
Research Article
J Schizophr Res. 2015;2(1): 1009.
Face Detection and Its Relationship with Visual Contrast Detection in Schizophrenia
Tor Ekstrom, Stephen Maher and Yue Chen*
Mclean Hospital, Harvard Medical School, Belmont
*Corresponding author: Yue Chen, Mclean Hospital, Harvard Medical School, MS 303, 115 Mill Street, Belmont, MA 02478
Received: December 03, 2014; Accepted: April 22, 2015; Published: April 23, 2015
Abstract
Face perception is impaired in schizophrenia. While a socially and ecologically important function, face perception receives inputs from visual processing, a more basic perceptual function also impaired in schizophrenia. How basic visual processing impairment contributes to face perception impairment is not well understood. In this study we examined face detection, an early stage of face perception, as a function of visual contrast in schizophrenia. We also examined visual contrast detection, a basic visual process. For face detection, subjects indentified the location (left or right) of a face imbedded in a larger image. For contrast detection, subjects indentified the location (left or right) of a low-contrast grating within a uniform luminance background. To vary task difficulty, the contrast level of the images used for both tasks was systematically manipulated. Performance of patients (n=27) and controls (n=20) were acquired and compared. Performance accuracies of patients were significantly lower than those of controls for face detection (p=.039) but not contrast detection. In patients, performance accuracies were significantly correlated between face detection and contrast detection (r=0.70). Patients’ face detection performance was moderately correlated with PANSS negative subscale scores (r=-0.42). This pattern of results suggests the contributions of basic visual signal to impaired face processing in schizophrenia. These results also suggest a potential association of face perception impairment with negative psychotic symptom status.
Keywords: Face perception; Schizophrenic; Vision; Psychotic symptom
Introduction
The ability to efficiently and accurately identify faces in the visual world is crucial to social functioning. While socializing, people identify and acquire information transmitted via facial expression. This ability, referred to as face perception, is impaired in schizophrenia [1-3]. Patients suffering from this mental disorder perform worse than healthy controls on identifying subtle differences in facial identity [4,5] and struggle to detect low level facial emotions [6-8], two main aspects of face perception. Schizophrenia patients have also been shown to be deficient at detecting the presence of faces [9]. While face perception impairment in schizophrenia is established, the underlying perceptual and cognitive processes are not clearly understood.
Face processing is special relative to the processing of other visual objects in that it engages specialized visual and cognitive mechanisms [10]. While these mechanisms are supported by the core face processing system that includes Fusiform Face Area, Occipital Face Area and Superior Temporal Sulcus [11], the basic visual processing system provides necessary sensory information conveyed from faces [12]. Because complex cognitive and social processes (such as face perception) rely on information fed from basic perceptual inputs (such as visual contrast detection), impairment in basic perceptual processing may jeopardize the proper functioning of these social and cognitive abilities downstream. In schizophrenia, whether and how the face processing system is specifically implicated remain unsettled [2,3]. On the other hand, patients’ basic visual processing capacities are compromised [13-17]. The putative link between face perception and basic visual detection has yet to be established in this psychiatric disorder.
To address this issue, we explored face detection as a function of visual contrast in patients with schizophrenia. Face detection is a face perception task that tests participants’ ability to identify a visual target as a face in a visual environment, without extracting more specific facial information such as emotion or identity. An examination of how face detection is modulated by visual contrast will illustrate the role of basic visual processing in identifying the presence of faces. Contrast detection is a basic visual task measuring participants’ ability to efficiently identify the presence of a grating (visual stimulus). An examination of how a visual stimulus is detected at minimal contrast levels will illustrate basic perceptual sensitivity to visual signals. Together these two tasks query different but interactive levels of visual and face processing to probe into the factors underlying face processing dysfunction in schizophrenia.
Previous work on face detection found degraded performance in schizophrenia patients [5,9,18], yet whether and to what extent the visual contrast factor is involved is unclear. Given the putative links between visual and face processing and previous work on face detection in schizophrenia, we hypothesize that performance on the face detection task is deficient in patients and patients’ performance on the contrast detection task predicts a significant portion of performance on the face detection task.
Methods
Subjects
Twenty-seven schizophrenia patients participated in this study. All patients met DSM-IV criteria for schizophrenia or schizoaffective disorder, based on a standardized interview [19] and a review of all available psychiatric hospital records. Their average illness duration was 22.8 years (std: 14.3 years). Twenty three patients were medicated with antipsychotic drugs (avg CPZ dose equivalent= 654.66mg, std: 525.74mg) [20]. Patients’ psychotic status was assessed using Positive and Negative Symptom Scales [21].
