
Research Article
Sarcoma Res Int. 2024; 9(1): 1052.
Preferences on Treatment Decision Making in Sarcoma Patients. Prevalence and Associated Factors – Results from the Prosa Study
Hanna Salm1,2*; Markus K Schuler3; Leopold Hentschel4; Stephan Richter12; Peter Hohenberger5; Bernd Kasper6; Dimosthenis Andreou7; Daniel Pink1,2; Luise Mütze9; Karin Arndt10; Christine Hofbauer4; Klaus-Dieter Schaser4; Jürgen Weitz4; Jochen Schmitt4,11; Martin Eichler4
1Department of Internal Medicine C, University Hospital Greifswald, Germany
2Sarcoma Center Berlin-Brandenburg, Helios Hospital Bad Saarow, Germany
3Clinic and Polyclinic for Internal Medicine I, University Hospital Carl Gustav Carus, TU Dresden, Germany
4National Center for Tumor Diseases (NCT/UCC), Dresden University of Technology, Dresden, Germany
5Division of Surgical Oncology & Thoracic Surgery, Mannheim University Medical Center, University of Heidelberg, Germany
6Sarcoma Unit, Mannheim Cancer Center, Mannheim University Medical Center, University of Heidelberg, Mannheim, Germany
7Department of General Orthopedics and Tumor Orthopedics, University Hospital Münster, Germany
8Department of Orthopedics and Trauma, Medical University of Graz, Graz, Austria
9Hospital Emmaus Niesky, Niesky, Germany
10German Sarcoma Foundation, Woelfersheim, Germany
11Center for Evidence-based Healthcare, University Hospital Carl Gustav Carus and Medical Faculty, Technical University Dresden, Germany
12Department of Medicine 1, NCT/UCC, Sarcoma Center, University Hospital Carl Gustav Carus, Dresden University of Technology, Dresden, Germany
*Corresponding author: Hanna Salm Department of Internal Medicine C, University Hospital Greifswald, Germany.Email: hanna.s.salm@gmail.com
Received: May 27, 2023 Accepted: June 13, 2023 Published: June 20, 2023
Abstract
Introduction: The impact of being diagnosed with a life-threatening illness may influence preferences to participate in treatment decisions. The objective of this analysis was to identify factors that are associated with sarcoma patients wanting to take a more active or passive role.
Methods: Data was obtained as part of a nationwide multicenter study (PROSa) aiming to investigate the structure and quality of medical care of sarcoma patients in Germany and their determinants. The study was conducted between 2017 and 2020 in 39 study centers.
For the present analysis, cross-sectional data of adult patients with sarcoma of any entity were analyzed. Control preference was measured with the Control Preference Scale (CPS). Preferences were divided in patient-led, shared, or physician-led-decision-making.
Associated factors were analyzed exploratively using multivariable multinominal logistic regression models. We included socio-economical and medical variables with stepwise backward variable selection.
Results: We included 1081 patients (48.6% female). 402 patients (37.2%) preferred to be in control about treatment decisions, while 400 patients (37.0%) favored shared responsibility. 25.8% (n = 279) wished to rather leave the control to the treating physician.
When comparing the patients who preferred physician-led decision making with those who favored shared responsibility, older patients were more likely to prefer shared decision-making compared to those 18 to > 40 years old (age group: > 75 years: Odds Ratio (OR) .53, 95% confidence interval (95% CI) .28; .99). Patients with a metastatic tumor desired shared decision making compared to those without metastases (metastasis: OR 1.61, 95% CI 1.09; 2.38).
When comparing the patients who preferred physician-led decision making with those who favored to be in control, older patients also preferred leaving the control to the physician and were less inclined to make the decisions by themselves: (18 to > 40 years vs > 75 years: OR .28, 95% CI .15; .55). With secondary school (8/9 years) as reference, patients holding a high school degree were more likely to prefer patient-led decision-making over physician-led decision making (OR 2.00, 95% CI 1.26; 3.09). Patients with sarcoma of the abdomen/retroperitoneum were more predisposed to taking control in treatment decisions compared to those with sarcoma of the back/spine or lower limb (back/spine: OR .18, 95% CI .06; .54, lower limb: OR .56, 95% CI .37; .85). With an income of 1250 €/month as reference, patients with a higher income were more likely to take control (> 2750€/month: OR 1.7, 95% CI 1.0; 3.1).
Conclusion: The findings of our study demonstrate that patients with metastatic disease are more likely to seek a joint decision, while those of higher age and lower education level are less likely to actively participate in treatment decisions. The results suggest that the impact of advanced illness may influence preferences to participate. Additionally, our findings indicate differences in participation preferences based on the tumor’s location, a finding that should be further investigated.
Introduction
In recent decades, the relationship between physician and patient has evolved from a classic paternalistic model to an interactive interaction [1]. This evolution towards information sharing and collaborative decision-making, aligned with patients’ preferences, represents a significant paradigm shift in medicine [2]. Furthermore, this topic is gaining increasing importance in health care policy [3,4]. Shared decision making has been defined as: “an approach where clinicians and patients share the best available evidence, when faced with the task of making decisions, and where patients are supported to consider options to achieve informed preference” [4]. Patient consultations now serve not only to inform patients about their condition and treatment options but also to ensure that treatments are tailored to patients’ preferences and needs [5].
