
Research Article
J Schizophr Res. 2014;1(1): 6.
Prostate Cancer Related JAZF1 Gene is Associated with Schizophrenia
Ke-Sheng Wang1*, Lingjun Zuo2, Daniel Owusu1,Yue Pan3 and Xingguang Luo2
1Department of Biostatistics and Epidemiology, East Tennessee State University, USA
2Department of Psychiatry, Yale University School of Medicine, USA
3Department of Public Health Sciences, University of Miami, USA
*Corresponding author: Kesheng Wang, Department of Biostatistics and Epidemiology, College of Public Health, East Tennessee State University, PO Box 70259, Lamb Hall, Johnson City, TN 37614-1700, USA
Received: July 29, 2014; Accepted: August 08, 2014; Published: August 11, 2014
Abstract
Background: Epidemiological studies have shown that there is a reduced risk of prostate cancer among persons diagnosed with Schizophrenia (SCZ). However, the mechanism of such relationship is not clear. The reduced incidence of cancer observed in SCZ patients may be related to differences in genetic background. Recently, the JAZF1 gene is found to be associated with prostate cancer and type 2 diabetes. However, no study has focused on the association of JAZF1 with the risk of SCZ.
Methods: We examined genetic associations of 118 Single-Nucleotide Polymorphisms (SNPs) within the JAZF1 gene with SCZ using one European American (EA) sample of 1,149 cases and 1,347 controls. Logistic regression analysis of SCZ as a binary trait was performed using PLINK software.
Results: The most significant association with SCZ was observed with rs10258132 (p = 0.0011); while the next best signal was rs17156259 (p = 0.0031). The third best associated SNP was rs7791865 (p = 0.00889). In addition, haplotype analyses revealed that the A-C haplotype from rs10244184 and rs10258132 was associated with SCZ (p = 0.00093); and the G-G haplotype from rs17156238 and rs17156259 was associated with SCZ (p = 0.00455).
Conclusion: These findings provide evidence of several genetic variants in JAZF1 gene influencing the risk of SCZ and will serve as a resource for replication in other populations.
Keywords: Schizophrenia; Prostate cancer; JAZF1; Single nucleotide polymorphism; Pleiotropy
Abbreviations
BMI: Body Mass Index; CNS: Central Nervous System; GWAS: Genome-wide Association Study; JAZF1: Juxtaposed with another Zinc Finger Protein 1; JJAZ1: Joined to JAZF1 protein 1; MAF: Minor Allele Frequency; LD: Linkage Disequilibrium; PrCa: Prostate Cancer; SCZ: Schizophrenia; TIP27: TAK1-interacting protein 27; T2D: Type 2 Diabetes; ZNF802: Zinc Finger Protein 802
Introduction
Schizophrenia (SCZ) is the most tragic psychiatric disorder with high degree of genetic and clinical heterogeneity. The prevalence of SCZ is approximately 1% worldwide and currently affects 1.1% of the Unites States (US) population over the age of 18 [1-3]. SCZ is known to be a multifactorial disorder with a demonstrated heritability of 80% in family studies and meta-analysis of multiple twin studies [4,5]. Prostate Cancer (PrCa) is the most common non-skin malignancy cancer in the developed world and the second leading cause of cancer death in men [6,7]. In the US, approximately 240, 890 new cases and 33, 720 deaths were expected in 2011 [8]. The established risk factors for PrCa are age, ethnicity and family history [9]. Twin studies suggest that about 42% of the disease risk may be attributed to heritable factors [10].
It has been reported that there is a reduced risk of some cancers among persons diagnosed with SCZ [11-13]. For example, the incidence of PrCa in individuals with SCZ is significantly lower than expected [14,15]. Cancer risks in schizophrenic patients were negatively associated with age in the general populations (e.g. stomach cancer, pancreatic cancer and PrCa) [16]. Furthermore, patients with SCZ showed higher co-occurrence of SCZ with breast cancer, yet lower co-occurrence of melanoma and PrCa with SCZ [17]. Additionally, Ibáñez et al [18] found inverse expression deregulations between Central Nervous System (CNS) disorders (Alzheimer's disease, Parkinson's disease, and SCZ) and three cancer types (lung, prostate, and colorectal cancers). However, other studies found no evidence that SCZ confers protection against cancer in general [19].
The reduced incidence of cancer observed in SCZ patients could potentially attributed to differences in genetic background [20]. For example, Catts and Catts [21] suggested that the reduced incidence of cancer observed in SCZ patients might be linked to differences in apoptosis, and proposed p53, a tumor suppressor gene, which is considered as a candidate gene for the susceptibility. Park et al [20] found that the p53 polymorphism specifically identified in Korean SCZ patients may be associated with reduced vulnerability to lung cancer.
