
Research Article
Sarcoma Res Int. 2023; 8(1): 1051.
Impact of Nodal Metastasis on Survival in Clear Cell Sarcoma
Hironari Tamiya1,2; Yoshinori Imura1; Toru Wakamatsu1; Hidetatsu Outani3; Shigeki Kakunaga1,4; Satoshi Takenaka1
1Department of Orthopedic Surgery, Osaka International Cancer Institute, Japan
2Department of Rehabilitation, Osaka International Cancer Institute, Japan
3Department of Orthopedic Surgery, Osaka University Hospital, Japan
4Department of Orthopedic Surgery, Osaka National Hospital, Japan
*Corresponding author: Hironari Tamiya, MD, PhD Department of Orthopedic Surgery, Osaka International Cancer Institute, 3-1-69 Otemae, Chuoku, Osaka 541-8567, Japan. Telephone: +81-6-6945-1181 Email: hironari.tamiya@oici.jp
Received: November 20, 2023 Accepted: December 18, 2023 Published: December 23, 2023
Abstract
Aim: Clear Cell Sarcoma (CCS) of soft tissue is a high-grade malignancy that often occurs on the distal extremities. The five-year overall survival is reported to be around 40%. Lymph node metastasis, that is categorized into stage IV in the latest American Joint Committee on Cancer (AJCC, 8th edition) staging system, is characteristic in CCS. We here focused on the impact of nodal metastasis on prognosis in CCS.
Methods: We performed a retrospective analysis of 37 patients with CCS who were treated between 1982 and 2023. This study investigated age, gender, tumor size, tumor location, AJCC stage (7th and 8th edition), presence of lymph node or distant metastasis, presence of surgery, chemotherapy, radiotherapy, and surgical margin. Statistical analysis was conducted to evaluate the impact of each factor on survival.
Results: In our cohort, tumor location, tumor size, and stage were significant prognostic factors. Notably, the disease-Specific Survival (DSS) in patients with nodal and/or distant metastasis was significantly shorter than those with no metastasis while DSS in the patients with nodal metastasis was not significantly different from those with distant metastasis.
Conclusion: Consistent with the latest AJCC 8th edition in which nodal and distant metastasis are both categorized into stage IV, there was no difference in DDS between nodal metastasis and distant metastasis in CCS in the present study. This result highlights the importance of nodal metastasis in CCS.
Keywords: Clear Cell Sarcoma; Nodal Metastasis; Distant Metastasis
Introduction
Clear Cell Sarcoma (CCS) of soft tissue is a rare type of high-grade malignant tumor arising from tendons and aponeurosis. It accounts for less than 1% of all soft tissue tumors. This rare tumor is also known as malignant melanoma of soft parts because tumor tissue tests positive for melanocytic markers such as MITF, Melan A, and HMB45. CCS mostly affects adults in their third to fourth decades of life. Approximately 90% of CCS occurs in the extremities. Notably, the occurrence of this tumor in the foot and ankle and the distal portion of the leg are around 40%. Its size is relatively small (2–6 cm) [1]. These characteristics make the tumors unique compared to other relatively common sarcomas such as myxofibrosarcoma, synovial sarcoma, or leiomyosarcoma.
Clinical outcomes of CCS remain poor. As previously reported, five-year survival ranges from 30% to 67% [2-4]. It is challenging to treat CCS even if a multidisciplinary treatment approach is used. To date, surgical resection is the most reliable treatment option as the effectiveness of chemotherapy and radiotherapy is unclear. Chemotherapy for CCS is not necessary if complete surgical resection can be achieved [4] and is indicated only in patients with metastatic disease; however, its efficacy appears poor [5], and further investigation to validate its usefulness is required. CCS also metastasizes rapidly throughout the body. Predominant lymph node metastasis is a unique clinical trait for CCS, in contrast to the generally blood-borne metastasis seen in other types of sarcoma. Recently, the American Joint Committee on Cancer (AJCC, 8th edition) staging system recently mentioned that any lymph node metastasis is categorized into stage IV, indicating strong association with poor prognosis.
Established factors for poor prognosis include necrosis, the extent of mitosis, resection margin, anatomic location, and tumor size [2], with tumor size being the most strongly correlated to survival [3-5,7]. Tumors larger than 5 cm tend to recur more frequently due to micrometastases [4,8]. However, one report has demonstrated that tumor size was not significant to prognosis and that tumor location, such as presence in trunk, predicted a worse prognosis [9].
The present study aimed to determine whether there is difference in survival in patients with nodal metastasis as opposed to distant metastasis in CCS.