As the physician-patient relationship transitions towards a more collaborative partnership, there is interest in identifying factors associated with patients seeking either a more active or passive role. Research suggests that active patient participation in decision-making can positively impact well-being, increase satisfaction with care, and reduce anxiety [6-8]. Furthermore, patient involvement in decision-making has been found to contribute to better quality of care [9,10]. However, not all patients necessarily desire involvement in the decision-making process [11,12]. Understanding patients’ preferences for participation is crucial for customizing patient involvement in healthcare [4,13]. However, in clinical practice, patients’ preference is often unknown, so physicians may not actively engage those who would prefer to be involved in decisions about their health, and vice versa [14]. The identification of patients’ preferences for information and control was found to be particularly important in oncology to avoid often occurring conflicts between patients’ expectation and physicians’ decision-making practices [15,16]. Patients who desire more involvement may be harmed from information deficits, while those expected to take more control than desired may experience higher distress [17]. Overall, a shared approach to decision-making in healthcare is preferred by most patients [18].
Several years ago, Degner and colleagues [19] introduced the Control Preference Scale (CPS), an instrument designed to assess the degree of control an individual patient prefers in health care decision-making. The CPS is among the most commonly used instruments for measuring patients’ Decision Control Preferences (DCPs). Research on DCP has shown that sociodemographic factors, such as age, gender or education are associated with patients preferences regarding decision-making in healthcare [20]. However, data on DCP has been lacking for sarcoma patients.
The patient group studied in the present work consists exclusively of sarcoma patients, a rare form of cancer [21] with treatment strategies based on complex interventions [22]. Health-related quality of life among sarcoma patients has been understudied, prompting the initiation of the PROSa study. Preliminary findings from this study have been previously published [23-31].
In this analysis, we investigated the relevance of sociodemographic characteristics as well as disease-related factors, considering evidence suggesting that the preferred level of involvement may change over the course of the disease [32]. Existing research in this area is limited, with prior studies predominantly focusing on prostate and breast cancer [16]. To our knowledge, no studies on decision-making preferences in a sample of sarcoma patients currently exist.
Methods
Recruitment, Participation, and Data Sources
Data was obtained as part of nationwide cohort study (www.uniklinikum-dresden.de/prosastudie). The prospective PROSa (Burden and medical care of sarcoma in Germany: Nationwide cohort study focusing on modifiable determinants of Patient-Reported Outcome measures in Sarcoma patients) study was conducted between autumn 2017 and spring 2020 in 39 study centers. These centers comprised 8 office-based practices, 22 hospitals of maximum care, and 9 other hospitals. Recruitment efforts were initiated through various channels such as medical societies and research networks, utilizing tools like email lists, personal letters, and advertisements to engage participants. The study aimed to collect data on patient-reported outcomes, clinical information at the patient level, and structural details of the participating study centers. The study included incident and prevalent adult patients and survivors of all sarcoma subtypes, with a detailed list of included entities available in the paper by Eichler et al. [28]. Patients who were mentally or linguistically incapable of completing the questionnaires were excluded.
Eligible patients were approached for participation during visits to the study centers, as well as through phone calls or letters. Participants submitted patient-reported outcomes and sociodemographic data to the study coordination center at the University Hospital Dresden either by mail or online. Clinical information and structural data of the participating study centers were submitted online by the centers using case report forms. Data collection was facilitated through REDCap (Vanderbilt University, Nashville, TN, USA) electronic data capture tools hosted at the Technical University Dresden [33].
Measures
Patients’ DCPs of medical decisions were measured using the standardized and validated Control Preference Scale (CPS) [19]. Patients can pick one statement out of five that best describes their preferred involvement in medical decision-making ranging from active (“I prefer to make the decision about which treatment I will receive”) to passive (“I prefer to leave all decisions regarding my treatment to my doctor”) role. To maintain a sufficient number of cases for statistical analysis per category, adjoining answer options were grouped to three categories: patient-led, shared, or physician-led decision-making.
The control preference scale by Degner and colleagues [19] is shown below. To provide better clarity, answers 1 and 2 were defined as patient-led decision making, response 3 as shared decision making, and responses 4 and 5 as physician-led decision making.
Compliance with Ethical Standards
This study was approved by the ethics committee of the Technical University of Dresden (AZ: EK 1790422017) and the ethics committees of the participating centers, and it was conducted in accordance with the Declaration of Helsinki. The study participants gave written informed consent.
Statistical Analysis
For the description of the study population, we evaluated the variables from the multivariable model (see below), as well as disease status and treatment intention. Categorical variables were presented in absolute numbers and relative frequencies. The variables were stratified according to the grouping of the univariate analysis (see below).