The juxtaposed with another zinc finger protein 1 (JAZF1) also known as TAK1-interacting protein 27 (TIP27) or zinc finger protein 802 (ZNF802) gene is located at 7p15 [22] and is highly expressed in testis, followed by colon, ovary, prostate, and placenta, but lower expressed in pancreas, brain, and liver [23]. Li et al [24] stated that the first 3 exons of JAZF1 are joined to the last 15 exons of Joined to JAZF1 protein 1 gene (JJAZ1) in the JAZF1/JJAZ1 fusion transcript, and suggested that there is a genetic pathway for progression of a benign precursor to a sarcoma involving increased cell survival, followed by accelerated cellular proliferation upon allelic exclusion of the unrearranged copy of that gene. JAZF1 has been associated with somatic fusion proteins in endometrial tumors [22,25-27]. Using a large genome-wide association study (GWAS), Thomas et al [28] found that Single-Nucleotide Polymorphisms (SNP) rs10486567 within JAZF1 was associated PrCa. Later, this variant was found to be associated with PrCa in a multiethnic sample of 2,768 incident PrCa cases and 2,359 controls from a multiethnic cohort (African Americans, European Americans, Latinos, Japanese Americans, and Native Hawaiians) [29]. Eeles et al [30] confirmed the associaiton of this SNP with PrCa from the second stage of genotyped 43,671 SNPs among 3,650 PrCa cases and 3,940 controls. Another study established rs10486567 as a bona-fide marker for association with susceptibility to PrCa in individuals of European ancestry [31]. Recently, the association of rs10486567 was valided in a African American populaiton [32] and confirmed by a meta-analysis [33]. Other studies showed the JAZF1 locus was associated with height[34], and type 2 diabetes (T2D) [35,36].
Previous studies have shown that zinc finger protein genes such as Zinc Finger Protein 74 (ZNF74) and 804A (ZNF804A) are associated with SCZ [37-40]. However, no study has focused on the association of JAZF1 with the risk of SCZ. This study explored the association of 118 SNPs within JAZF1 gene with the risk of SCZ in a European American (EA) sample (1,149 cases and 1,437 controls) from the Molecular Genetics of Schizophrenia - non GAIN Sample.
Material and Methods
Samples
NonGAIN sample is part of the Molecular Genetics of Schizophrenia (MGS) genome wide association study of 3,972 cases and 3,629 controls after quality control (dbGaP Study Accession: phs000167.v1.p1). Unrelated adult cases with DSM-IIIR (SGI study) or DSM-IV (MGS1, MGS2 studies) SCZ or schizoaffective disorder were collected under institutional review board-approved protocols in three studies, Schizophrenia Genetics Initiative (SGI), Molecular Genetics of Schizophrenia Part 1 (MGS1), and MGS23. Cases selected met criteria for SCZ or schizoaffective disorder per the Diagnostic and Statistical Manual of Mental Disorders version IV (DSM-IV). The details about these subjects were described elsewhere [41-43]. Genotyping data using the Affymetrix Genome-wide human SNP Array 6.0 (total 729,454 SNPs) were available for the sample. 1,179 European American (EA) patients with SCZ and 1,364 EA controls were selected from the nonGAIN Sample. We investigated the genetic associations of 118 SNPs within the JAZF1 gene with the risk of SCZ.
Statistical methods
For the initial Analysis, HelixTree Software (https://www.goldenhelix.com/SNP—Variation/HelixTree/index.html, Golden Helix, Bozeman, MT) was used to assess control genotype data for conformity with Hardy-Weinberg equilibrium (HWE). To deal with population stratification, the principal-component analysis approach with ten principal components [44] in HelixTree was used to identify outlier individuals. Then, logistic regression analysis of SCZ as a binary trait, adjusted for age and sex, was performed for the nonGAIN sample using PLINK v1.07 [45]. The asymptotic p values for this test were observed while the Odds Ratios (ORs) and standard errors of ORs were estimated. For logistic regression, the additive model was applied. In addition to obtaining nominal p values, empirical p-values were generated by 100,000 permutation tests using Max (T) permutation procedure implemented in PLINK. Minor Allele Frequency (MAF) was determined for each SNP and the Linkage Disequilibrium (LD) structure was constructed using Haploview software [46]. Haplotype analysis based on a slide-window was performed using PLINK.
Results
Based on the analysis of the first ten principal components using HelixTree and other exclusion criteria, 1,149 cases and 1,347 controls were left for further analyses. Participant characteristics are presented in Table 1. The mean values of age are 42.9 and 49.8 years for cases and controls, respectively.
Variable
Cases
Controls
Number
1,149
1,347
Sex, N (%)
Males
803(69%)
669(50%)
Females
346(31%)
678(50%)
Age, years
Mean ± SD
42.9 ± 11.9
49.8 ± 15.8
Range
18-84
18-90
Table 1: Descriptive Characteristics of Schizophrenia Cases and Controls.