Twenty non-psychiatric control subjects participated in this study. Control subjects were recruited with advertisements posted in the local community. They were screened for exclusion of any psychiatric disorders among both themselves and their families using non-patient SCID [22].
Additional inclusion criteria for both groups were 1) no history of any neurological disorders (such as seizure or stroke) or head injuries, 2) IQ > 75, and 3) no substance dependence within the last six months. The two groups did not differ in age or verbal IQ score [23]. Table 1 provides demographic information for all of the subjects. The study protocol was approved by the Institutional Research Board (IRB) of McLean Hospital. Written informed consent was obtained from all subjects prior to participation.
Group
Sex
Age (years)
Verbal IQ
Eyes Test*
PANSS+
PANSS-
PANSSgen
Healthy Control n= 20
Male= 12
Female= 8
41.45
(16.17)
114.50
(16.06)
75.00
(11.15)
N/A
N/A
N/A
SZ Patient n= 27
Male= 12
Female= 15
46.41
(13.24)
105.50
(15.53)
64.42
(14.53)
16.96
(4.79)
13.96
(3.98)
32.30
(4.59)
* indicating a significant group difference at p<0.05.
Table 1: Clinical and Demographic information of the sample.
Procedure
We designed and administered two psychophysical tasks, face detection and contrast detection. We also administered a standardized social cognitive task, the Eyes Test [24].
Stimulus
For the face detection task, participants were asked to judge whether the target image, a face, was located at the left or right side (a two-alternative forced choice) of a presented stimulus. The target face images were rendered to resemble line drawings and embedded among other scrambled “line drawing” patterns (Figure 1). For each presentation, participants gave their judgments (left or right) by pressing one of two designated keys on a keyboard. To vary task difficulty, contrast levels of stimulus were systematically manipulated (0%, 1%, 2%, 4%, 8%, 16% and 100%).

Figure 1: Illustration of visual stimuli used for Face Detection and
Contrast Detection tasks. In top panels, stimuli for face detection - line
drawn face images among scrambled lines –are shown. The faces are
presented on either the right or left side of the stimulus. The top left panel
shows a face detection stimulus presented at the 2% contrast level whereas
the top right panel shows a stimulus at the 100% contrast level. In bottom
panels, stimuli for contrast detection - gratings within a background of the
same average luminance –are shown. During the Contrast Detection task the
grating can appear on either the right or left side of the stimulus. The bottom
left panel shows a grating at the 2% contrast level whereas the bottom right
panel shows a grating at the 16% contrast level.
For the contrast detection task, participants were asked to judge whether the target image, a contrast grating, was located at the left or right side (a two-alternative forced choice) of a presented stimulus. The target contrast grating was imposed over part of a larger gray rectangle of the same average luminance. For each presentation, subjects gave their judgments (left or right) by pressing one of two designated keys on a keyboard. The target grating image was presented at 0%, 1%, 2%, 4%, 8% and 16% contrast.
Trials
The face detection task consisted of 84 trials for 7 contrast levels with 12 trials per contrast level. The contrast detection task consisted of 72 trials for 6 contrast levels, also with 12 trials per contrast level. Each face detection stimulus was presented for 300 msec whereas each contrast detection stimulus was presented for 100 msec. For both tasks, stimuli were presented in the center of the screen. The size of the stimuli subtended 13 degrees of visual angle. The presentation order of stimuli were randomized across task (contrast vs. face), target location within the images (left vs. right), and stimulus contrast level (6 for contrast detection, 7 for face detection).
Percents of correct trials, or accuracy scores, were collected and analyzed as the measure of performance. Reaction Time (RT) was also collected and analyzed for the face detection task.
For the Eyes Tests, designed for measuring Theory of Mind [24], participants were presented with an image of an actor’s eyes and were instructed to select one of four available words that best describe the emotion conveyed by the image. Participants were provided with the meanings of words they did not know. The task consisted of 36 trials. Accuracy scores were collected and analyzed as a performance measure.
Results
To analyze face detection accuracy data a repeated measure ANOVA was performed, with factors of group (patient vs. control) and contrast (6 levels, excluding 0%). A significant main effect was found for contrast (F(5, 225)=12.49, p<0.001) and group (F(1,45)=4.51, p=.039). There was a trend of interaction between contrast and group, but it was not statistically significant (F(5,45)=1.69, p=.138) (Figure 2).