Univariate group comparisons were performed comparing patients who preferred patient-led, shared and physician-led decision making. Significance tests were conducted utilizing chi-square tests. A p-value less than 0.05 was considered to be statistically significant.
Associated factors with patient control preference were analyzed by multinominal logistic regression models with stepwise variable selection to control for potentially confounding variables. We compared patients with a physician-led control preference to a) patients with a patients-led preference and b) patients with a shared control preference. Odds Ratios (OR), 95% confidence intervals, (95% CI) and p-values were evaluated in a model that was adjusted for age at baseline, sex, education, income, occupational status, tumor site, received treatments, metastatic disease, tumor size, aftercare status and time since diagnosis.
Results
Participation
Patients were contacted between September 2017 and January 2019. A total of 1309 patients participated in the study, as shown in Figure 1.
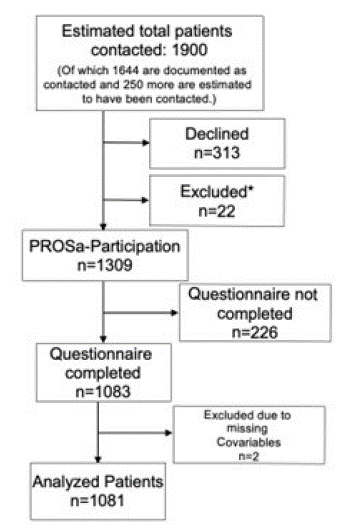
Figure 1: Study participation. Number of patients contacted overall had to be extrapolated from numbers of reporting study centers because not every study center documented contacted patients. Reasons for exclusion: language (2), mental capacity (1), double recruitment (8), withdrawal consent (2), histology (9).
Study Population
A total of 1081 patients completed the CPS, 525 (48.6%) were female and 556 (51.4%) were male. The majority of patients either had sarcoma of the abdomen/retroperitoneum (26.8%) or the lower limbs (36.4%). 31.0% of the tumors were metastasizing. At study entry, treatment intention was more often curative than palliative (74.7% vs. 23.2%), as shown in Table 1.
Variable
Value
N
%
Sex
female
525
48.6
male
556
51.4
Age
18-<40
181
16.7
=40-<55
257
23.8
=55-<65
293
27.1
=65-<75
221
20.4
=75
129
11.9
Tumor site
abdomen/retroperitoneum
290
26.8
thorax
87
8.0
pelvis/urogenital
158
14.6
lower limbs
394
36.4
upper limbs
84
7.8
head & neck
33
3.1
back/spine
29
2.7
other/unknown
6
0.6
Metastasis
no metastasis
596
55.1
metastasis
335
31.0
unknown
150
13.9
Disease status
complete remission
482
44.6
partial remission + stable disease
320
29.6
tumor progress
155
14.3
unknown
124
11.5
Treatment intent
palliative
251
23.2
curative
808
74.7
unknown
22
2.1
Table 1: Description of study population at baseline.
Preference in Treatment Decision-Making
The majority of patients preferred patient-led (37.2%) or shared responsibility (37.0%) with regard to treatment decision making. Twenty six percent favored to rather leave the control to the treating physician (Table 2). Table 2 also shows preferences in treatment decision making including all five CPS response options.
Preference in treatment decision-making
N
%
Patient-led
402
37.2
Shared
400
37.0
Physician-led
279
25.8
All
1081
100.0
Preference in treatment decision-making including all five CPS response options
N
%
Decision by the patient alone
16
1.5
Decision by the patient with the involvement of the physician
386
35.7
Shared decision
400
37.0
Decision by the physician with the involvement of the patient
232
21.5
Decision by the physician alone
47
4.3
All
1081
100.0
Table 2: Preference in treatment decision.
Univariate Analysis
Sociodemographic factors: Higher age (65-<75 years) was associated with increased willingness to leave the control to the treating physician (34.4% vs. 16.6%, p < 0.001), as shown in Table 3.