Methods
Between 1982 and 2023, we treated 37 patients with CCS in three facilities: Osaka International Cancer Institute, Osaka University Hospital, and Osaka National Hospital. To identify significant prognostic factors, the following variables were considered: age, gender, tumor location, tumor size, chemotherapy, radiotherapy, TNM classification and stage at initial presentation, follow-up duration, and status at the last follow-up. Staging was categorized according to the AJCC 7th or 8th edition. Chemotherapeutic regimens were determined based on the patient’s wishes and discussions among the attending orthopedists. Chemotherapy dose was adjusted based on the degree of adverse effects. The Kaplan-Meier method was used to calculate overall and Disease-Specific Survival (DSS). The logrank test was used to evaluate statistical significance. Differences at p<0.05 were considered significant. Statistical analyses were conducted using EZR (64 bit) [10].
Results
Demographic data was obtained from 37 patients. The age of the study population ranged from 8 to 83 years (median, 38) with 18 males (49%) and 19 (51%) females. Tumors were predominant in the extremities (n=28, 76%), whereas nine (24%) cases exhibited tumors in the trunk, including in the buttocks, chest wall, small intestine, groin, axilla, perineum, and head. The ankle/foot/toe was the most common (n = 16, 43%) location, followed by thigh/knee (n=6, 16%), and the hand/finger (n=4, 11%). All tumors were located in the deep layers and varied from 0.5 to 13 cm (median, 3 cm) in size. Tumors =2 cm were seen in 11 cases (30%), between 2 and 5 cm in 11 cases (30%), between 5 and 10 cm in 8 cases (22%), and >10 cm only in two cases (5%). There were no records of tumor size in five cases. Twenty patients (54%) presented with lymph node and/or distant metastases, whereas 17 cases (46%) displayed localized lesions at the initial visit. The AJCC staging system 7th edition categorized 16 cases as stage IIA (high-grade deep tumors in =5 cm without metastases), 5 as stage III (any tumors with lymph node metastases), and 16 as stage IV (any tumors with distant metastases) whereas 8th edition classified 16 cases into stage II (high-grade tumors in =5 cm without metastases), only one case as stage IIIA (high-grade tumors in 5-10 cm without metastases), and 20 into stage IV (any tumors with nodal and/or distant metastases).
Thirty-one (84%) patients underwent surgical resection of the local lesions; among them, histology of resected tissue revealed 22 cases with negative margins, including 5 amputations and 3 additional wide resections, whereas a positive margin was seen in 9 cases. Wide resection and intraoperative irradiated bone and/or tendon graft surgery (IORBG) was performed in three cases. Six patients (16%) did not undergo surgery due to the severity of disease progression. In these patients, supportive care was provided in two cases; two patients underwent just one cycle of chemotherapy, and the remaining two underwent more than two cycles of chemotherapy that resulted in disease progression. Nineteen surgical procedures were performed in ten patients and included marginal resection for local recurrence (n=2), lymph node dissection (n=5), marginal resection for distant metastasis (n=5), wide resection for distant metastasis (n=1), pulmonary resection (n=7), and hip disarticulation (n=1). Chemotherapy was administered in 27 patients (73%), and 4 patients underwent chemotherapy alone without surgical procedures. Radiotherapy was administered in 14 patients (38%).
The follow-up time ranged from 0.5 to 239 months (median, 41 months). At final follow-up, 9 (24%) patients were classified as Continuous Disease Free (CDF), 3 (8%) had No Evidence of Disease (NED), 23 (62%) were dead of disease, and 2 (5%) were dead of other causes (DOC). The disease-Specific Survival (DSS) rates at five years were 46% (Figure 1). There was a significant difference in five-year DSS among stage IIA (79%), III (53%), and IV (12%) patients in accordance with AJCC 7th edition standards (Figure 2A). The data according to the latest AJCC 8th edition was shown though the number o3f stage IIIA was only one (Figure 2B).
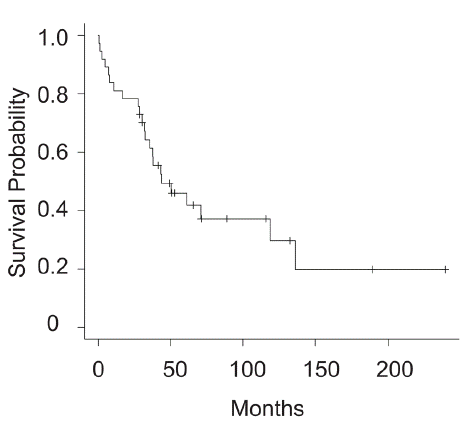
Figure 1: Disease-specific survival in overall clear cell sarcoma patients.