All 118 SNPs are in Hardy-Weinberg equilibrium in the controls. The results of the top 15 SNPs with p<0.05 are summarized in Table 2. From single marker analysis, the strongest association with SCZ was observed with rs10258132 (p = 0.0011); while the next best signal was rs17156259 (p = 0.0031). The third best associated SNP was rs7791865 (p = 0.00889). All 15 SNPs had empirical pointwise p–values <0.05 using a permutation procedure (Table 2).
Using Haploview software, we identified 4 haplotype blocks for 20 flanking SNPs including rs10258132, rs7791865 and rs17156259. Figure 1 shows the LD structure. The numbers indicate the D' values between the corresponding two SNPs. Haplotype analyses showed that the A-C haplotype from rs10244184 and rs10258132 (D'=0.91) was associated with SCZ (p = 0.00093); while the C-G haplotype from rs10258132 and rs916857 (D'=0.91) was associated with SCZ (p = 0.00326). Furthermore, the G-G haplotype from rs17156238 and rs17156259 (D'=0.97) was associated with SCZ (p = 0.00455); while the G-G haplotype from rs17156259 and rs17156271 (D'=0.97) was associated with SCZ (p = 0.00535) (Table 2).
SNP
Position
Allelea
MAFb
HWEc
ORd
Pe
EMP1f
rs10258132
27892688
C
0.10
0.50
1.39(1.14-1.70)
0.00127
0.0011
rs916857
27896645
G
0.41
0.78
1.16(1.03-1.31)
0.0139
0.0147
rs7791865
27919811
A
0.08
0.63
1.33(0.07-1.64)
0.00825
0.00889
rs17156100
27968765
G
0.09
0.93
1.24(1.02-1.52)
0.0343
0.0299
rs17156102
27969077
C
0.09
0.93
1.24(1.02-1.52)
0.0343
0.0299
rs17156173
28012128
C
0.02
0.71
0.62(0.41-0.93)
0.0213
0.0194
rs17156205
28020586
T
0.02
0.70
0.62(0.41-0.93)
0.0213
0.0194
rs6946007
28021711
G
0.02
0.21
0.65(0.44-0.96)
0.0305
0.0287
rs6956395
28032626
G
0.03
0.27
0.68(0.49-0.96)
0.028
0.0241
rs6943061
28033081
T
0.03
0.28
0.68(0.49-0.96)
0.028
0.0241
rs6961522
28033437
T
0.02
0.76
0.61(0.41-0.92)
0.0179
0.017
rs17156238
28035553
G
0.03
0.28
0.67(0.48-0.95)
0.0238
0.02
rs17156259
28052747
G
0.03
0.49
0.54(0.36-0.81)
0.003
0.0031
rs17156271
28054284
G
0.03
0.61
0.61(0.41-0.91)
0.0153
0.0145
rs10951191
28083358
C
0.25
0.12
0.86(0.75-0.99)
0.0374
0.0399
a Minor allele; b Minor allele frequency; c Hardy-Weinberg equilibrium p-value; d Odds ratio; e p-value based on logistic regression; f empirical p-value generated by 100,000 permutation tests using Max (T) permutation procedure implemented in PLINK.
Table 2: 15 SNPs within JAZF1 gene associated with schizophrenia.
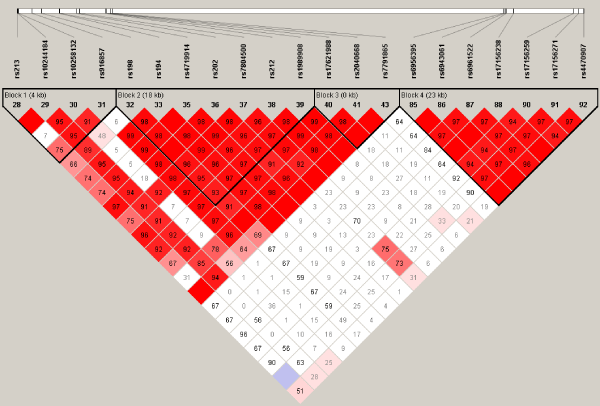
Figure 1: Linkage disequilibrium structure of 20 SNPs within JAZF1 including rs10258132, rs7791865 and rs17156259. The numbers indicate the D' values between the corresponding two SNPs.
Discussion
We conducted a candidate gene association study to identify possible SNPs within JAZF1 gene for the risk of SCZ. Totally, 15 SNPs showed significant associations with SCZ (p<0.05). Haplotype analysis further supported the single marker analysis results. Results provide support for the candidacy of JAZF1 as a potential target region that contributes to the risk of SCZ. To our knowledge, this is the first candidate gene study which investigates the associations between JAZF1 polymorphisms and SCZ.