Variable
Value
Patient-led N (%)
Shared
N (%)Physician-led N (%)
All
N (100%)p-value
sex
female
200 (38.1)
202 (38.5)
123 (23.4)
525 (48.6)
0.22
male
202 (36.3)
198 (35.6)
156 (28.1)
556 (51.4)
Age
18-<40
83 (45.9)
68 (37.6)
30 (16.6)
181 (16.7)
< 0.001
=40-<55
113 (44.0)
90 (35.0)
54 (21.0)
257 (23.7)
=55-<65
101 (34.5)
120 (41.0)
72 (24.6)
293 (27.3)
=65-<75
72 (32.6)
73 (33.0)
76 (34.4)
221 (20.4)
=75
33 (25.6)
49 (38.0)
47 (36.4)
129 (11.9)
Education
secondary school (8-9 years)
72 (29.5)
87 (35.7)
85 (34.8)
244 (22.6)
< 0.001
secondary school (10 years)
121 (33.0)
148 (40.3)
98 (26.7)
367 (34.0)
vocational baccalaureate/ high school
201 (46.5)
149 (34.5)
82 (19.0)
432 (39.1)
other
8 (21.1)
16 (42.1)
14 (36.8)
38 (3.5)
Income
<1250 €/month
79 (34.8)
81 (35.7)
67 (29.5)
227 (21.0)
0.002
1250-<1750 €/month
78 (37.0)
70 (33.2)
63 (29.9)
211 (19.5)
1750-<2250 €/month
79 (32.6)
106 (43.8)
57 (23.6)
242 (22.4)
2250-<2750 €/month
41 (46.1)
34 (38.2)
14 (15.7)
89 (8.2)
>2750 €/month
86 (47.8)
59 (32.8)
35 (19.4)
180 (16.7)
unknown
39 (29.5)
50 (37.9)
43 (32.6)
132 (12.2)
Occupational status
blue collar worker
56 (27.6)
78 (28.4)
69 (34.0)
203 (18.8)
0.066
civil servant
32 (40.5)
32 (40.5)
15 (19.0)
79 (7.3)
white collar worker
234 (38.9)
221 (36.8)
146 (24.3)
601 (55.6)
self employed
42 (40.4)
38 (36.5)
24 (31.1)
104 (9.6)
not applicable/unknown
38 (40.4)
31 (33.0)
25 (26.6)
94 (8.7)
Table 3: Socio-demographic factors. The p-values were calculated using the Chi-Square test.
Patients with an education level of high school were more likely to make decisions by themself than those with 8 or 9 years of school education (46.5% vs. 29.5%, p 0.001).
Patients with a low income (<1250 €/month) were less likely to take control compared to those with a higher income (>2750 €/month): 34.8% vs. 47.8%, p 0.002.
Blue collar workers were more likely to prefer physician-led decision making than white collar workers (34.0% vs. 24.3%, p 0.066).
Disease-related factors: If the tumor had not metastasized, patients were more likely to take control in treatment decision-making as if the tumor had already spread (40.6% vs. 32.5%, p. 0.010).
Further differences could be shown in regard of the tumor location. Patients with sarcoma of the abdomen/retroperitoneum were more likely to take control than patients with sarcoma of the back/spine or lower limb (41.0% vs.17.2% (back/spine) and 34.8% (lower limbs), p. 0.049).
Patients treated with palliative intent were more likely to prefer shared responsibility with their treating physician than patients treated with curative intent (41.8% vs. 35.3%, p 0.285).
Further details of disease-related factors that are associated with patient’s role in treatment decision making are shown in table 4 below.
Variable
Value
Patient-led N (%)
Shared
N (%)Physician-led N (%)
All
N (100%)p-value
Tumor site
abdomen/retroperitoneum
119 (41.0)
98 (33.8)
73 (25.2)
290 (26.8)
0.049
thorax
28 (32.2)
36 (41.4)
23 (26.4)
87 (8.0)
pelvis
65 (41.1)
62 (39.2)
31 (19.6)
158 (14.6)
lower limbs
137 (34.8)
150 (38.1)
107 (27.2)
394 (36.4)
upper limbs
34 (40.5)
26 (31.0)
24 (28.6)
84 (7.8)
head and neck
12 (36.4)
17 (51.5)
4 (12.1)
33 (3.1)
back/spine
5 (17.2)
10 (34.5)
14 (48.3)
29 (2.7)
unknown
2 (33.3)
1 (16.7)
3 (50.0)
6 (0.6)
Treatment combinations
all other (radiotherapy + systemic therapy/no therapy (yet)/other therapy)
22 (33.3)
26 (39.4)
18 (27.3)
66 (6.1)
0.173
systemic therapy only
17 (26.2)
32 (49.2)
16 (24.6)
65 (6.0)
OP + radiotherapy + systemic therapy
76 (33.5)
88 (38.8)
63 (27.8)
227 (21.1)
OP + radiotherapy
56 (34.8)
67 (41.6)
38 (23.6(
161 (14.9)
OP + systemic therapy
107 (38.9)
98 (35.6)
70 (25.5)
275 (25.4)
OP only
124 (43.2)
89 (31.0)
74 (25.8)
287 (26.5)
Metastasis
no metastasis
242 (40.6)
199 (33.4)
155 (26.0)
596 (55.1)
0.010
metastasis
109 (32.5)
148 (44.2)
78 (23.3)
335 (31.0)
unknown
51 (34.0)
53 (35.3)
46 (30.7)
150 (13.9)
T-stage
small (T1)
68 (40.0)
62 (36.5)
40 (23.5)
170 (15.7)
0.306
large (T2-T4)
186 (37.1)
173 (34.5)
142 (28.3)
501 (46.3)
other/unknown
148 (36.1)
165 (40.2)
97 (23.7)
410 (40.0)
Disease status
complete remission
194 (40.2)
168 (34.9)
120 (24.9)
482 (44.6)
0.019
partial remission + stable disease
129 (40.3)
108 (33.8)
83 (25.9)
320 (29.6)
tumor progress
42 (27.1)
73 (47.1)
40 (25.8)
155 (14.3)
unknown
37 (29.8)
51 (41.1)
36 (29.0)
124 (11.5)
In aftercare
not in aftercare
149 (32.7)
184 (40.4)
123 (27.0)
456 (42.1)
0.053
in aftercare
247 (40.5)
213 (34.9)
150 (24.6)
610 (56.4)
unknown
6 (40.0)
3 (20.0)
6 (40.0)
15 (1.5)
Treatment intent
palliative
82 (32.7)
105 (41.8)
64 (25.5)
251 (23.2)
0.285
curative
312 (38.6)
285 (35.3)
211 (26.1)
808 (74.7)
unknown
8 (36.4)
10 (45.5)
4 (18.2)
22 (2.1)
Time since diagnosis
0-<0.5 years
74 (36.1)
72 (35.1)
59 (28.8)
205 (19.0)
0.559
0.5-<1 years
50 (40.3)
39 (31.5)
35 (28.2)
124 (11.5)
1-<2 years
51 (32.5)
62 (39.5)
44 (28.0)
157 (14.5)
2-<5 years
115 (40.2)
103 (36.0)
68 (23.8)
286 (26.5)
more than 5 years
112 (36.2)
124 (40.1)
73 (23.6)
309 (28.5)
Table 4: Disease-related factors. The p-values were calculated using the Chi-Square test.
