS herpesvirus in vivo and in vitro [8]. The effectiveness of oral administration has been confirmed in the treatment of renal transplant KS patients [9]. However, so far, it has not been used in the treatment of KS who refuse chemotherapy or chemotherapy intolerance. Here, we report 3 patients who achieved long-term disease control after oral sirolimus.
Patients and Methods
Patient Preparation
We treated 3 cases of classic KS with sirolimus, and evaluated the effectiveness and related side effects. Written informed consent was obtained from each patient. Participants’ demographics, clinicopathological data were collected from hospital electronic medical records and follow-up phone inquiries.
Ethics Approval and Consent to Participate
The Ethics Committee of Xiamen Branch, Zhongshan Hospital, Fudan University Biomedical Research Department provided ethical approval. This study was carried out in accordance with the principles of the Helsinki declaration and informed consent for collecting and preserving samples and details was obtained from each patient.
Efficacy Evaluation
The efficacy was evaluated according to recist1. 1, which was divided into complete response (CR), partial response (PR), and disease stable (SD) and disease progression (PD). The progression free survival (PFS) and overall survival (OS) of patients were counted.
Results
Baseline Characteristics of Patients
The features of the patients are shown in (Table 1). Two were not suitable for chemotherapy, andone refused chemotherapy.
Patient
Age
Gender
Combined diseases
Sirolimus oral dose
Side effect
PFS (months)
OS (months)
Patient 1
62
male
Focal proliferative IgA nephropathy and chronic kidney disease
1mg / d
No obvious adverse
25
NA
Patient 2
56
male
Hypertension
1mg / d
Mild lower limb edema
24
NA
Patient 3
74
male
Rheumatoid arthritis,hypertension and chronic kidney disease
1mg / d
Grade 1 oral mucosa
4
4
Table 1: Features of patients.
Case Presentation
Patient 1, male, 62 years old, and was referred to local hospital in September 2018 dueto the increased urine foam. Ultrasound in the external hospital: left / right kidney length 120/110mm, double kidney multiple cysts, urine protein 3+, urine occult blood 3+, 24h urine protein 4. 65g/L, 2018-10-12 serum creatinine 106umol/L, albumin 21. 91g/L, nephritic syndrome diagnosed clearly. On October 24, 2018, renal biopsy was performed in our hospital. Combined with pathological diagnosis, focal proliferative IgA nephropathy and stage 2 chronic kidney diseases were given prednisone acetate 60mg/d orally. From May 2019, it was found that the left leg was swollen without diagnosis and treatment. After that, multiple purplish red warty nodules were added to the left leg, gradually increasing, involving the lower limbs (Figure 1A). Skin biopsy was performed in an external hospital on July 15, 2019. The postoperative pathology showed vascular tumors. Immunohistochemistry showed positive expressions of CD31, CD34, HHV8 and ERG, Ki-67 (60%). KS was considered. No obvious tumor metastasis was found on plain computed tomography (CT) scan of thoracoabdominal basin. Sirolimus was given orally for 1mg / d instead of chemotherapy due to the patient’s nephrotic syndrome. This patient achieved symptom relief within three days. After administration for 3 months, the rash subsided, local scab and skin swelling subsided significantly (Figure 1B), and the condition PR was evaluated. During this period, the glucocorticoids treatment of nephrotic syndrome continued and gradually decreased the dosage and completed withdrawn in October 2019. There was no obvious adverse reaction during the oral administration of sirolimus. After half a year, the rash continued to shrink, and the local skin did not reswell (Figure 1C). After 1 year, the focus continued to PR, leaving only pigmentation (Figure 1D).