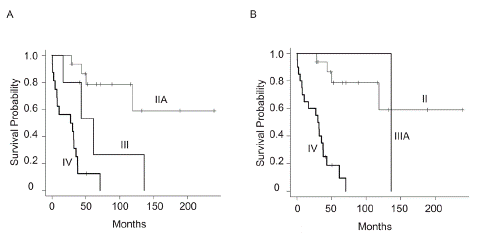
Figure 2: Disease-specific survival in clear cell sarcoma patients in each stage according to (A) AJCC 7th edition or (B) AJCC 8th edition.
We then aimed to identify prognostic factors to impact DSS. As shown in Table 1, tumor location, tumor size, and stage were significant prognostic factors. The benefits of therapies other than surgery, such as chemotherapy or radiotherapy, were not clearly demonstrated in this study. Notably, DSS in patients with nodal and/or distant metastasis was significantly shorter than those with no metastasis.
Number
5-year DSS (%)
p-value
Age
0.11
=40
21
61
>40
16
27
Gender
0.80
M
18
53
F
19
42
Location
<0.001
Extremity
28
58
Trunk
9
11
Size
<0.01
=5cm
22
60
>5cm
10
30
Stage
<0.001
IIA
16
79
III
5
53
IV
16
12
Chemotherapy
0.39
Yes
27
44
No
10
48
Radiotherapy
0.95
Yes
14
47
No
23
47
Margin
0.053
Negative
21
65
Positive
9
40
Metastasis
<0.001
No
17
80
Nodal
4
37
Distant
16
12
Table 1: Statistical analysis of several parameters in relation to 5-year DSS. The 7Th edition was adopted as AJCC staging.
Finally, we investigated the impact of nodal metastasis on survival. As a result, DSS was not statistically different between the patients with nodal metastasis and those with distant metastasis at the initial visit (median survival, nodal: 43.3 months [n=4] vs distant: 28.9 months [n=16], p=0.194) (Figure 3A). Furthermore, DSS from the occurrence of nodal metastasis during the follow-up was not significantly different from those of distant metastasis (median survival, nodal: 43.3 months [n=9] vs distant: 30.3 months [n=19], p=0.125) (Figure 3B).
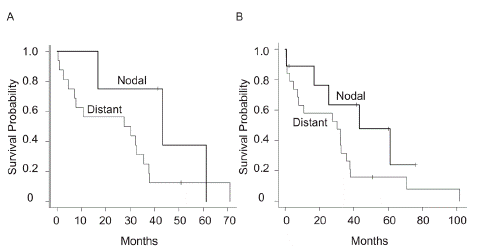
Figure 3: (A) Disease-specific survival in the patients with nodal (any T N1 M0) or distant metastasis (any T N0/N1 M1). (B) Disease-specific survival from the occurrence of nodal or distant metastasis during the follow-up.
Discussion
CCS is also known as malignant melanoma of soft parts as these lesions also express melanocytic markers. CCS often occurs in young adults, particularly in the distal extremities, and is predominantly fatal. In our cohort, the five-year survival rate was 46%, a finding that is comparable to that of previous reports (30–67%) [2,4,11]. A unique characteristic of CCS is the preference for lymph node and/or distant metastasis, which has been associated with poor clinical outcomes. In contrast, we showed that stage at presentation, tumor size, and location were significant prognostic factors, although tumor size, tumor location, surgical margin status, and perhaps adjuvant radiotherapy have all been reported as prognostic factors [3-5,7]. Notably, several reports on CCS have suggested that tumor size is significantly correlated with prognosis which is compatible with our results. However, as seen in the described cases, small lesions do not necessarily guarantee good clinical outcomes. In our cohort, stage III and IV lesions (AJCC 7th edition) were seen in 4 of the 21 cases (11%) with lesion size <5 cm. Kawai et al. found that classifying patients based on lesion size as greater or lesser than 5 cm may help identify patients with CCS at greater risk of metastases, suggesting the possibility of micrometastasis at the initial presentation, that is, no evidence of metastasis at presentation but the occurrence of a detectable mass in the later phase [3]. We report a large difference in five-year disease-specific survival between stage IIA (79%), III (53%), and IV (12%) (AJCC 7th edition) patients, suggesting that patients without lymph node/distant metastases may benefit from complete surgical resection, including amputation, regardless of tumor size, even though larger tumors generally tend to metastasize compared to smaller ones.