Associated Factors in Multivariate Multinomial Logistic Regression
Physician-led versus patient-led decision-making: Patients in the higher age groups were significantly less likely to make decisions by themselves (65 - < 75 years: OR .37, p <.001; more than 75 years: OR .28, p <.001) compared to those in the youngest age group.
With increased educational status, patients favored to make decisions by themselves. Patients with a high school education level were more likely to make decisions of their own than those with 8/9 years of secondary school education: vocational baccalaureate/high school: OR 1.97, p .003.
Higher income was associated with patients preferring patient-led decisions compared to those in the low-income group. With an income of 1250 €/month as reference, patients with a higher income were more likely to take control: >2750€/month: OR 1.7, p .042.
We found two significant differences regarding tumor site and disease status. With abdomen as the reference, patients with sarcomas of the back/spine and lower limbs showed a higher preference for physician-led decision making: back/spine: OR .17, p .002; lower limbs: OR .55, p .006 (all results are shown in table 5).
Variable
Value
OR
95% CI
(l;u)p
Age at study entry
18 - < 40 years reference
40 - < 55 years
.75
.43; 1.32
.332
55 - < 65 years
.47
.27; .82
.008
65 - < 75 years
.37
.21; .66
<.001
more than 75 years
.28
.14; .54
<.001
Sex
male vs. female
.84
.60; 1.17
.311
Education
secondary school (8/9 years) reference
secondary school (10 years)
1.08
.69; 1.68
.712
vocational baccalaureate/high school
1.97
1.26; 3.09
.003
other (non (yet)/unknown)
.62
.24; 1.62
.336
Income
< 1250 € reference
1250 € - < 1750 €
1.17
.71; 1.92
.526
1750 € - < 2250 €
1.10
.67; 1.82
.694
2250 € - < 2750 €
2.01
.97; 4.17
.060
> 2750 €
1.79
1.02; 3.14
.042
unknown
.87
.49; 1.55
.659
Tumor location
abdomen reference
back/spine
.17
.05; .53
.022
head&neck
1.40
.41; 4.74
.586
upper limbs
.72
.38; 1.36
.315
lower limbs
.55
.36; .84
.006
pelvis
1.07
.62; 1.83
.806
thorax
.59
.30; 1.16
.129
unknown
.41
.06; 2.87
.375
Metastasis
no metastasis reference
metastasis
1.01
.67; 1.52
.954
unknown
.82
.50; 1.32
.423
Aftercare
not in aftercare reference
in aftercare
1.38
.96; 1.98
.080
unknown
1.01
.29; 3.54
.981
Note: Results of multinominal logistic regression models. OR= Odds Ratio, 95% CI: 95% confidence interval. Variables not included in the stepwise inclusion: time since diagnosis, occupational status, received treatments, tumor size.
Table 5: Physician-led versus patient-led decision-making.
Physician-led versus shared decision-making: Patients in the higher age groups were significantly less likely to make decisions by themselves: 65 - < 75 years: OR .50, p .01; more than 75 years: OR .532, p .04.
Patients with a metastatic tumor preferred shared decision making compared to those without metastases: OR 1.61, p .016 (results are shown in table 6).