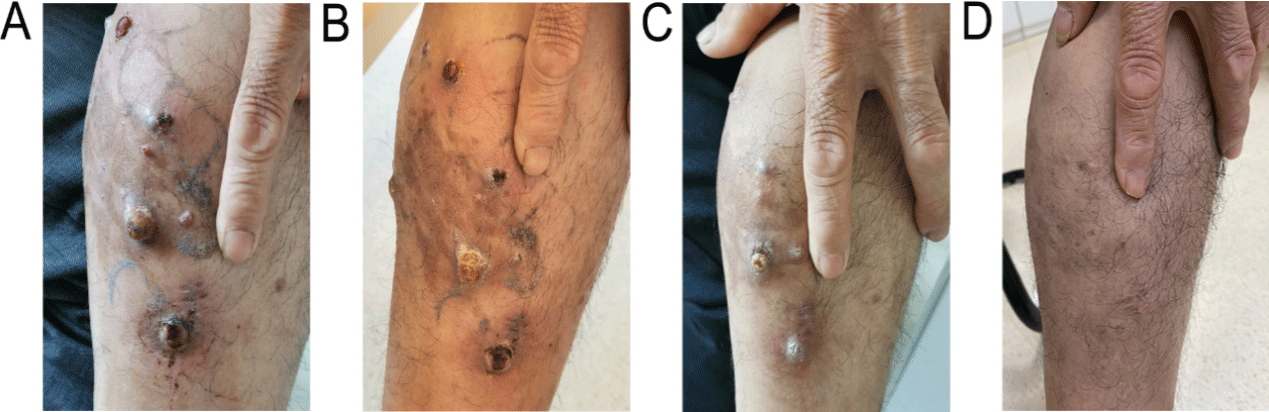
Figure 1:
Patient 2, male, 56 years old, went to the local hospital one and a half months ago because of redness and swelling of the left lower limb and pain after standing for a long time. There was no obvious improvement after symptomatic treatment, so he underwent skin biopsy of the left lower leg on July 29, 2019. The postoperative pathology showed: (rash of the left lower leg) vascular tumor, mitotic image was easy to see (Figure 2A), and immunohistochemistry showed CD31, CD34, ERG, FLI-1, Ki-67 positive expression (Figure 2B-2F). Referring to immunohistochemical and clinical findings, he was diagnosed with Kaposi’s sarcoma. CT revealed no recurrence and metastasis. Combined with personal will and the efficacy of oral sirolimus in the previous patient we observed, this patient took sirolimus 1mg/d orally from August 8, 2019 instead of chemotherapy. After only 2 days, the lower limb tumors were significantly smaller than before, and the lesions were improved. No obvious side effects were observed beside lower limb edema (G1) during the treatment. After 1 month, the lesions were improved. Compared with the focus in July 2019, edema and rash were significantly improved after two years. At present, the patient is ongoing the treatment of sirolimus and the disease is under control.
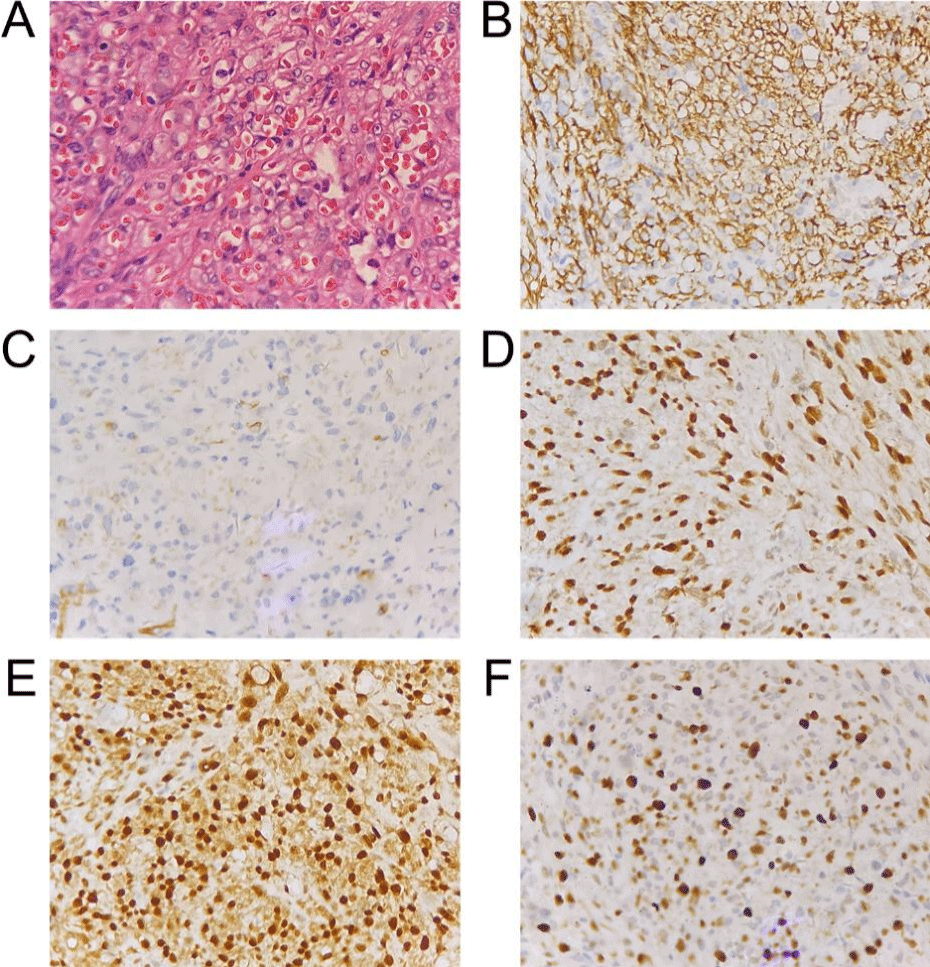
Figure 2:
Patient 3, male, 74 years old, found a purplish red lesion on the right plantar skin on July 6, 2020, with a size of about 3-4cm, local ulcer formation, accompanied by bleeding and exudation. Red patches on the skin of limbs and back, about 1-2cm in