
Research Article
J Schizophr Res. 2025; 12(1): 1051.
The Effect of Iloperidone on Hostility in Schizophrenia Patients: Post Hoc Analysis from a Randomized, Placebo-Controlled Trial
Carlin JL¹*, Xiao C² and Polymeropoulos MH¹
¹Clinical Research and Development, Vanda Pharmaceuticals, Inc., Washington, D.C., USA
²Biostatistics, Vanda Pharmaceuticals, Inc., Washington, D.C, USA
*Corresponding author: Jesse L. Carlin, Vanda Pharmaceuticals, Inc., 2200 Pennsylvania Avenue NW, Suite 300-E, Washington D.C. 20037, USA Tel: (202) 734-3431; Email: Jesse.Carlin@vandapharma.com
Received: May 08, 2025 Accepted: June 05, 2025 Published: June 10, 2025
Abstract
Objective: Individuals with schizophrenia have increased risk of hostile behavior. Iloperidone, a dopamine D2 receptor and serotonin 5HT2A receptor antagonist was evaluated for acute exacerbations of schizophrenia in a phase 3 study. A posthoc analysis evaluated iloperidone’s antihostility effects in patients with schizophrenia.
Method: A phase 3, randomized, placebo-controlled, active-controlled study in adults with acute exacerbation of schizophrenia conducted between 2005 and 2006 was analyzed. The principal posthoc outcome was mean change from baseline to day 28 on the Positive and Negative Syndrome Scale (PANSS) hostility item (P7); separate analyses were stratified for baseline hostility score (P7) using ziprasidone as a positive control. Sedation was also analyzed as a factor. Analyses were based on the ITT population (N = 567) using mixed-effects model for repeated measures.
Results: The change from baseline to day 28 was statistically significant on PANSS hostility item in favor of iloperidone versus placebo: (–1.26, p = .0012; The magnitude of change for iloperidone increased with greater baseline hostility, however only baseline = 2 reached statistical significance (p=0.02). The magnitude of effect on PANSS hostility item was similar for iloperidone and ziprasidone compared to placebo. However, sedation occurred at a higher frequency in ziprasidone vs. iloperidone (12.7% vs. 27.8%, p=.0001).
Conclusions: Significant improvement on the hostility item was seen in iloperidone- vs placebo-treated patients with schizophrenia; the effect of iloperidone was similar to ziprasidone and was higher with in patients with baseline hostility. Ziprasidone’s effect on hostility appeared to be driven by sedation compared to iloperidone.
Keywords: Schizophrenia; Hostility; Antipsychotic; Iloperidone; PANSS; Aggression
Abbreviations
BID: Bis in Die (latin, twice daily); CATIE: Clinical Antipsychotic Trials of Intervention Effectiveness; CGI-S: Clinical Global Impression of Severity; D2: Dopamine D2 Receptor; 5HT2A: Serotonin Receptor 2A; NeA1: Noradrenergic Alpha 1; PANSS: Positive and Negative Syndrome Scale; DSM-IV: The Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; ITT: Intent-to-treat; MMRM: Mixed-effects Model Repeated Measures; LSMD: Least Square Mean Difference; LS: Least Square; Mg/day: Milligram Per Day; PANSS-EC: Positive and Negative Syndrome Scale-Excited Component; BPRS: Brief Psychiatric Rating Scale; PANSS-P: PANSS positive subscale; PANSS-N: PANSS negative subscale; PANSS-GP: PANSS General Psychopathology Subscale; CDSS: Calgary Depression Scale for Schizophrenia.
Introduction
Hostility is defined as having unfriendly attitudes manifested by overt behaviors including irritability, anger, resentment, or aggression. Symptoms of hostility are present in some patients during acute exacerbations of schizophrenia and have been associated with subsequent aggressive or violent behavior [1]. Violent or threatening behavior is a frequent reason for admission to a psychiatric inpatient facility and the presence of these behaviors can prolong time to discharge. Moreover, hostility is associated with non-adherence to treatment [2]. Post hoc analyses of data from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) study in patients with schizophrenia suggested that second-generation antipsychotics may have specific effects on hostility, which are independent of their effects on positive schizophrenia symptoms [3]. The anti-aggressive effect of antipsychotics has been extensively studied and drugs such as clozapine [4], risperidone [5], aripiprazole [6] and cariprazine [7] have been shown to reduce hostility in clinical studies. Iloperidone was approved by the FDA for the treatment of adults with schizophrenia in May 2009 for acute exacerbations and the label was later expanded to include long term relapse prevention. Iloperidone showed efficacy in 2 short-term 4- week and 1 short term 6-week acute schizophrenia trials [8-10]. In addition, iloperidone was shown to be comparable to haloperidol in three 52-week maintenance studies [11] and was effective in preventing relapse in subjects previously stabilized on iloperidone [12].