Variable
Value
OR
95% CI
(l;u)p
Age at study entry
18 - < 40 years reference
40 - < 55 years
.779
.44; 1.37
.38
55 - < 65 years
.743
.42; 1.28
.29
65 - < 75 years
.509
.28; .89
.01
more than 75 years
.532
.28; .99
.04
Sex
male vs. female
.787
.56; 1.08
.14
Education
secondary school (8/9 years) reference
secondary school (10 years)
1.184
.77; 1.80
.43
vocational baccalaureate/high school
1.444
.92; 2.24
.10
other (non (yet)/unknown)
1.191
.53; 2.65
.67
Income
< 1250 reference
1250 - < 1750
1.044
.63; 1.70
.86
1750 - < 2250
1.554
.96; 2.50
.07
2250 - < 2750
1.809
.86; 3.76
.13
> 2750
1.281
.72; 2.26
.39
unknown
1.041
.60; 1.79
.88
Tumor location
abdomen reference
back/spine
.522
.21; 1.28
.15
head&neck
2.925
.91; 9.32
.07
upper limbs
.794
.41; 1.51
.49
lower limbs
.998
.65; 1.51
.99
pelvis
1.334
.77; 2.29
.29
thorax
1.035
.55; 1.93
.91
unknown
.250
.02; 2.59
.24
Metastasis
no metastasis reference
metastasis
1.612
1.09; 2.38
.01
unknown
1.012
.63; 1.62
.96
Aftercare
not in aftercare reference
in aftercare
1.076
.75; 1.52
.68
unknown
.366
.08; 1.56
.17
Note: Results of multinominal logistic regression models. OR= Odds Ratio, 95% CI: 95% confidence interval. Variables not included in the stepwise inclusion: time since diagnosis, occupational status, received treatments, tumor size.
Table 6: Physician-led versus patient-led decision-making.
Discussion
The purpose of this study was to examine the decision-making preferences in a sample of sarcoma patients and to explore associations between these preferences and sociodemographic characteristics as well as disease-related factors.
To our knowledge, this study is the first to explore the impact of disease related factors, such as tumor site, associated with preferences in treatment decision making in sarcoma patients.
The majority of our sample showed a preference for active (37.2%) or collaborate (37.0%) participation in medical decision-making, while only 25.8% preferred the health care professionals to take control. This demonstrates the value of personal autonomy and aligns with prior research indicating that a collaborative approach to decision-making in healthcare is the preferred choice for the majority of patients [18].
Sociodemographic Factors
We found that being younger was associated with increased desire for involvement in medical decisions. This aligns with previous studies showing younger age [14,34-37] to be a predictor for active involvement. Additionally, higher age was shown to be significantly associated with an increased willingness to leave the decision control to the physician [38]. Moreover, a correlation emerged between decision preferences and educational background. Those in the highest educational category tended to express a greater inclination towards making decisions autonomously, while patients in the lowest educational category showed a preference for a more passive role, entrusting control to the physician. This this in line with previous research showing higher educational status [37,39] to be a predictor for active involvement. Furthermore, higher income was associated with patients preferring patient-led decisions compared to those in the low-income group. This finding aligns with previous studies [40].
The relationship between gender and preferences in decision-making was less clear, which is consistent with previous research demonstrating a difference by gender [34,41] but mostly reporting no gender differences [35,36,42,43].
Disease-Related Factors
Significant variations were observed among different tumor sites, underlining the notable heterogeneity of the disease. However, explaining these differences in relation to tumor location are not easy.
Patients with sarcomas of the abdomen/retroperitoneum were more inclined to take an active role in decision-making compared to patients with sarcomas of the back/spine or lower limb. As patients with sarcomas of the back/spine comprised only 2.7% of the patient population, we cannot draw reliable conclusion from this result.
With respect to the tumor location in the lower limb, this observation may find partial explanation by results from the main PROSa study [28], which indicates differences in quality of life and symptom burden across different tumor sites: Patients with sarcomas of the lower extremities felt generally worse, possibly influenced by the functional limitations associated with the tumor location. Moreover, patients with sarcomas of the trunk (thorax, abdomen) demonstrated better outcomes in terms of symptom burden than those with lower extremity tumors. Therefore, it is conceivable that the reduced participation in decision-making of patients with tumors in the extremities is related to their poorer well-being in general.
Moreover, the specific location of a tumor can influence the range of available treatment options, often necessitating more in-depth discussions and collaborative decision-making between healthcare providers and patients. Consequently, it is reasonable to suggest that shared decision-making becomes more prevalent when addressing sarcomas at certain sites, where treatment strategies tend to be more extensive and intricate.
Strength and Limitations
Through the PROSa study, we established an extensive network of recruiting study centers across Germany, enabling us to enroll a significant number of sarcoma patients. Participation rates were notably high, estimated at 69%, reflecting strong patient motivation. Our descriptive data highlight the wide spectrum of the disease, demonstrating recruitment across all sarcoma subtypes, except for skin sarcomas. Given that most participants were recruited from high-volume centers, it’s important to acknowledge that our sample may not fully represent the typical landscape of sarcoma care in Germany, suggesting a potential selection bias at the institutional level. Consequently, it’s likely that small and well-differentiated tumors were underrepresented in our study cohort.
Primarily, participants were recruited during hospital or practice visits, with clinical contacts becoming less frequent over time for survivors. This trend raises the possibility of a sick survivor bias, wherein selected patients with more severe disease courses were included in our study.
Conclusion
While decision control preference has been investigated in different patient populations, there has been no evidence regarding sarcoma patients. Therefore, our analysis provides important new insights in a large sample of patients with sarcoma. Our study demonstrated that patients’ preferences in decision-making are associated with both disease and sociodemographic factors, providing valuable insights for understanding patients’ involvement in clinical decisions.