diameter higher than the skin surface. Pathological biopsy in the external hospital on August 13, 2020 showed that the tumor was of vascular origin. Immunohistochemistry: positive expression of CD31, CD34, D2-40, FLI-1 and ERG, HHV8 (+) and Ki-67 (30%). KS was considered in pathological consultation. Anti-infective treatment was given for more than 20 days, but the lesion did not improve significantly. The patient was referred to our hospital on August 31, 2020. Rheumatoid arthritis has been treated with prednisone 5mg/d for more than 10 years; with a history of hypertension for 8 years, valsartan and amlodipine were given to reduce blood pressure, and the blood pressure could be controlled; chronic kidney disease complicated with renal insufficiency and cardiac insufficiency. Sirolimus 1mg/d was taken orally from September 2, 2020. The lesions of lower limbs were improved 3 days after treatment. After 2 months, the skin lesions of the right plantar were significantly improved and the exudate was significantly absorbed. Grade 1 oral mucosa appears during oral sirolimus, which can be relieved after symptomatic treatment. On January 13, 2021, the patient died of lung infection. Therefore, the final OS of the patient after taking sirolimus was 4.0 months.
Discussion
The average age of onset of KS varies by subtype. Typical KS usually occurs in elderly men, while epidemic KS may occur earlier, which is consistent with the age of three patients in this study [2,6]. The diagnosis of KS depends on pathological biopsy. Common endothelial cell markers such as CD34 and CD31 were all expressed in three patients of this study.