Figure 2: Performance Accuracy in Face Detection. Each data point
represents the group average of the accuracy scores during the face
detection Task. Stimulus contrast level is represented on the X axis which is
on a logarithmic scale. The Y axis represents performance accuracy. Error
bars represent ±1 standard error.
A repeated measure ANOVA for contrast detection accuracy data was performed, with factors of group (patient vs. control) by contrast (5 levels, excluding 0%). A main effect of contrast was significant (F(4, 156)=15.37, p<0.001). Neither the group effect, nor the interaction between group and contrast was significant (Figure 3).
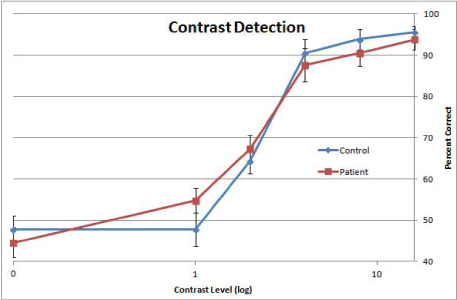
Figure 3: Performance Accuracy in Contrast Detection. Each data point
represents the group average of the proportion of correct responses collected
during the Contrast Detection Task. Stimulus contrast level is represented
on the X axis which is on a logarithmic scale. The Y axis represents percent
correct. Error bars represent ±1 standard error.
For patients but not controls, average face detection accuracy was significantly correlated with average contrast detection accuracy (r=.70, p<0.05).
Among clinical and demographic factors, the patient group’s PANSS negative subscale were significantly correlated with average face detection accuracy (r=-.42, p<0.05) (Figure 4). Patients’ face detection accuracy scores were also significantly correlated with age (r=-.42, p<0.05). Patients’ face detection accuracy scores were not correlated with CPZ score (equivalent daily dose of antipsychotic drugs).
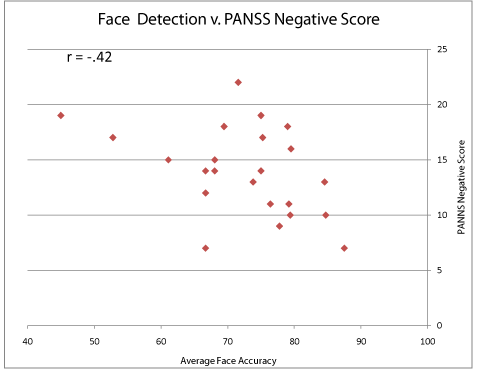
Figure 4: The relationship between Face Detection Performance and
Psychotic Symptoms. Each data point represents one subject. The X axis
indicates the face Detection performance whereas the Y axis indicates the
PANSS Negative Subscale score.
A repeated measure ANOVA of face detection reaction time was performed with factors of group (patient vs. control) and contrast (6 levels, excluding 0%). A significant effect was found for group (F(1, 45)=4.10, p=.049) and for an interaction of group and contrast (F(5, 225)=3.09, p=.01). This indicates that while controls responded with similar speed across stimulus contrast levels, patients response speed decreased with stimulus contrast.
A two-tailed t-test showed that patients performed significantly worse than controls on the Eyes Test task (t(36)=2.47, p<0.05).
Discussion
This study showed that face detection performance for both groups improved with increasing contrast level, suggesting a critical role of visual contrast for face detection. Moreover, patients showed impaired face detection performance, primarily at high contrast levels, suggesting that contrast alone can only account for a portion of the impaired face detection performance in patients. In patients, the face detection performance was moderately correlated with PANSS negative scores, suggesting a potential link between face processing and negative psychotic symptoms.
Face detection and basic visual processing
Previous work on face perception in schizophrenia used mostly salient visual stimuli [5,7,25-27]. Whether and to what extent these face perception impairments are attributed to basic visual processing could not be inferred. Contrast detection is a precursor to most perceptual and cognitive tasks involving visual targets including face. Thus, to understand the factors underlying face perception impairment in schizophrenia, it is crucial to evaluate the link between face perception and basic visual processing. To address this issue, this study used a two-prong approach –face detection as a function of contrast and association between face detection and contrast detection. First, patients showed impaired face detection performance at all contrast levels save for the two lowest (1 and 2%) (Figure 2). The data indicate that face detection is deficient not just for salient stimuli but also for non-salient stimuli provided that they are above perceptual threshold. Note that for these participants, average perceptual threshold for contrast detection was between 2 and 4% (Figure 3). Second, a significant correlation was found between performances on face detection and contrast detection in patients. This correlation suggests that a significant portion of their face detection impairment can be accounted for by the contrast detection performance. That is, patients who have low contrast sensitivities also have low performance levels in face detection. Taken together, these two aspects of data provide independent evidence for the notion that basic visual processing contributes to face detection impairments in schizophrenia.