The insights gained could assist physicians in adjusting their approach to decision-making to meet the specific needs of sarcoma patients. Doctors should be mindful of how these factors may impact decision processes to ensure care that is centered around the patient. A more thorough integration of the patient perspective has the potential to enhance both care and communication between physicians and patients.
Author Statements
Funding
The PROSa study was funded by the German Cancer Aid (grant No. 111713).
References
- Emanuel EJ, Emanuel LL. Four Models of the Physician-Patient Relationship. JAMA J Am Med Assoc. 1992; 267: 2221-6.
- Mihalj M, Carrel T, Urman RD, Stueber F, Luedi MM. Recommendations for Preoperative Assessment and Shared Decision-Making in Cardiac Surgery. Current Anesthesiology Reports. 2020; 10: 185-195.
- O’Connor AM, Wennberg JE, Legare F, Llewellyn-Thomas HA, Moulton BW, Sepucha KR, et al. Toward the “tipping point”: Decision aids and informed patient choice. Health Affairs. 2007; 26: 716-25.
- Elwyn G, Laitner S, Coulter A, Walker E, Watson P, Thomson R. Implementing shared decision making in the NHS. BMJ (Online). 2010; 341: c5146.
- O’Mahony S, Mazur E, Charney P, Wang Y, Fine J. Use of multidisciplinary rounds to simultaneously improve quality outcomes, enhance resident education, and shorten length of stay. J Gen Intern Med. 2007; 22: 1073-1079.
- Ford S, Fallowfield L, Lewis S. Doctor-patient interactions in oncology. Soc Sci Med. 1996; 42: 1511-9.
- Flynn D, Knoedler MA, Hess EP, Murad MH, Erwin PJ, Montori VM, et al. Engaging patients in health care decisions in the emergency department through shared decision-making: A systematic review. Acad Emerg Med. 2012; 19: 959-67.
- López ME, Kaplan CP, Nápoles AM, Hwang ES, Livaudais JC, Karliner LS. Satisfaction with treatment decision-making and treatment regret among Latinas and non-Latina whites with DCIS. Patient Educ Couns. 2014; 94: 83-9.
- Entwistle VA, Carter SM, Cribb A, McCaffery K. Supporting patient autonomy: The importance of clinician-patient relationships. Journal of General Internal Medicine. 2010; 25: 741-745.
- Coulter A, Entwistle V, Gilbert D. Sharing decisions with patients: Is the information good enough? BMJ. 1999; 318: 318-22.
- Levinson W, Kao A, Kuby A, Thisted RA. Not all patients want to participate in decision making:A national study of public preferences. Journal of General Internal Medicine. 2005; 20: 531-5.
- Deber RB, Kraetschmer N, Irvine EJ. What role do patients wish to play in treatment decision making? Arch Intern Med. 1996; 156: 1414-20.
- Towle A, Godolphin W. Framework for teaching and learning informed shared decision making. British Medical Journal. 1999; 319: 766-71.
- Degner LF, Kristjanson LJ, Bowman D, Sloan JA, Carriere KC, O’Neil J, et al. Information needs and decisional preferences in women with breast cancer. JAMA. 1997; 277: 1485-92.
- Blinman P, Hughes B, Crombie C, Christmas T, Hudson M, Veillard AS, et al. Patients’ and doctors’ preferences for adjuvant chemotherapy in resected non-small-cell lung cancer: What makes it worthwhile? Eur J Cancer. 2015; 51: 1529-37.
- Tariman JD, Berry DL, Cochrane B, Schepp AD. Preferred and actual participation roles during health care decision making in persons with cancer: A systematic review. Annals of Oncology. 2009; 21: 1145-1151.
- Gattellari M, Voigt KJ, Butow PN, Tattersall MHN. When the treatment goal is not cure: Are cancer patients equipped to make informed decisions? J Clin Oncol. 2002; 20: 503-13.
- Frosch DL, Kaplan RM. Shared decision making in clinical medicine: Past research and future directions. Am J Prev Med. 1999; 17: 285-94.
- Degner LF, Sloan JA, Venkatesh P. The Control Preferences Scale. Can J Nurs Res. 1997; 29: 21-43.
- Florin J, Ehrenberg A, Ehnfors M. Clinical decision-making: Predictors of patient participation in nursing care. J Clin Nurs. 2008; 17: 2935-2944.
- Ressing M, Wardelmann E, Hohenberger P, Jakob J, Kasper B, Emrich K, et al. Strengthening health data on a rare and heterogeneous disease: Sarcoma incidence and histological subtypes in Germany. BMC Public Health. 2018; 18: 235.
- Casali PG, Abecassis N, Bauer S, Biagini R, Bielack S, Bonvalot S, et al. Soft tissue and visceral sarcomas: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018; 29: iv51-iv67;
- Jakob J, Hentschel L, Richter S, Kreisel I, Hohenberger P, Kasper B, et al. Transferability of Health-Related Quality of Life Data of Large Observational Studies to Clinical Practice: Comparing Retroperitoneal Sarcoma Patients from the PROSa Study to a TARPS-WG Cohort. Oncol Res Treat. 2022; 45: 660-669.