JAZF1 is a large gene of approximately 350 kb. Previous studies have identified SNP rs10486567 located in intron 2 was associated with risk of PrCa [28-31,47]. Recently, several SNPs located close to each other in another LD block within intron 1 and ˜210 Kb centromeric from rs10486567, have been associated with body stature [34], T2D [35,36], height [48], and arteriolosclerosis [49]. Epidemiological studies have shown an inverse correlation between T2D and PrCa [50-53], although some studies showed inconsistent results [54]. However, the basis for this association is unclear. It has been proposed that JAZF1 may be a possible direct causal link between T2D and PrCa [55,56]. Recently, it was found that the risk associated with the G allele of rs10486567 in the JAZF1 intron created an NKX3-1 binding site while destroying a FOXA1 binding site in line JAZF1 is a large gene of approximately 350 kb. Previous studies have identified SNP rs10486567 located in intron 2 was associated with risk of PrCa [28-31,47]. Recently, several SNPs located close to each other in another LD block within intron 1 and ~210 Kb centromeric from rs10486567, have been associated with body stature [34], T2D [35,36], height [48], and arteriolosclerosis [49]. Epidemiological studies have shown an inverse correlation between T2D and PrCa [50-53], although some studies showed inconsistent results [54]. However, the basis for this association is unclear. It has been proposed that JAZF1 may be a possible direct causal link between T2D and PrCa [55,56]. Recently, it was found that the risk associated with the G allele of rs10486567 in the JAZF1 intron created an NKX3-1 binding site while destroying a FOXA1 binding site in line
Reduced risk of some cancers among persons diagnosed with SCZ was identified [11-13]. Others observed that patients with SCZ have a significantly higher risk of colon cancer [61], breast cancer [62,63] and lung cancer [62]. Moreover, decreased risks for several types of cancer such as PrCa and malignant melanoma were observed in both siblings and parents of schizophrenic patients when compared to the general population, providing further support for genetic protection against cancer in families with SCZ [62,64]. Gal et al [65] observed a statistically significant reduction of risks for overall cancer in SCZ among patients for both parents combined and for both fathers and mothers taken singly and a marginal reduction effect for PrCa; whereas the familial aggregation did not show an association with a significant increased risk for cancer. However, the mechanism of such association is not clear. The shared genetic variations may explain part of the associations among these diseases. Closer attentions to the shared network between core cell cycle regulators and non-cell cycle functions in neurons will be informative for neuroscience [66]. Furthermore, Wang et al [67] suggested that these mechanisms may be important clues for the schizophrenia research community in understanding the links between SCZ and cancer. It has been shown that p53 is a well characterized tumor suppressor protein with prominent roles in apoptosis. Several studies have shown an association between TP53 (the gene encoding p53) function and SCZ [21, 68-70]. Especially, Park et al [20] found that the p53 polymorphism specifically identified in Korean SCZ patients may be associated with reduced vulnerability to lung cancer. The JAZF1 gene also known as zinc finger protein 802 (ZNF802) encodes a nuclear protein with three C2H2-type zinc fingers, and functions as a transcriptional repressor. Previous studies have shown that other zinc finger protein genes such as zinc finger protein 74 (ZNF74) and 804A (ZNF804A) are associated with SCZ [37-40]. To our knowledge, this is the first candidate gene study which investigates the associations between JAZF1 polymorphisms and SCZ. Although the JAZF1 gene is expressed in brain, the exact role of this gene product is not known. Laity and Andrews [71] reviewed that zinc is an essential nutrient, but toxic when accumulated to excess and provides a structural scaffold for many proteins such as zinc fingers, zinc clusters, and nuclear hormone receptors. Zinc deficiency has been linked to abnormal embryonic and fetal development [72], immune dysfunction, and neurological problems including T2D and SCZ [71,73-77]. Furthermore, the co-occurrence of SCZ and T2D has been well documented and may be, at least in part, driven by shared genetic factors [78-81]. Recently, several shared genes and common genetic pathways for T2D and SCZ have been proposed [78- 81]. Considering the association of JAZF1 with T2D [35,36], it may be hypothesized that the JAZF1 may be part of mechcanism of the cormorbidity of PrCa, SCZ and T2D.
There are a number of strengths in this study. First, our sample size was relatively large for candidate gene association studies. Second, the haplotype analyses results further supported the single marker analysis findings. We also realized some limitations in this study. First, our current findings might be spurious or subject to type I error. Second, these findings need to be replicated in additional samples. Third, this study focused on association between the JAZF1 gene and SCZ rather than functional study.
Conclusion
Our results demonstrate that several genetic variants in PrCa related JAZF1 gene were associated with the risk of SCZ. These findings may serve as a resource for replication in other populations. Future functional study of this gene may help to better characterize the genetic architecture of the risk of SCZ.