According to previous studies, for KS with local lesions, radiotherapy, intralesional chemotherapy and electrochemical therapy have good curative effects. Imiquimod or 9-cis retinoic acid can also be used locally, but there is no randomized trial comparison. Radiotherapy is one of the most efficient treatments for all forms of localised KS with overall response rates range from 47% to 99% [10-14]. However, it has a possible risk of off-site recurrence and skin toxicity (telangiectasia, pigmentation, skin atrophy and fibrosis). Surgical resection is only suitable for some patients, and the recurrence rate is high. Generally, KS also needs systematic treatment to control disease and reduce lesions. Previously reported chemotherapeutic drugs mainly include pegylated liposomal doxorubicin (PLD), paclitaxel, vinblastine, etoposide, and interferon a-2A/2B and antiangiogenic drugs (pomalidomide / lenalidomide / bevacizumab) [6]. In a study of twenty-eight HIV patients with moderate-advanced KS, a better response rate was observed in the HAART plus PLD group after 48 weeks (76% versus 20%) [15]. PLD is approved as first-line therapy of HIV-related KS. Another study included 73 analyzable patients, including 36 in the paclitaxel arm and 37 in the PLD arm. Comparing the paclitaxel and PLD arms revealed comparable response rates (56% vs 46%; P=0. 49), median progression-free survival (17. 5 months vs 12. 2 months; P=0. 66), and 2-year survival rates (79% vs 78%; P=0. 75), but somewhat more grade 3 to 5 toxicity for paclitaxel (84% vs 66%; P=0. 077) [16]. In general, 80 mg/m2 weekly on a continuous basis or 3 weeks on 1 week off is the preferred schedule [17,18]. In 9 out of the 11 Classic KS patients, interferon alfa (3 million units 5 times a week for 2 weeks then 2-6 million units 3-6 times a week) was evaluated. Maximum response was achieved after 4-6 months. Remission lasted 4-72 months. Recurrences were retreated, with additional remissions after only 5-8 weeks of treatment. Side effects included fever and fatigue, which were overcome by dose reduction [19]. Pomalidomide was tested in 15 HIV-infected patients and 7 HIV-uninfected patients with an overall response rate (ORR) of 60% (95% confidence interval [CI], 32%-84%) and 100% (95% CI, 59%-100%), respectively; median progression-free survival was 16. 6 months [20]. Other antiangiogenic drugs such as bevacizumab showed a 31% ORR (95% CI, 11%-58. 7%) in a 17- patient phase II trial and deserves further evaluation. Other reported alternative drugs include sodium tetradecyl sulfate, topical timolol, interleukin-12, imatinib, liposome retinoic acid, nivolumab, pembrolizumab, sorafenib, etc. In HIV related KS, topical 9-cis-retinoid acid (alitretinoin gel 0. 1%) in association with highly active antiretroviral therapy showed 37% partial or total response rate [21]. In conclusion, as for Kaposi’s sarcoma, chemotherapy drugs have some side effects. At present, there is no unified, sustained and effective treatment for some patients with KS cannot tolerate chemotherapy or refuse chemotherapy due to multiple diseases.
It is well known that mTOR inhibitor is a serine threonine protein kinase, which plays a central regulatory role in important processes such as gene transcription, protein synthesis and apoptosis [22,23]. Sirolimus (or rapamycin), the first identified inhibitor of mTOR, has recently emerged as an active cancer treatment due to its ability to block the KS herpesvirus-encoded G protein-coupled receptor (vGPCR) oncogenesis[8]. RCC was the first cancer for which an mTOR inhibitor was approved [24]. Previous clinical studies also support the importance of mTOR pathway in soft tissue sarcoma [25- 27]. In the Stallone study, sirolimus was used to replace cyclosporine A in the treatment of 10 KS patients after renal transplantation, which promoted the regression of lesions, confirming the clinical efficacy and good safety of sirolimus [28]. Guenova et al. also reported that oral rapamycin treatment achieved good clinical results in a patient with disseminated KS with normal immune function [29]. In addition, a recent case report showed that the lesion completely subsided after 16 weeks of local treatment with sirolimus in a 73-year-old male patient with classic KS [30]. Other similar cases of KS treated with sirolimus have also been reported [31-33]. However, the number of cases in all previous reports is relatively small, some are found accidentally in patients with renal transplantation, and there is no effective evidence on KS with multiple complications unsuitable for chemotherapy, which can be explained by our study.