- Eichler M, Pink D, Menge F, Jakob J, Hentschel L, Richter S, et al. Quality of life of GIST patients with and without current tyrosine kinase inhibitor treatment: Cross-sectional results of a German multicentre observational study (PROSa). Eur J Cancer Care (Engl). 2021; 30: e13484.
- Eichler M, Hentschel L, Singer S, Hornemann B, Hohenberger P, Kasper B, et al. Distress in soft-tissue sarcoma and gastrointestinal stromal tumours patients—Results of a German multicentre observational study (PROSa). Psychooncology. 2022; 31: 1700-1710.
- Eichler M, Singer S, Hentschel L, Richter S, Hohenberger P, Kasper B, et al. The association of Health-Related Quality of Life and 1-year-survival in sarcoma patients—results of a Nationwide Observational Study (PROSa). Br J Cancer. 2022; 126: 1346-1354;
- Büttner M, Singer S, Hentschel L, Richter S, Hohenberger P, Kasper B, et al. Financial toxicity in sarcoma patients and survivors in Germany: results from the multicenter PROSa study. Support Care Cancer. 2022; 30: 187-196.
- Eichler M, Hentschel L, Richter S, Hohenberger P, Kasper B, Andreou D, et al. The health-related quality of life of sarcoma patients and survivors in germany—cross-sectional results of a nationwide observational study (Prosa). Cancers (Basel). 2020; 12: 3590.
- Eichler M, Andreou D, Golcher H, Hentschel L, Richter S, Hohenberger P, et al. Utilization of Interdisciplinary Tumor Boards for Sarcoma Care in Germany: Results from the PROSa Study. Oncol Res Treat. 2021; 44: 301-312.
- Eichler M, Singer S, Hentschel L, Hornemann B, Hohenberger P, Kasper B, et al. The psycho-oncological care situation of patients with soft tissue sarcomas: Results of a German multicenter observational study (PROSa). Onkologie. 2023.
- Eichler M, Schmitt J, Schuler MK. Die Dauer von Ethikvoten in Deutschland - am Beispiel einer nicht-interventionellen Beobachtungsstudie mit 44 teilnehmenden Zentren (PROSa). Z Evid Fortbild Qual Gesundhwes. 2019: 146.
- Brom L, Pasman HRW, Widdershoven GAM, Van Der Vorst MJDL, Reijneveld JC, Postma TJ, et al. Patients’ preferences for participation in treatment decision-making at the end of life: Qualitative interviews with advanced cancer patients. PLoS One. 2014; 25: e100435;
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)-A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009; 42: 377-81.
- Arora NK, McHorney CA. Patient preferences for medical decision making: Who really wants to participate? Med Care. 2000; 38: 335-41.
- Mah HC, Muthupalaniappen L, Chong WW. Perceived involvement and preferences in shared decision-making among patients with hypertension. Fam Pract. 2016; 33: 296-301.
- Mckinstry B. Do patients wish to be involved in decision making in the consultation? A cross sectional survey with video vignettes. BMJ. 2000; 321: 867-71.
- Wallberg B, Michelson H, Nystedt M, Bolund C, Degner LF, Wilking N. Information needs and preferences for participation in treatment decisions among Swedish breast cancer patients. Acta Oncol (Madr). 2000; 39: 467-76.
- Schuler M, Schildmann J, Trautmann F, Hentschel L, Hornemann B, Rentsch A, et al. Cancer patients’ control preferences in decision making and associations with patient-reported outcomes: a prospective study in an outpatient cancer center. Support Care Cancer. 2017; 25: 2753-2760.
- Janz NK, Wren PA, Copeland LA, Lowery JC, Goldfarb SL, Wilkins EG. Patient-physician concordance: Preferences, perceptions, and factors influencing the breast cancer surgical decision. J Clin Oncol. 2004; 22: 3091-8.
- Gu XF, Xu HF, Liu Y, Li L, Yu YQ, Zhang X, et al. Involvement in treatment decision-making and self-reported efficacy among patients with advanced colorectal cancer: a nationwide multi-center cross-sectional study. Front Oncol. 2023; 13: 1168078.
- Seo J, Goodman MS, Politi M, Blanchard M, Kaphingst KA. Effect of Health Literacy on Decision-Making Preferences among Medically Underserved Patients. Med Decis Mak. 2015; 36: 550-6.
- Bruera E, Willey JS, Lynn Palmer J, Rosales M. Treatment decisions for breast carcinoma: Patient preferences and physician perceptions. Cancer. 2002; 94: 2076-80.
- Wong F, Stewart DE, Dancey J, Meana M, McAndrews MP, Bunston T, et al. Men with prostate cancer: Influence of psychological factors on informational needs and decision making. J Psychosom Res. 2000; 49: 13-9.