Acknowledgement
Funding support for the companion studies, Genome- Wide Association Study of Schizophrenia (GAIN) and Molecular Genetics of Schizophrenia - nonGAIN Sample (MGS_nonGAIN), was provided by Genomics Research Branch at NIMH and the genotyping and analysis of samples was provided through the Genetic Association Information Network (GAIN) and under the MGS U01s: MH79469 and MH79470, and K01 DA029643, R21 AA021380, R21 AA020319, and the National Alliance for Research on Schizophrenia and Depression (NARSAD) Award 17616. Assistance with data cleaning was provided by the National Center for Biotechnology Information. The MGS dataset(s) used for the analyses described in this manuscript were obtained from the database of Genotype and Phenotype (dbGaP) found at https://www.ncbi.nlm.nih.gov/gap through dbGaP accession number phs000167.v1.p1 (nonGAIN). Samples and associated phenotype data for the MGS GWAS study were collected under the following grants: NIMH Schizophrenia Genetics Initiative U01s: MH46276 (CR Cloninger), MH46289 (C Kaufmann), and MH46318 (MT Tsuang); and MGS Part 1 (MGS1) and Part 2 (MGS2) R01s: MH67257 (NG Buccola), MH59588 (BJ Mowry), MH59571 (PV Gejman), MH59565 (Robert Freedman), MH59587 (F Amin), MH60870 (WF Byerley), MH59566 (DW Black), MH59586 (JM Silverman), MH61675 (DF Levinson), and MH608`79 (CR Cloninger). Further details of collection sites, individuals, and institutions may be found in data supplement Table 1 of Sanders et al. (2008). This study was approved by Internal Review Board (IRB), East Tennessee State University.
Haplotype
Frequencya
ORb
p-Valuec
rs10244184
rs10258132
A
C
0.1
1.42
0.00093
G
A
0.26
0.93
0.315
A
A
0.64
0.93
0.254
rs10258132
rs916857
C
G
0.09
1.37
0.00326
A
G
0.33
1.04
0.518
A
A
0.58
0.86
0.0128
rs17156283
rs17156259
G
G
0.02
0.54
0.00455
A
A
0.93
1.58
0.0102
rs17156259
rs17156271
G
G
0.02
0.54
0.00535
A
A
0.98
1.76
0.00535
a Haplotype frequency in the sample.
b Odds ratio for each haplotype.
c p-value for the single haplotype.
Table 3: Haplotype analysis of JAZF1 gene.
References
- Regier DA, Narrow WE, Rae DS, Manderscheid RW, Locke BZ, Goodwin FK. The de facto mental and addictive disorders service system. Epidemiologic Catchment Area prospective 1-year prevalence rates of disorders and services. Archives of General Psychiatry. 1993; 50: 85-94.
- Mowry BJ, Nancarrow DJ. Molecular genetics of schizophrenia. Clin Exp Pharmacol Physiol. 2001; 28: 66-69.
- U.S. Census Bureau Population Estimates by Demographic Characteristics. Table 2: Annual Estimates of the Population by Selected Age Groups and Sex for the United States: April , 2000 to July , 2004 (NC-EST2004-02) Source: Population Division, U.S. Census Bureau Release Date: June 9, 2005.
- Sullivan PF, Kendler KS, Neale MC. Schizophrenia as a complex trait: evidence from a meta-analysis of twin studies. Arch Gen Psychiatry. 2003; 60: 1187-1192.
- Gejman PV, Sanders AR, Duan J. The role of genetics in the etiology of schizophrenia. Psychiatr Clin North Am. 2010; 33: 35-66.
- Ferlay J, Autier P, Boniol M, Heanue M, Colombet M, Boyle P. Estimates of the cancer incidence and mortality in Europe in 2006. Ann Oncol. 2007; 18: 581-592.
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009; 59: 225-249.
- Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011; 61: 212-236.
- Crawford ED. Epidemiology of prostate cancer. Urology. 2003; 62: 3-12.
- Lichtenstein P, Holm NV, Verkasalo PK, Iliadou A, Kaprio J, Koskenvuo M, et al. Environmental and heritable factors in the causation of cancer--analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med. 2000; 343: 78-85.
- Jablensky A, Lawrence D. Schizophrenia and cancer: is there a need to invoke a protective gene? Arch Gen Psychiatry. 2001; 58: 579-580.
- Cohen M, Dembling B, Schorling J. The association between schizophrenia and cancer: a population-based mortality study. Schizophr Res. 2002; 57: 139-146.
- Barak Y, Achiron A, Mandel M, Mirecki I, Aizenberg D. Reduced cancer incidence among patients with schizophrenia. Cancer. 2005; 104: 2817-2821.
- Torrey EF. Prostate cancer and schizophrenia. Urology. 2006; 68: 1280-1283.
- Raviv G, Laufer M, Baruch Y, Barak Y. Risk of prostate cancer in patients with schizophrenia. Compr Psychiatry. 2014 [In press].
- Lin CY, Lane HY, Chen TT, Wu YH, Wu CY, Wu VY. Inverse association between cancer risks and age in schizophrenic patients: a 12-year nationwide cohort study. Cancer Sci. 2013; 104: 383-390.