To our knowledge, this study is one of the few studies to evaluate the clinical efficacy of sirolimus in the treatment of classic KS in our country. In this study, the first patient was complicated with nephrotic syndrome. In oral hormone therapy, sirolimus was individualized in consideration of chemotherapy-related nephrotoxicity and the patient’s economic situation. The third patient was complicated with hypertension, rheumatoid arthritis and renal insufficiency. On the basis of the successful application of the first two patients, the second person with grade-1 hypertensives who refused chemotherapy chose to take sirolimus orally. The local lesions of the three patients were rapidly relieved three days after oral administration of sirolimus. Our results are consistent with previous studies. One patient died of pulmonary infection complications, and the overall survival time was 12 months. The other two patients have not yet reached disease progression. It is worth noting that the progression free survival time of one patient reached more than 18 months. Sirolimus, as an oral mTOR inhibitor, has good tolerance in clinical practice. The recommended dose of sirolimus is 2–5 mg per day [22]. The most common adverse reactions are skin reaction, stomatitis, thrombocytopenia, diarrhea, fatigue, hyperlipidemia and hyperglycemia [34]. Less common effects include renal insufficiency, peripheral edema, interstitial pneumonitis and infections [34]. In this study, three patients with comorbidities took sirolimus1mg/d orally, both of which were within the recommended dose. The main adverse reaction was grade 1-2 oral mucositis, and there were no grade 3-4 serious side effects. Our study directly confirmed the longterm remission of lesions and satisfactory safety of oral sirolimus in the treatment of KS who were intolerant to chemotherapy or refused chemotherapy.
Although the number of cases in this study is small, sirolimus shows satisfactory efficacy and less side effects, which also provides clinical real cases and reference data for the clinical diagnosis and treatment of KS patients. In the future, further large sample studies are still needed to verify the efficacy and safety of sirolimus in this population.
Acknowledgments
We thank Fuyu Chen for excellent and constructive advice.
References
- Antman K, Chang Y. Kaposi’s sarcoma. N Engl J Med. 2000; 342: 1027-38.
- Schneider JW, Dittmer DP. Diagnosis and Treatment of Kaposi Sarcoma. American Journal of Clinical Dermatology. 2017; 18(4): 529-539. doi:10.1007/ s40257-017-0270-4.
- Cesarman E, Damania B, Krown SE, Martin J, Bower M, Whitby D. Kaposi sarcoma. Nature Reviews Disease Primers. 2019; 5(1): 1-21. doi:10.1038/ s41572-019-0060-9.
- Lebbé C. [Humanes herpesvirus 8 (HHV-8) and Kaposi sarcoma]. Hautarzt. 2008; 59: 18-25.
- Franceschi S, Arniani S, Balzi D, Geddes M. Survival of classic Kaposi’s sarcoma and risk of second cancer. British Journal of Cancer. 1996; 74(11): 1812-1814. doi:10.1038/bjc.1996.635.
- Lebbe C, Garbe C, Stratigos AJ, Harwood C, Peris K, Marmol VD, et al. Diagnosis and treatment of Kaposi’s sarcoma: European consensus-based interdisciplinary guideline (EDF/EADO/EORTC). European journal of cancer. 2019; 114: 117-127. doi:10.1016/j.ejca.2018.12.036.
- Dancey J. mTOR signaling and drug development in cancer. Nature Reviews Clinical Oncology. 2010; 7(4): 209-219. doi:10.1038/nrclinonc.2010.21.
- Sodhi A, Montaner S, Patel V, Gómez-Román JJ, Li Y, Sausville EA, et al. Akt plays a central role in sarcomagenesis induced by Kaposi’s sarcoma herpesvirus-encoded G protein-coupled receptor. Proceedings of the National Academy of Sciences of the United States of America. 2004; 101(14): 4821- 4826. doi:10.1073/PNAS.0400835101.