In the present study, we attempted to validate the AJCC staging system in which nodal metastasis was grouped together in distant metastasis as stage IV. Importantly, statistical significance in DSS was eliminated when compared between the patients with nodal metastasis and those with distant metastasis as shown in Figure 3. This may emphasize greater validity of the latest AJCC (8th edition) in CCS.
One of the reasons for poor prognosis in CCS is the lack of effective chemotherapy regimens. One study has demonstrated that doxorubicin combined with cisplatin may provide greater benefit in CCS [3]. However, our data could not be used to prove the effectiveness of doxorubicin and cisplatin. Similarly, Jones et al. stated that the effectiveness of chemotherapy is limited, with a response rate of 4% and progression-free survival of 11 weeks [12]. In our study, there were few cases of NED and alive with disease, which may reflect the ineffectiveness of chemotherapy as prognosis is poor once lymph node and/or distant metastases have occurred. Overall, we believe that chemotherapy does not confer a significant advantage with respect to prognosis.
There are some limitations to our study. First, because the number of cases is small, statistical weakness should be considered. Next, this is a retrospective study with some missing data on tumor size, which may alter the conclusions. Nevertheless, we emphasize that additional research is required to clarify the impact of lymph node metastasis on survival.
Conclusion
Nodal metastasis is a significant negative prognostic factor in CCS from the result showing that there was no difference in DDS between nodal metastasis and distant metastasis in CCS in the present study.
Author Statements
Conflict of Interest
The authors declare that they have no conflicts of interest.
Funding
This paper was supported by Takeda Science Foundation.
Data Availability
The data that support the findings of this study are available from the corresponding author, HT, upon reasonable request.
Ethics Approval
This study was approved by the institutional review board of Osaka International Cancer Institute.
Patient Consent
Informed consent was obtained from all individual participants included in the study.
References
- Fletcher CDM, Unni KK, Mertens F. Pathology and genetics of tumours of soft tissue and bone. World Health Organ Classif Tumours. 2002.
- Deenik W, Mooi WJ, Rutgers EJ, Peterse JL, Hart AA, Kroon BB. Clear cell sarcoma (malignant melanoma) of soft parts: A clinicopathologic study of 30 cases. Cancer. 1999; 86: 969-75.
- Kawai A, Hosono A, Nakayama R, Matsumine A, Matsumoto S, Ueda T, et al. Clear cell sarcoma of tendons and aponeuroses: A study of 75 patients. Cancer. 2007; 109: 109-16.
- Ferrari A, Casanova M, Bisogno G, Mattke A, Meazza C, Gandola L, et al. Clear cell sarcoma of tendons and aponeuroses in pediatric patients: A report from the Italian and German Soft Tissue Sarcoma Cooperative Group. Cancer. 2002; 94: 3269-76.
- Kim YJ, Song SY, Choi W, Je HU, Ahn JH, Chung HW, et al. Postoperative radiotherapy after limb-sparing surgery for soft-tissue sarcomas of the distal extremities. Anticancer Res. 2016; 36: 4825-31.
- Finley JW, Hanypsiak B, Mcgrath B, Kraybill W, Gibbs JF. Clear cell sarcoma: the Roswell Park experience. J Surg Oncol. 2001; 77: 16-20.
- Lee HL, Ho WY, Lam YL, Ng TP, Li KH, Shek T, et al. Prognostic factors associated with clear cell sarcoma in 14 Chinese patients. J Orthop Surg (Hong Kong). 2014; 22: 236-9.
- Lucas DR, Nascimento AG, Sim FH. Clear cell sarcoma of soft tissues: Mayo Clinic experience with 35 cases. Am J Surg Pathol. 1992; 16: 1197-204.
- Bianchi G, Charoenlap C, Cocchi S, Rani N, Campagnoni S, Righi A, et al. Clear cell sarcoma of soft tissue: A retrospective review and analysis of 31 cases treated at Istituto Ortopedico Rizzoli. Eur J Surg Oncol. 2014; 40: 505-10.
- Kanda Y. Investigation of the freely available easy-to-use software ’EZR’ for medical statistics. Bone Marrow Transplant. 2013; 48: 452-8.
- Hocar O, Le Cesne A, Berissi S, Terrier P, Bonvalot S, Vanel D, et al. Clear cell sarcoma (malignant melanoma) of soft parts: A clinicopathologic study of 52 cases. Dermatol Res Pract. 2012; 2012: 984096.
- Jones RL, Constantinidou A, Thway K, Ashley S, Scurr M, Al-Muderis O, et al. Chemotherapy in clear cell sarcoma. Med Oncol. 2011; 28: 859-63.