- Catalá-López F, Suárez-Pinilla M, Suárez-Pinilla P, Valderas JM, Gómez-Beneyto M, Martinez S, et al. Inverse and direct cancer comorbidity in people with central nervous system disorders: a meta-analysis of cancer incidence in 577,013 participants of 50 observational studies. Psychother Psychosom. 2014; 83: 89-105.
- Ibáñez K, Boullosa C, Tabarés-Seisdedos R, Baudot A, Valencia A. Molecular evidence for the inverse comorbidity between central nervous system disorders and cancers detected by transcriptomic meta-analyses. PLoS Genet. 2014; 10: e1004173.
- Goldacre MJ, Kurina LM, Wotton CJ, Yeates D, Seagroat V. Schizophrenia and cancer: an epidemiological study. Br J Psychiatry. 2005; 187: 334-338.
- Park JK, Lee HJ, Kim JW, Park YH, Lee SS, Chang HI, et al. Differences in p53 gene polymorphisms between Korean schizophrenia and lung cancer patients. Schizophr Res. 2004; 67: 71-74.
- Catts VS, Catts SV. Apoptosis and schizophrenia: is the tumour suppressor gene, p53, a candidate susceptibility gene? Schizophr Res. 2000; 41: 405-415.
- Koontz JI, Soreng AL, Nucci M, Kuo FC, Pauwels P, van Den Berghe H, et al. Frequent fusion of the JAZF1 and JJAZ1 genes in endometrial stromal tumors. Proc Natl Acad Sci U S A. 2001; 98: 6348-6353.
- Nakajima T, Fujino S, Nakanishi G, Kim YS, Jetten AM. TIP27: a novel repressor of the nuclear orphan receptor TAK1/TR4. Nucleic Acids Res. 2004; 32: 4194-4204.
- Li H, Ma X, Wang J, Koontz J, Nucci M, Sklar J. Effects of rearrangement and allelic exclusion of JJAZ1/SUZ12 on cell proliferation and survival. Proc Natl Acad Sci U S A. 2007; 104: 20001-20006.
- Micci F, Panagopoulos I, Bjerkehagen B, Heim S. Consistent rearrangement of chromosomal band 6p21 with generation of fusion genes JAZF1/PHF1 and EPC1/PHF1 in endometrial stromal sarcoma. Cancer Res. 2006; 66: 107-112.
- Micci F, Walter CU, Teixeira MR, Panagopoulos I, Bjerkehagen B, Saeter G, et al. Cytogenetic and molecular genetic analyses of endometrial stromal sarcoma: nonrandom involvement of chromosome arms 6p and 7p and confirmation of JAZF1/JJAZ1 gene fusion in t(7;17). Cancer Genet Cytogenet. 2003; 144:119-124.
- Oliva E, de Leval L, Soslow RA, Herens C. High frequency of JAZF1-JJAZ1 gene fusion in endometrial stromal tumors with smooth muscle differentiation by interphase FISH detection. Am J Surg Pathol. 2007; 31: 1277-1284.
- Thomas G, Jacobs KB, Yeager M, Kraft P, Wacholder S, Orr N, et al. Multiple loci identified in a genome-wide association study of prostate cancer. Nat Genet. 2008; 40: 310-315.
- Waters KM, Henderson BE, Stram DO, Wan P, Kolonel LN, Haiman CA. Association of diabetes with prostate cancer risk in the multiethnic cohort. Am J Epidemiol. 2009; 169: 937-945.
- Eeles RA, Kote-Jarai Z, Al Olama AA, Giles GG, Guy M, Severi G, et al. Identification of seven new prostate cancer susceptibility loci through a genome-wide association study. Nat Genet. 2009; 41: 1116-1121.
- Prokunina-Olsson L, Fu YP, Tang W, Jacobs KB, Hayes RB, Kraft P, et al. Refining the prostate cancer genetic association within the JAZF1 gene on chromosome 7p15.2. Cancer Epidemiol Biomarkers Prev. 2010; 19: 1349-1355.
- Chang BL, Spangler E, Gallagher S, Haiman CA, Henderson B, Isaacs W, et al. Validation of genome-wide prostate cancer associations in men of African descent. Cancer Epidemiol Biomarkers Prev. 2011; 20: 23-32.
- Liu H, Wang B, Han C. Meta-analysis of genome-wide and replication association studies on prostate cancer. Prostate. 2011; 71: 209-224.
- Soranzo N, Rivadeneira F, Chinappen-Horsley U, Malkina I, Richards JB, Hammond N, et al. Meta-analysis of genome-wide scans for human adult stature identifies novel Loci and associations with measures of skeletal frame size. PLoS Genet. 2009; 5: e1000445.
- Zeggini E, Scott LJ, Saxena R, Voight BF, Marchini JL, Hu T, et al. Meta-analysis of genome-wide association data and large-scale replication identifies additional susceptibility loci for type 2 diabetes. Nat Genet. 2008; 40: 638-645.