Face detection and psychotic symptoms
The finding of correlation between face detection accuracy and negative psychotic symptom (Figure 4) highlights the notion that social functioning problems (such social withdrawal) in patients are related to more fundamental problems in face perception. This finding is further nuanced by the lack of correlation between contrast detection and psychotic symptom status, suggesting that the social impairments do not arise at the level of basic visual processing. Our sample consisted of patients with chronic schizophrenia, presumably being clinically stabilized in terms of psychosis. The presence of the relationship in such a sample suggests that face detection is a sensitive assessment of social functioning problem in this psychiatric disorder.
Limitations and future studies
This study has several limitations. First, the sample size is moderate. Although group difference in face detection was found, the interaction between group and contrast is equivocal. This interaction would more clearly illustrate whether and how face detection impairment in patients is modulated by contrast level. Second, potential effects of antipsychotic medication, if existing, have not been clearly illustrated although the CPZ measurement was not associated with the face detection performance in this group of patients. In general, only no to modest effects of antipsychotic drugs (especially the second-generation ones) were reported on visual perception [28,29] and cognition [30]. Verbal IQ and CPZ of patients in this study were not correlated. One study however showed that administration of risperidone benefits facial emotion perception in patients [31]. Whether face detection in patients who receive little to no antipsychotic medication is affected remains to be seen. Third, the relationship of face detection with other perceptual and cognitive functions (such as perception of visual form) has not been examined in patients. Given the existence of multiple types of perceptual and cognitive deficits in patients, examination of the relationships would help clarify the specificity and generalizability of the face perception impairments.
Improved face detection performance with increase of stimulus contrast (Figure 2) suggests that boosting visual saliency benefits the detection of faces in the visual world. Although this relationship between face detection and stimulus contrast was somewhat compromised in patients (less increase of performance accuracy with stimulus contrast, Figure 2), focusing on the detection of face with low visual saliency can still provide a feasible target for remediation and cognitive training [32,33]. This focus will allow a new approach to improving face perception and social functioning in this psychiatric disorder.
Acknowledgement
This work was supported by a grant from the NIH (R01 MH 096793). We thank Dr. Ongur for supervision of clinical assessment of patients, and Drs. Norton and McBain for discussion in the early stage of this study.
References
- Phillips M, David A. Facial processing in schizophrenia and delusional misidentification: cognitive neuropsychiatric approaches. Schizophr Res. 1995; 17:109-114.
- Chen Y. Face perception in schizophrenia spectrum disorders: interface between cognitive and social cognitive functioning. Ritsner M, editor. In: Handbook of Schizophrenia Spectrum Disorders New York: Springer. 2011; 111-120.
- Darke H, Peterman JS, Park S, Sundram S, Carter O. Are patients with schizophrenia impaired in processing non-emotional features of human faces? Front Psychol. 2014; 4: 529.
- Feinberg TE, Rifkin A, Schaffer C, Walker E. Facial discrimination and emotional recognition in schizophrenia and affective disorders. Arch Gen Psychiatry. 1986; 43: 276-279.
- Chen Y, Norton D, McBain R, Ongur D, Heckers S. Visual and cognitive processing of face information in schizophrenia: detection, discrimination and working memory. Schizophr Res. 2009; 107: 92-98.
- Addington J, Addington D. Facial affect recognition and information processing in schizophrenia and bipolar disorder. Schizophr Res. 1998; 32: 171-181.
- Kohler CG, Turner TH, Bilker WB, Brensinger CM, Siegel SJ, Kanes SJ, et al. Facial emotion recognition in schizophrenia: intensity effects and error pattern. Am J Psychiatry. 2003; 160: 1768-1774.
- Norton D, McBain R, Holt DJ, Ongur D, Chen Y. Association of impaired facial affect recognition with basic facial and visual processing deficits in schizophrenia. Biol Psychiatry. 2009; 65: 1094-1098.