Iloperidone is a second-generation atypical antipsychotic available as an oral tablet. Iloperidone’s primary mechanism of action is Dopamine D2 receptor and Serotonin 5HT2A receptor antagonism, with greater affinity for the 5HT2A receptor than the D2 receptor (Fanapt package insert, 2016). Iloperidone also has a strong affinity for the noradrenergic alpha 1 (NEa1) receptor [13]. It is thought that antagonism at these receptors can relieve acute symptoms of schizophrenia.
Iloperidone has been reported to have beneficial effects on the cluster of excitement/hostility symptoms derived from the Positive and Negative Syndrome Scale (PANSS) in early pooled clinical trials [14]. To observe the effect of iloperidone specifically on hostility, a post hoc analysis of the PANSS Hostility factor items was performed from data from the last 4-week short-term acute schizophrenia clinical trial.
Material and Methods
Study Design
This data was taken from a randomized, double-blind, placebo and ziprasidone-controlled, parallel group, multi-center study to evaluate the efficacy and safety of fixed doses of iloperidone and ziprasidone in patients with schizophrenia conducted between 2005 and 2006 (ClinicalTrials.gov identifier: NCT00254202). This study included 3 treatment groups: iloperidone 24 mg/day, ziprasidone 160 mg/day, and placebo.
The study consisted of a screening period of up to 14 days to determine eligibility, a baseline visit followed by a 7 day study drug titration period, and a 21 day maintenance period. Patients were randomized at baseline to study drug to receive iloperidone, ziprasidone, or placebo in a 2:1:1 ratio. Study assessments occurred daily during titration and on days 10, 14, 21, and 28 of the maintenance period or upon termination/discontinuation.
Patients were randomized in a ratio of 2:1:1 to receive b.i.d. treatment with iloperidone, ziprasidone, or placebo, respectively. Patients were hospitalized during the 4 weeks of the short-term double blind phase of the study (Days 1 to 28). The primary efficacy measure of this study was the PANSS total rating after 4 weeks of double blind treatment. The protocol was approved by an institutional review board, ICH-E6 Good Clinical Practice guidelines were followed and written informed consent was obtained from all participants before any study procedures occurred.
Patients
Patients were men and women 18–65 years of age, inclusive, and had a diagnoses of schizophrenia according to Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria, Clinical Global Impression of Severity (CGI-S) score of 4 or greater at baseline, overall Positive and Negative Syndrome Scale (PANSS) total score of 70 or greater at screening and baseline, and a rating of 4 (moderate) or greater on at least 2 of the following PANSS Positive (PANSS-P) symptoms: delusions, conceptual disorganization, hallucinations, and suspiciousness/ persecution at screening and baseline. Exclusionary criteria included a diagnosis of schizoaffective disorder, bipolar disorder, a cognitive disorder, treatment-resistant schizophrenia, substance abuse, suicidal or homicidal intent, congenital long QT syndrome, or clinically significant gastrointestinal, hepatic, or renal disease.
Study Drug
Patients received placebo twice daily or ascending twice-daily doses of iloperidone 1, 2, 4, 6, 8, 10, and 12 mg (days 1–7, respectively) or ziprasidone 20 mg (days 1–2), 40 mg (days 3–4), 60 mg (days 5–6), and 80 mg (day 7) BID as indicated on the ziprasidone package insert. Study medication was titrated to final doses of 24 mg/d total for iloperidone or 160 mg/d total for ziprasidone. These dosages were continued during the maintenance period (days 8–28). The ziprasidone titration schedule and maximum dose were based on the agent’s prescribing information (Geodon package insert, 2002). All doses were given with food.