- Voight BF, Scott LJ, Steinthorsdottir V, Morris AP, Dina C, Welch RP, et al. Twelve type 2 diabetes susceptibility loci identified through large-scale association analysis. Nat Genet. 2010; 42: 579-589.
- Takase K, Ohtsuki T, Migita O, Toru M, Inada T, Yamakawa-Kobayashi K, et al. Association of ZNF74 gene genotypes with age-at-onset of schizophrenia. Schizophr Res. 2001; 52: 161-165.
- Riley B, Thiselton D, Maher BS, Bigdeli T, Wormley B, McMichael GO, et al. Replication of association between schizophrenia and ZNF804A in the Irish Case-Control Study of Schizophrenia sample. Mol Psychiatry. 2010; 15: 29-37.
- Williams HJ, Norton N, Dwyer S, Moskvina V, Nikolov I, Carroll L, et al. Fine mapping of ZNF804A and genome-wide significant evidence for its involvement in schizophrenia and bipolar disorder. Mol Psychiatry. 2011; 16: 429-441.
- Hess JL, Glatt SJ. How might ZNF804A variants influence risk for schizophrenia and bipolar disorder? A literature review, synthesis, and bioinformatic analysis. Am J Med Genet B Neuropsychiatr Genet. 2014; 165: 28-40.
- O'Donovan MC, Craddock N, Norton N, Williams H, Peirce T, Moskvina V, et al. Identification of loci associated with schizophrenia by genome-wide association and follow-up. Nat Genet. 2008; 40: 1053-1055.
- Sanders AR, Duan J, Levinson DF, Shi J, He D, Hou C, et al. No significant association of 14 candidate genes with schizophrenia in a large European ancestry sample: implications for psychiatric genetics. Am J Psychiatry. 2008; 165: 497-506.
- Shi J, Levinson DF, Duan J, Sanders AR, Zheng Y, Pe'er I, et al. Common variants on chromosome 6p22.1 are associated with schizophrenia. Nature. 2009; 460: 753-757.
- Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 2006; 38: 904-909.
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007; 81: 559-575.
- Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005; 21: 263-265.
- Waters KM, Le Marchand L, Kolonel LN, Monroe KR, Stram DO, Henderson BE, et al. Generalizability of associations from prostate cancer genome-wide association studies in multiple populations. Cancer Epidemiol Biomarkers Prev. 2009; 18: 1285-1289.
- Johansson A, Marroni F, Hayward C, Franklin CS, Kirichenko AV, Jonasson I, et al. Common variants in the JAZF1 gene associated with height identified by linkage and genome-wide association analysis. Hum Mol Genet. 2009; 18: 373-380.
- Chou SH, Shulman JM, Keenan BT, Secor EA, Buchman AS, Schneider J, et al. Genetic susceptibility for ischemic infarction and arteriolosclerosis based on neuropathologic evaluations. Cerebrovasc Dis. 2013; 36: 181-188.
- Kasper JS, Giovannucci E. A meta-analysis of diabetes mellitus and the risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 2006; 15: 2056-2062.
- Gong Z, Neuhouser ML, Goodman PJ, Albanes D, Chi C, Hsing AW, et al. Obesity, diabetes, and risk of prostate cancer: results from the prostate cancer prevention trial. Cancer Epidemiol Biomarkers Prev. 2006; 15: 1977-1983.
- Calton BA, Chang SC, Wright ME, Kipnis V, Lawson K, Thompson FE, et al. History of diabetes mellitus and subsequent prostate cancer risk in the NIH-AARP Diet and Health Study. Cancer Causes Control. 2007; 18: 493-503.
- Spurdle AB, Thompson DJ, Ahmed S, Ferguson K, Healey CS, O'Mara T, et al. Genome-wide association study identifies a common variant associated with risk of endometrial cancer. Nat Genet. 2011; 43: 451-454.
- Will JC, Vinicor F, Calle EE. Is diabetes mellitus associated with prostate cancer incidence and survival? Epidemiology. 1999; 10: 313-318.
- Frayling TM, Colhoun H, Florez JC. A genetic link between type 2 diabetes and prostate cancer. Diabetologia. 2008; 51: 1757-1760.
- Machiela MJ, Lindström S, Allen NE, Haiman CA, Albanes D, Barricarte A, et al. Association of type 2 diabetes susceptibility variants with advanced prostate cancer risk in the Breast and Prostate Cancer Cohort Consortium. Am J Epidemiol. 2012; 176: 1121-1129.
- Hazelett DJ, Rhie SK, Gaddis M, Yan C, Lakeland DL, Coetzee SG. Ellipse/GAME-ON consortium; Practical consortium, Henderson BE5. Comprehensive functional annotation of 77 prostate cancer risk loci. PLoS Genet. 2014; 10: e1004102.