- Chen Y, Norton D, Ongur D, Heckers S. Inefficient face detection in schizophrenia. Schizophr Bull. 2008 ; 34: 367-374.
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci. 1997; 17: 4302-4311.
- Haxby JV, Hoffman EA, Gobbini MI. The distributed human neural system for face perception. Trends Cogn Sci. 2000; 4: 223-233.
- Bruce V, Young A. Understanding face recognition. Br J Psychol. 1986; 77: 305-327.
- Butler PD, Silverstein SM, Dakin SC. Visual perception and its impairment in schizophrenia. Biol Psychiatry. 2008; 64: 40-47.
- Chen Y. Abnormal visual motion processing in schizophrenia: a review of research progress. Schizophr Bull. 2011; 37: 709-715.
- Green MF, Butler PD, Chen Y, Geyer MA, Silverstein S, Wynn JK, et al. Perception Measurement in Clinical Trials of Schizophrenia: Promising Paradigms From CNTRICS. Schizophr Bull. 2009; 35: 163-181.
- Butler PD, Chen Y, Ford JM, Geyer MA, Silverstein SM, Green MF. Perceptual measurement in schizophrenia: promising electrophysiology and neuroimaging paradigms from CNTRICS. Schizophr Bull. 2012; 38: 81-91.
- Silverstein SM, Keane BP. Vision science and schizophrenia research: toward a re-view of the disorder. Editors' introduction to special section. Schizophr Bull. 2013; 37: 681-689.
- Butler PD, Tambini A, Yovel G, Jalbrzikowski M, Ziwich R, Silipo G, et al. What's in a face? Effects of stimulus duration and inversion on face processing in schizophrenia. Schizophr Res. 2008; 103: 283-292.
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV Disorders (SCID). Washington, DC: American Psychiatric Press. 1994.
- Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003; 64: 663-667.
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987; 13: 261-276.
- First MB, Spitzer RL, Gibbon M, William JB. Structure Clinical Interview for DSM -IV-TR Axis I Disorders - Non-patient Edition (SCID-I/NP, 11/2002 revision) New York, NY Biometric Research Department, New York State Psychiatric Institute. 2002.
- Wechsler D. Manual for the Adult Intelligence Scale-Revised. New York: Psychological Corporation. 1981.
- Baron-Cohen S, Wheelwright S, Hill J, Raste Y, Plumb I. The "Reading the Mind in the Eyes" Test revised version: a study with normal adults, and adults with Asperger syndrome or high-functioning autism. J Child Psychol Psychiatry. 2001; 42: 241-251.
- McBain R, Norton D, Chen Y. Differential roles of low and high spatial frequency content in abnormal facial emotion perception in schizophrenia. Schizophr Res. 2010; 122: 151-155.
- Calkins ME, Gur RC, Ragland JD, Gur RE. Face recognition memory deficits and visual object memory performance in patients with schizophrenia and their relatives. Am J Psychiatry. 2005; 162: 1963-1966.
- Lee J, Gosselin F, Wynn JK, Green MF. How Do Schizophrenia Patients Use Visual Information to Decode Facial Emotion? Schizophr Bull. 2011; 37: 1001-1008.
- Chen Y, Levy DL, Sheremata S, Nakayama K, Matthysse S, Holzman PS. Effects of typical, atypical, and no antipsychotic drugs on visual contrast detection in schizophrenia. Am J Psychiatry. 2003; 160: 1795-1801.
- Brenner CA, Wilt MA, Lysaker PH, Koyfman A, O'Donnell BF. Psychometrically matched visual-processing tasks in schizophrenia spectrum disorders. J Abnorm Psychol. 2003; 112: 28-37.
- Harvey PD, McClure MM. Pharmacological approaches to the management of cognitive dysfunction in schizophrenia. Drugs. 2006; 66: 1465-1473.
- Fakra E, Salgado-Pineda P, Besnier N, Azorin JM, Blin O. Risperidone versus haloperidol for facial affect recognition in schizophrenia: Findings from a randomised study. World J Biol Psychiatry. 2009; 10: 719-728.
- Fisher M, Holland C, Merzenich MM, Vinogradov S. Using Neuroplasticity-Based Auditory Training to Improve Verbal Memory in Schizophrenia. Am J Psychiatry. 2009; 166: 805-811.
- Norton DJ, McBain RK, Ongur D, Chen Y. Perceptual training strongly improves visual motion perception in schizophrenia. Brain Cogn. 2011; 77: 248-256.