There was no placebo washout period; patients could take their current antipsychotic medications up until Day 0. Zolpidem (or similar bob-benzodiazepine with short half-life medication) was permitted for insomnia; after administration, a minimum of 8 hours had to elapse before completing efficacy evaluations. Lorazepam (or similar injectable short half-life benzodiazepine medication) was permitted for agitation/severe restlessness, according to specified schedules, doses, and routes; after administration, a minimum of 4 hours had to elapse before completing efficacy evaluations. Benztropine (or similar medication) was permitted for extrapyramidal symptoms but only after assessment with the Extrapyramidal Symptom Rating Scale (ESRS) was performed [15].
Outcome Assessments
The primary efficacy endpoint was change from baseline in PANSS total (PANSS-T) scores. Secondary endpoints included change from baseline on the PANSS-derived Brief Psychiatric Rating Scale (BPRS); PANSS-P; PANSS negative subscale (PANSS-N); PANSS general psychopathology subscale (PANSS-GP); Calgary Depression Scale for Schizophrenia (CDSS)17; CGI-S (1 = normal/not at all ill; 7 = among most extremely ill); and the Clinical Global Impression of Change (4 = no change; <4 = improvement; and >4 = worsening).
Assessment of Hostility
The post hoc efficacy assessment of hostility was mean change from baseline to day 28 on the PANSS hostility item, which measures verbal and nonverbal expressions of anger and resentment, including sarcasm, passive-aggressive behavior, verbal abuse, and assaultiveness [16]. Scores range from 1 (no hostility) to 7 (extreme hostility characterized by marked anger that results in extreme uncooperativeness, preventing other interactions, or physical assault). Mean change from baseline on the PANSS hostility item (P7) was assessed in the overall patient population and in sub group of patients with increasing levels of baseline hostility. Baseline hostility levels were defined by baseline cut off scores of 2 (minimally severe; questionable pathology; may be at the upper extreme of normal limits), 3 (mildly severe; indirect or restrained communication of anger such as sarcasm, disrespect, hostile expressions, and occasional irritability), 4 (moderately severe; overtly hostile attitude, showing frequent irritability and direct expression of anger or resentment).
Supporting analyses included change from baseline to day 28 on PANSS-Excited Component (PANSS-EC), a subscale of the PANSS used to evaluate acute agitation and aggression in psychiatric patients. PANSS-EC items (, uncooperativeness [G8], poor impulse control [G14], excitement [P4], and hostility [P7]) are rated from 1 (not present) to 7 (extremely severe).
Statistical Analysis
Efficacy analyses were based on the intent-to treat (ITT) population, comprising all patients who received 1 or more doses of study medication and underwent a baseline and 1 or more postbaseline PANSS efficacy evaluations. 10 patients were randomized in error at a second site after already being randomized at a first site. Data from the second site were excluded from analysis in the ITT population for efficacy. Primary efficacy outcome was mean change from baseline to last scheduled observation in PANSS-T score for iloperidone versus placebo, analyzed using mixed-effects model repeated measures (MMRM).
The PANSS-EC and hostility item (P7) were analyzed using a mixed-effects model for repeated measures approach with study pooled sites, treatment group, time, and treatment group–by-time interaction as categorical fixed effects; baseline value and baselineby- time interaction were included as covariates. The effect of other symptoms of schizophrenia was not controlled for. The principal post hoc outcome was mean change from baseline to day 28 on the Positive and Negative Syndrome Scale (PANSS) hostility item (P7); separate analyses were stratified for baseline hostility score (P7: = 2, = 3, = 4) and ziprasidone was used as a positive control. Sedation was also analyzed as a factor. Analyses were based on the intent-to-treat population (N = 567)
Results and Discussion
Of the 913 patients screened and the 606 patients assigned to randomization, 593 patients were randomized to the study. Of the randomized patients, 381 (64.2%) completed the initial doubleblind phase (4-week treatment). A total of 193 (65.4%), 98 (65.8%), and 90 (60.4%) patients treated with iloperidone, ziprasidone, and placebo completed the study, respectively. The most common reason for discontinuation in all groups was withdrawal of consent. Demographics and clinical characteristics were similar in each group (Table 1).