- Yaich S, Charfeddine K, Zaghdane S, Aoud NE, Jarraya F, Kharrat M, et al. Sirolimus for the treatment of Kaposi sarcoma after renal transplantation: a series of 10 cases. Transplantation proceedings. 2012; 44(9): 2824-2826. doi:10.1016/j.transproceed.2012.09.025.
- Stelzer KJ, Griffin TW. A randomized prospective trial of radiation therapy for AIDS-associated Kaposi’s sarcoma. International journal of radiation oncology, biology, physics. 1993; 27(5): 1057-1061. doi:10.1016/0360- 3016(93)90523-X.
- Kigula-Mugambe JB, Kavuma A. Epidemic and endemic Kaposi’s sarcoma: a comparison of outcomes and survival after radiotherapy. Radiother Oncol. 2005; 76: 59-62.
- Yildiz F, Genc M, Akyurek S, Cengiz M, Ozyar E, Selek U, et al. Radiotherapy in the management of Kaposi’s sarcoma: comparison of 8 Gy versus 6 Gy. J Natl Med Assoc. 2006; 98(7): 1136-9.
- Caccialanza M, Marca S, Piccinno R, Eulisse G. Radiotherapy of classic and human immunodeficiency virus-related Kaposi’s sarcoma: results in 1482 lesions. Journal of the European Academy of Dermatology and Venereology. 2008; 22(3): 297-302. doi:10.1111/j.1468-3083.2007.02405.x.
- Singh NB, Lakier RH, Donde B. Hypofractionated radiation therapy in the treatment of epidemic Kaposi sarcoma--a prospective randomized trial. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2008; 88(2): 211-216. doi:10.1016/j. radonc.2008.03.009.
- Martin-Carbonero L, Barrios A, Saballs P, Sirera G, Santos J, Palacios R, et al. Pegylated liposomal doxorubicin plus highly active antiretroviral therapy versus highly active antiretroviral therapy alone in HIV patients with Kaposi’s sarcoma. AIDS. 2004; 18(12): 1737-1740. doi:10.1097/01. AIDS.0000131385.60974.B9.
- Cianfrocca M, Lee S, Roenn JV, Tulpule A, Dezube BJ, Aboulafia DM, et al. Randomized trial of paclitaxel versus pegylated liposomal doxorubicin for advanced human immunodeficiency virus-associated Kaposi sarcoma. Cancer. 2010; 116(16): 3969-3977. doi:10.1002/cncr.25362.
- Fardet L, Stoebner P, Bachelez H, Descamps V, Kerob D, Meunier L, et al. Treatment with taxanes of refractory or life-threatening Kaposi sarcoma not associated with human immunodeficiency virus infection. Cancer. 2006; 106(8): 1785-1789. doi:10.1002/cncr.21791.
- Brambilla L, Romanelli A, Bellinvia M, Ferrucci S, Vinci M, Boneschi V, et al. Weekly paclitaxel for advanced aggressive classic Kaposi sarcoma: experience in 17 cases. British Journal of Dermatology. 2008; 158(6): 1339- 1344. doi:10.1111/j.1365-2133.2008.08517.x.
- Tur E, Brenner S. Classic Kaposi’s sarcoma: low-dose interferon alfa treatment. Dermatology. 1998; 197: 37-42.
- Polizzotto MN, Uldrick TS, Wyvill KM, Aleman K, Peer CJ, Bevans M, et al. Pomalidomide for Symptomatic Kaposi’s Sarcoma in People With and Without HIV Infection: A Phase I/II Study. J Clin Oncol. 2016; 34(34): 4125- 31.
- Bodsworth NJ, Bloch M, Bower M, Donnell D, Yocum R, Group TIPGKS. Phase III Vehicle-Controlled, Multi-Centered Study of Topical Alitretinoin Gel 0.1% in Cutaneous AIDS-Related Kaposi’s Sarcoma. American Journal of Clinical Dermatology. 2001; 2(2): 77-87. doi:10.2165/00128071-200102020- 00004.