- Xie S, Lee YF, Kim E, Chen LM, Ni J, Fang LY, et al. TR4 nuclear receptor functions as a fatty acid sensor to modulate CD36 expression and foam cell formation. Proc Natl Acad Sci U S A. 2009; 106: 13353-13358.
- Jang WY, Bae KB, Kim SH, Yu DH, Kim HJ, Ji YR, et al. Overexpression of Jazf1 reduces body weight gain and regulates lipid metabolism in high fat diet. Biochem Biophys Res Commun. 2014; 444: 296-301.
- Stevens VL, Ahn J, Sun J, Jacobs EJ, Moore SC, Patel AV, et al. HNF1B and JAZF1 genes, diabetes, and prostate cancer risk. Prostate. 2010; 70: 601-607.
- Hippisley-Cox J, Vinogradova Y, Coupland C, Parker C. Risk of malignancy in patients with schizophrenia or bipolar disorder: nested case-control study. Arch Gen Psychiatry. 2007; 64: 1368-1376.
- Catts VS, Catts SV, O'Toole BI, Frost AD. Cancer incidence in patients with schizophrenia and their first-degree relatives - a meta-analysis. Acta Psychiatr Scand. 2008; 117: 323-336.
- Bushe CJ, Bradley AJ, Wildgust HJ, Hodgson RE. Schizophrenia and breast cancer incidence: a systematic review of clinical studies. Schizophr Res. 2009; 114: 6-16.
- Lichtermann D, Ekelund J, Pukkala E, Tanskanen A, Lönnqvist J. Incidence of cancer among persons with schizophrenia and their relatives. Arch Gen Psychiatry. 2001; 58: 573-578.
- Gal G, Goral A, Murad H, Gross R, Pugachova I, Barchana M, et al. Cancer in parents of persons with schizophrenia: is there a genetic protection? Schizophr Res. 2012; 139: 189-193.
- Frank CL, Tsai LH. Alternative functions of core cell cycle regulators in neuronal migration, neuronal maturation, and synaptic plasticity. Neuron. 2009; 62: 312-326.
- Wang Y, He G, He L, McGrath J. Do shared mechanisms underlying cell cycle regulation and synaptic plasticity underlie the reduced incidence of cancer in schizophrenia? Schizophr Res. 2011; 130: 282-284.
- Ni X, Trakalo J, Valente J, Azevedo MH, Pato MT, Pato CN, et al. Human p53 tumor suppressor gene (TP53) and schizophrenia: case-control and family studies. Neurosci Lett. 2005; 388: 173-178.
- Lung FW, Shu BC, Kao WT, Chen CN, Ku YC, Tzeng DS. Association of DRD4 uVNTR and TP53 codon 72 polymorphisms with schizophrenia: a case-control study. BMC Med Genet. 2009; 10: 147.
- Feigenson KA, Kusnecov AW, Silverstein SM. Inflammation and the two-hit hypothesis of schizophrenia. Neurosci Biobehav Rev. 2014; 38: 72-93.
- Laity JH, Andrews GK. Understanding the mechanisms of zinc-sensing by metal-response element binding transcription factor-1 (MTF-1). Arch Biochem Biophys. 2007; 463: 201-210.
- Bloxam DL, Bax CM. Zinc deficiency and abnormal fetal development: assessment of maternal or fetal zinc status. Am J Obstet Gynecol. 1996; 175: 1078.
- Andrews RC. Diabetes and schizophrenia: genes or zinc deficiency? Lancet. 1992; 340: 1160.
- Black MM. Zinc deficiency and child development. Am J Clin Nutr. 1998; 68: 464S-469S.
- Salgueiro MJ, Weill R, Zubillaga M, Lysionek A, Caro R, Goldman C, et al. Zinc deficiency and growth: current concepts in relationship to two important points: intellectual and sexual development. Biol Trace Elem Res. 2004; 99: 49-69.
- Richardson-Andrews RC. The sunspot theory of schizophrenia: further evidence, a change of mechanism, and a strategy for the elimination of the disorder. Med Hypotheses. 2009; 72: 95-98.
- Meyer U. Developmental neuroinflammation and schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2013; 42: 20-34.
- Bellivier F. Schizophrenia, antipsychotics and diabetes: Genetic aspects. Eur Psychiatry. 2005; 20 Suppl 4: S335-339.
- Rouillon F, Sorbara F. Schizophrenia and diabetes: epidemiological data. Eur Psychiatry. 2005; 20 Suppl 4: S345-348.
- Lin PI, Shuldiner AR. Rethinking the genetic basis for comorbidity of schizophrenia and type 2 diabetes. Schizophr Res. 2010; 123: 234-243.
- Liu Y, Li Z, Zhang M, Deng Y, Yi Z, Shi T. Exploring the pathogenetic association between schizophrenia and type 2 diabetes mellitus diseases based on pathway analysis. BMC Med Genomics. 2013; 6: S17.