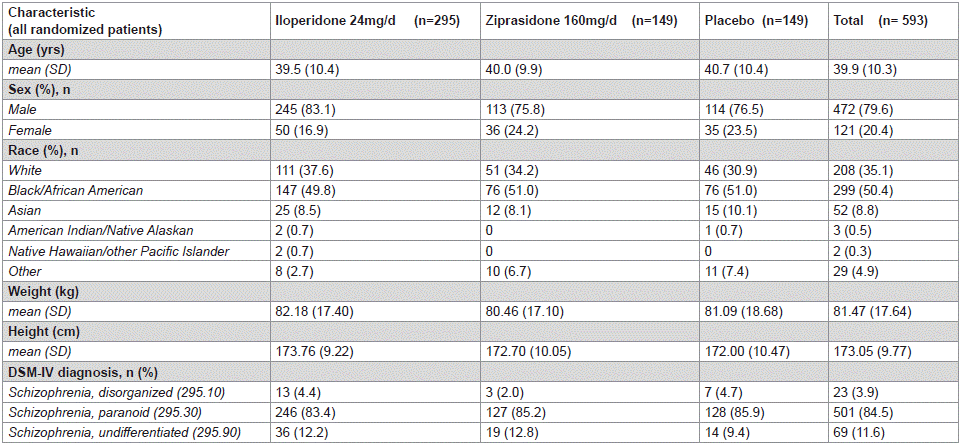
Table 1: All Randomized Patients Demographics and Clinical Characteristics.
Overall Efficacy
Patients treated with iloperidone had significantly greater improvement in PANSS-T score at day 28 compared to those receiving placebo (-12.0, iloperidone; -7.1, placebo; P < 0.01) (Table 2). Patients receiving ziprasidone also had significantly greater improvement versus those receiving placebo (-12.3; P < 0.05 vs placebo), confirming the sensitivity of the measure (Table 2).
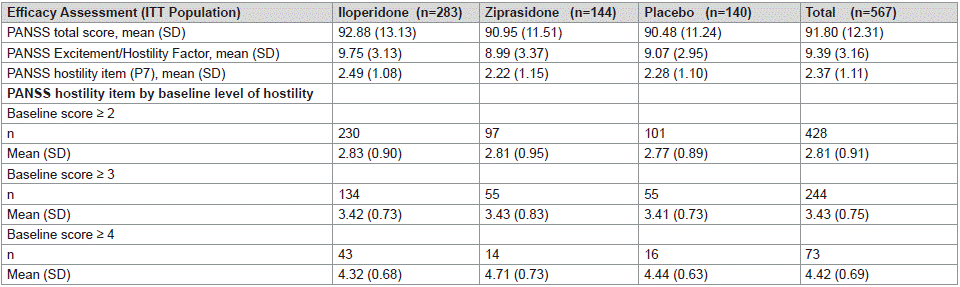
Table 2: Baseline Efficacy Scores in ITT Population.
PANSS Excitement Component
Baseline levels of PANSS Excitement Component was similar between all treatment groups (Table 2). Patients treated with iloperidone had significantly greater improvement in PANSS Excitement/Hostility Factor score at day 28 compared to those receiving placebo (-0.74, iloperidone; 0.52, placebo; P =0.0012) (Table 3).
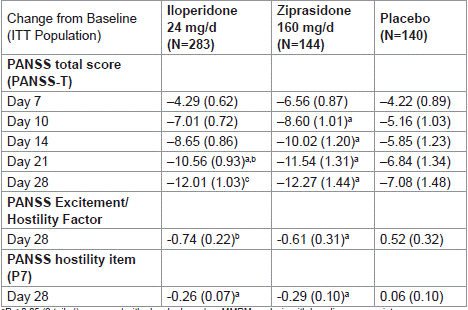
Table 3: Mean change from baseline to Day 28 (MMRM analysis, ITT
population).
PANSS Excitement Component score decreases in patients treated with iloperidone by day 14 (p=0.05) and is statistically different from placebo by day 21 (p=0.0114) and continued decreasing through day 28 (p=0.0012) (Figure 1). In patients treated with ziprasidone, PANSS Excitement Component score is statistically different from placebo by day 14 (p=0.0375) and continued decreasing through day 21 (p=0.0379) and day 28 (p=0.011) similar to iloperidone and further confirming assay sensitivity (Figure 1).