- Chen YL, Law P, Loh HH. Inhibition of PI3K/Akt signaling: an emerging paradigm for targeted cancer therapy. Current medicinal chemistry. Anticancer agents. 2005; 5(6): 575-589. doi:10.2174/156801105774574649.
- Wan X, Helman LJ. The biology behind mTOR inhibition in sarcoma. The oncologist. 2007; 12(8): 1007-1018. doi:10.1634/ THEONCOLOGIST.12-8-1007.
- Motzer RJ, Bukowski RM. Targeted therapy for metastatic renal cell carcinoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006; 24(35): 5601-5608. doi:10.1200/JCO.2006.08.5415.
- Hofbauer G, Marcollo-Pini A, Corsenca A, Kistler A, French L, Wüthrich R, et al. The mTOR inhibitor rapamycin significantly improves facial angiofibroma lesions in a patient with tuberous sclerosis. British Journal of Dermatology. 2008; 159(2): 473-475. doi:10.1111/j.1365-2133.2008.08677.x.
- Hage JVD. Faculty Opinions recommendation of Diagnosis and treatment of Kaposi’s sarcoma: European consensus-based interdisciplinary guideline (EDF/EADO/EORTC). Faculty Opinions – Post-Publication Peer Review of the Biomedical Literature. 2019. doi:10.3410/F.735777963.793560422.
- Zhang S, Liang L, Ji Y, Wang Z, Zhou Y. The benefit of everolimus in recurrent/ epithelioid angiosarcoma patients: Case reports and literature review. Oncotarget. 2017; 8(55): 95023-95029. doi:10.18632/oncotarget.21832.
- Salido R, Garnacho-Saucedo G, Cuevas-Asencio I, Ruano J, Galán-Gutierrez M, Vélez A, et al. Sustained clinical effectiveness and favorable safety profile of topical sirolimus for tuberous sclerosis – associated facial angiofibroma. Journal of the European Academy of Dermatology and Venereology. 2012; 26(10): 1315-1318. doi:10.1111/j.1468-3083.2011.04212.x.
- Bissler JJ, McCormack FX, Young LR, Elwing JM, Chuck G, Leonard JM, et al. Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis. The New England journal of medicine. 2008; 358(2): 140-151. doi:10.1056/NEJMoa063564.
- Guenova E, Metzler G, Hoetzenecker W, Berneburg M, Rocken M. Classic Mediterranean Kaposi’s sarcoma regression with sirolimus treatment. Archives of dermatology. 2008; 144(5): 692-3. doi:10.1001/archderm.144.5.692.
- Ho CM, Huang SF, Hu RH, Ho MC, Wu YM, Lee PH. Sirolimus-induced signaling modifications in Kaposi’s sarcoma with resolution in a liver transplant recipient. Clin Transplant. 2010; 24(1): 127-32.
- Lebbe C, Garbe C, Stratigos AJ, Harwood C, Peris K, Marmol VD, et al. Diagnosis and treatment of Kaposi’s sarcoma: European consensus-based interdisciplinary guideline (EDF/EADO/EORTC). European journal of cancer. 2019; 114: 117-127. doi:10.1016/j.ejca.2018.12.036.
- Wu W, Li C, Lee RA, Hata T. The use of sirolimus to treat Kaposi’s sarcoma in an HIV-negative rheumatoid arthritis patient on disease-modifying drug therapies. Int J Dermatol. 2015; 54(10): 1188-90.
- Duran I, Siu LL, Oza AM, Chung T, Sturgeon J, Townsley CA, et al. Characterisation of the lung toxicity of the cell cycle inhibitor temsirolimus. European journal of cancer. 2006; 42(12): 1875-1880. doi:10.1016/J. EJCA.2006.03.015.