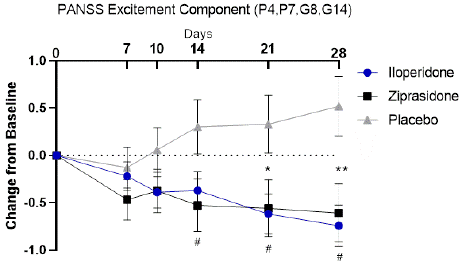
Figure 1: PANSS Excitement Component. Change from Baseline to Day
28 in the ITT population analyzed by MMRM. Iloperidone (Blue circles),
ziprasidone (black squares), and placebo (gray triangles). *p<0.05, **p<0.01
iloperidone vs. placebo; #p<0.05 ziprasidone vs placebo.
PANSS Hostility Item
A statistically significant difference in change from baseline to DAY 28 on the PANSS Excitement/Hostility subscale in favor of iloperidone versus placebo Least Square Mean Difference (LSMD) was –1.26, p = .0012) supported the principal PANSS hostility item analysis.
Baseline levels of PANSS hostility item (P7) was similar between all treatment groups (Table 3). Patients treated with iloperidone had significantly greater improvement in PANSS Hostility item score at day 28 compared to those receiving placebo (-0.25, iloperidone; 0.06, placebo; p= 0.012) (Table 3). PANSS hostility item score in patients treated with iloperidone is statistically different from placebo by day 10 (p=0.014) and continues decreasing through day 14 (p=0.11), day 21 (p=0.015) and day 28 (p=0.012) (Figure 2). In patients treated with ziprasidone, PANSS hostility item score is statistically different from placebo by day 10 (p=0.0265) and continued decreasing through day 14 (p=0.083), day 21 (p=0.012) and day 28 (p=0.013) similar to iloperidone and further confirming assay sensitivity (Figure 2).
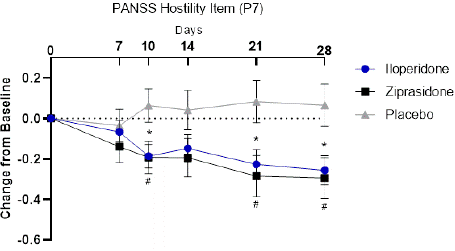
Figure 2: PANSS Hostility Item (P7). Change from Baseline to Day 28 in the
ITT population analyzed by MMRM. Iloperidone (Blue circles), ziprasidone
(black squares), and placebo (gray triangles). *p<0.05, iloperidone vs.
placebo; #p<0.05 ziprasidone vs. placebo.
Subgroup Analysis by Baseline Hostility
PANSS baseline hostility subgroup analyses revealed no differences between treatment groups at baseline (Table 3). Statistically significant improvement in mean change from baseline to day 28 was seen in patients treated with iloperidone compared with placebo in PANSS baseline hostility = 2 (-0.57, iloperidone; -0.22, placebo; p=0.02) (Figure 3). The LS mean difference was -0.34 for iloperidone vs. placebo. A similar improvement was seen in patients treated with ziprasidone with a PANSS baseline hostility = 2 (-0.58, ziprasidone) compared to placebo (p=0.044). Other sub-group analyses were not significant due to the small sample size. Change from baseline in PANSS baseline hostility = 3 subgroup LS mean difference was -0.17 for iloperidone compared to placebo (p=0.405) and change from baseline in PANSS baseline hostility = 4 subgroup LS mean difference was -0.36 for iloperidone compared to placebo (p=0.36).
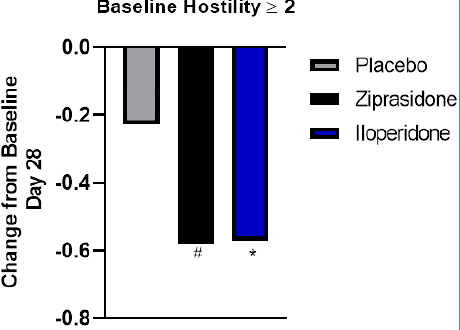
Figure 3: PANSS Hostility Item (P7) in Baseline Hostility Score = 2.
Change from Baseline to Day 28 in Baseline Hostility Score = 3 sub group.
Iloperidone (Blue bar), ziprasidone (black bar), and placebo (gray bar).
*p<0.05, iloperidone vs. placebo; #p<0.05 ziprasidone vs. placebo.
Conclusion
There was significantly greater improvement in hostility assessed by mean change in the PANSS hostility item in iloperidone treated patients compared with placebo-treated patients in a post hoc analysis of phase 3 clinical trial VYV-683-3101. The improvement in hostility is associated with overall PANSS total score improvement in both iloperidone and ziprasidone treated patients. In a supportive analysis, significant improvement in mean change from baseline to day 28 was also seen for iloperidone versus placebo on the PANSS Excitability/ Hostility Factor subscale. Improvement in hostility was greater in patients presenting with higher levels of hostility at baseline.
In our analyses, a robust and persistent antihostility effect for iloperidone relative to placebo was detected early in the course of treatment. Statistically significant improvement for iloperidone versus placebo on the PANSS hostility item was seen as early as day 10. Differences from placebo remained statistically significant at each time point, indicating that improvement associated with iloperidone was partially independent of improvement in positive symptoms. These results point to a specific antihostility effect for iloperidone that is independent of a general antipsychotic effect. Therefore, iloperidone may be a useful option for patients with schizophrenia who present with hostility. Data from a number of studies have demonstrated that clozapine to have anti-aggressive effects [4] as well as multiple second generation atypical antipsychotics. Clozapine, olanzapine, and paliperidone-extended release are typically first line treatments in patients with schizophrenia presenting with high levels of hostility and violence [17,18]. Other antipsychotics have also demonstrated efficacy in hostility in schizophrenia [19] and would not come with the serious safety concerns and monitoring needed with clozapine [20,21]. Data from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) revealed 74% of patients had discontinued medication within 18 months due to insufficient efficacy, intolerable side effects or for other reasons [22]. Due to differing effect size, side effects, and safety profiles of atypical antipsychotics, there is still a need for new treatment options with efficacy against hostility and aggression in schizophrenia.
The findings from our post hoc analysis of an antihostility effect for iloperidone appear to be consistent with post hoc reports of other atypical antipsychotics compared with placebo in patients with schizophrenia [3] as well as reports of other clinical trials assessing iloperidone [14]. A 5-factor analysis evaluating 7 pooled clinical trials measured the efficacy of iloperidone versus placebo in excitement/ hostility, depression/anxiety, cognition, positive, and negative symptoms and demonstrated iloperidone improved excitement/ hostility from baseline; LS mean change (± SD) were 0.6 (± 0.43) for iloperidone vs. -1.0 (± 0.23) for placebo (p < 0.001) [14].
One limitation of this analysis is the presence of sedation or the overall change in positive PANSS symptoms were not adjusted for. The most common adverse events in this study were dizziness, somnolence, and weight gain with no apparent difference in the incidence of AEs between treatment groups). The presence of sedation was 13% in iloperidone, 27% in ziprasidone and 8% in placebo [9]. This suggests that the reduction in hostility for iloperidone-treated patients was not due to nonspecific sedative effects associated with iloperidone. Lower levels of sedation are a benefit of iloperidone over other antipsychotics since high levels of sedation can lead to nonadherence [23] or to increased need for monitoring [24]. Reduction in hostility also does not seem to be due to differences in resuce medication such as lorazepam which was allowable in the study; lorazepam was used by 9.5% of patients given iloperidone compared to 10.7% patients given placebo.
To assess the relationship between the amount of hostility at baseline and the effect of iloperidone, we performed analyses on subgroups in which patients had at least minimal, mild, or moderate hostility at baseline. Statistically significant improvement in favor of iloperidone versus placebo was observed only in mild baseline hostility subgroup. The magnitude of change on the hostility item was greater in iloperidone-treated patients with mild baseline hostility than the overall patient population. Statistically significant improvement was not seen in higher levels of baseline hostility, mainly due to the low sample size. However, the magnitude of change for iloperidone increased with greater baseline hostility.
Similar to iloperidone, ziprasidone 160mg/d also separated from placebo in the PANSS Excitement Component and the PANSS hostility item alone. The active control ziprasidone in this study was used for assay sensitivity; any dose response or drug-drug comparisons will require the conduct of appropriately controlled randomized controlled trials.
The clinical importance of improving hostility is reflected in the large number of pharmacological studies using hostility as the principal treatment target [18]. The causes of hostility and aggression in schizophrenia are complex and largely unknown. One hypothesis is that the development of hostility in schizophrenia may follow 2 distinct pathways—one associated with premorbid conditions, including antisocial conduct, and another associated with the acute psychopathology of schizophrenia [25]. The CATIE study revealed baseline risk factors predicting aggressive behavior over a 6 month period which included economic deprivation [22], substance use disorders, violent victimization, childhood conduct problems, and childhood sexual abuse [26]. Genotype can also be a potential predisposing factor to hostility in schizophrenia. A common polymorphism was found in the catechol O-methyltransferase (COMT) gene where a methionine is substituted for a valine (Val 158 Met), which has been shown to be associated with aggressive behavior in patients diagnosed with schizophrenia or schizoaffective disorder [27,28].
Hostility is present in other psychiatric disorders such as bipolar disorder, personality disorders, Alzheimer’s disease, subtypes of dementia, and irritability and aggression in autism [29-32]. Hostility is also observed in post traumatic stress disorder (PTSD). For example, veterans with diagnosed PTSD reported significantly higher levels of trait anger and hostility than both a control veteran group with below diagnostic threshold levels of PTSD symptoms and a healthy control group [33]. Further studies are needed to conclude iloperidone would be safe and effective in other indications presenting with hostility and aggression.
Iloperidone 24mg/d (12mg BID) is effective, safe, and generally well tolerated in patients with acute exacerbations of schizophrenia. In post hoc analyses of 1 phase 3 study in patients with schizophrenia, treatment with iloperidone had significantly greater effects on hostility than treatment with placebo. Many antipsychotics have shown to be useful in treating hostility in patients with schizophrenia, however new treatments are still needed due to the differing variability in efficacy and side effect profile of each drug. No treatment is appropriate for every patient and these results suggest iloperidone to be an effective option to help manage hostility in acute exacerbations of schizophrenia.
References
- Bartels SJ, Drake E, Wallach MA & Freeman DH. Characteristic Hostility in Schizophrenic Outpatients. Schizophr. Bull. 1991; 17.
- Lindenmayer J, et al. Medication nonadherence and treatment outcome in patients with schizophrenia or schizoaffective disorder with suboptimal prior response. J. Clin Psychiatry. 2009; 70: 990–906.
- Volavka J, Czobor P, Citrome L & Van Dorn R. Effectiveness of antipsychotic drugs against hostility in patients with schizophrenia in the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) study. CNS Spectr. 2014; 19: 374–381.
- Volavka J. The effects of clozapine on aggression and substance abuse in schizophrenic patients. J. Clin. Psychiatry. 1999; 60: 43–46.
- Czobor P, Volavka J & Meibach R. Effect of risperidone on hostility in schizophrenia. J Clin Psychopharmacol. 1995; 15: 243–249.
- Volavka J, et al. Efficacy of Aripiprazole Against Hostility in Schizophrenia and Schizoaffective Disorder: Data From 5 Double-Blind Studies. J. Clin. Psychiatry. 2005; 66: 1362–1366.
- Leslie Citrome MD, Durgam S, Lu K, Ferguson P & Laszlovszky I. The Effect of Cariprazine on Hostility Associated With Schizophrenia: Post Hoc Analyses From 3 Randomized Controlled Trials. 2016; 77: 109–115.
- Cutler A, Kalali A, Mattingly G, Kunovac J & Meng X. Long term safety and tolerability of iloperidone: results from a 25-week, open-label extension trial. CNS Spectr. 2013; 18: 43–54.
- Cutler A, Kalali A, Weiden P, Hamilton J & Wolfgang C. Four-week, doubleblind, placebo- and ziprasidone-controlled trial of iloperidone in patients with acute exacerbations of schizophrenia. J Clin Psychopharmacol. 2008; 28: S20-28.
- Weiden P, Cutler A & Polymeropoulos MH, Wolfgang C. Safety profile of iloperidone: a pooled analysis of 6-week acute phase pivotal trials. J Clin Psychopharmacol. 2008; 28: S12-19.
- Kane J, Lauriello J, Laska E, Di Marino M & Wolfgang C. Long-term efficacy and safety of iloperidone: results from 3 clinical trials for the treatment of schizophrenia. J Clin Psychopharmacol. 2008; 28: S29-35.
- Weiden PJ, et al. A Randomized Trial of Iloperidone for Prevention of Relapse in Schizophrenia: The REPRIEVE Study. CNS Drugs. 2016; 30: 735–747.
- Kalkman H, Subramanian N & Hoyer D. Extended radioligand binding profile of iloperidone: a broad spectrum dopamine/serotonin/norepinephrine receptor antagonist for the management of psychotic disorders. Neuropsychopharmacology. 2001; 25: 904–914.
- Citrome L, Meng X & Hochfeld M. Efficacy of iloperidone in schizophrenia: A PANSS five-factor analysis. Schizophr. Res. 2011; 131: 75–81.
- Chouinard G & Margolese H. Manual for the Extrapyramidal Symptom Rating Scale (ESRS). Schizophr Res. 2005; 76: 247–265.
- Kay S, Fiszbein A & Opler L. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987; 13.
- Krakowski M, Czobor P, Citrome L & Al E. Atypical antipsychotic agents in the treatment of violent patients with schizophrenia and schizoaffective disorde. Arch Gen Psychiatry. 2006; 63: 622–629.
- Victoroff J, Coburn K, Reeve A, Sampson S & Shillcutt S. Pharmacological management of persistent hostility and aggression in persons with schizophrenia spectrum disorders: A systematic review. J. Neuropsychiatry Clin. Neurosci. 2014; 26: 283–312.
- Citrome L, et al. Effects of clozapine, olanzapine, risperidone, and haloperidol on hostility among patients with schizophrenia. Psychiatr. Serv. 2001; 52: 1510–1514.
- Citrome L. Clozapine for schizophrenia: lifethreatening or life-saving treatment? clozapine, despite its side effect burden, may be the most effective and have the lowest mortality risk among all available antipsychotics. Curr Psychiatr. 2008; 8: 56–63.
- Cohen D & Monden M. White blood cell monitoring during long-term clozapine treatment. Am J Psychiatry. 2013; 170: 366–369.
- Lieberman JA, et al. Effectiveness of Antipsychotic Drugs in Patients with Chronic Schizophrenia. N. Engl. J. Med. 2005; 353: 1209–1223.
- Nonadherence with antipsychotic medication in schizophrenia: challenges and management strategies. Patient Relat. Outcome Meas. 2014; 4: 43–62.
- Citrome L & Volavka J. The psychopharmacology of violence: making sensible decisions. CNS Spectr. 2014; 19: 411–418.
- Volavka J & Leslie Citrome MD, M Jan Volavka MD. Pathways to aggression in schizophrenia affect results of treatment. Schizophr Bull. 2011; 37: 921– 929.
- Swanson J, et al. Comparison of antipsychotic medication effects on reducing violence in people with schizophrenia. Br J Psychiatry. 2008; 193: 37–43.
- Lachman HM, Nolan KA, Mohr P, Saito T & Volavka J. Association between catechol O-methyltransferase genotype and violence in schizophrenia and schizoaffective disorder. Am. J. Psychiatry. 1998; 155: 835–837.
- Bhakta S, Zhang J & Malhotra A. The COMT Met158 allele and violence in schizophrenia: A meta-analysis. Schizophr Res. 2012 September. Schizophr. Res. 2012; 140: 192–197.
- San L, et al. State of Acute Agitation at Psychiatric Emergencies in Europe: The STAGE Study. Clin. Pract. Epidemiol. Ment. Heal. 2016; 12: 75–86.
- Buckley PF, Noffsinger SG, Smith DA, Hrouda DR & Knoll JL. Treatment of the psychotic patient who is violent. Psychiatr. Clin. North Am. 2003; 26: 231–272.
- Ballard C & Corbett A. Agitation and aggression in people with Alzheimer’s disease. Curr. Opin. Psychiatry. 2013; 26.
- Troost PW, et al. Long-Term Effects of Risperidone in Children With Autism Spectrum Disorders: A Placebo Discontinuation Study. J. Am. Acad. Child Adolesc. Psychiatry. 2005; 44: 1137–1144.
- Jakupcak M, et al. Anger, hostility, and aggression among Iraq and Afghanistan war veterans reporting PTSD and subthreshold PTSD. J. Trauma. Stress. 2007; 20: 945–954.