
Case Report
J Schizophr Res. 2016; 3(1): 1021.
Chlorpromazine Induced Hypothermia in a Schizophrenic Patient Receiving Multiple Antipsychotic Medications: A Case Report
Lian NZ1# and Detweiler MB1,2,3*
¹Department of Psychiatry and Behavioral Medicine, Virginia Tech-Carilion School of Medicine, USA
²Department of Psychiatry, Veterans Affairs Medical Center, USA
³Geriatric Research Group, Veterans Affairs Medical Center, USA
#Fellow in the Child and Adolescence Psychiatry Fellowship Program at Hofstra Northwell School of Medicine, Glen Oaks, NY, USA
*Corresponding author: Detweiler MB, Department of Psychiatry, Veterans Affairs Medical Center, 1970 Roanoke Boulevard, Salem, Virginia, 24153, USA
Received: September 02, 2015; Accepted: March 30, 2016; Published: April 01, 2016
Abstract
We report multiple episodes of life threatening hypothermia in a patient with schizophrenia treated with chlorpromazine in combination with haloperidol and olanzapine. A 71-year-old African American male with schizophrenia taking chlorpromazine, haloperidol and olanzapine for more than 10 years developed successive episodes of hypothermia with altered consciousness. During four hospital admissions, his core temperature varied from 94°F to 89°F. After rechallenging the patient with each individual antipsychotic, chlorpromazine was found to be the source of the hypothermia. The patient improved following discontinuation of chlorpromazine and continuation of haloperidol and olanzapine.
Combination antipsychotic treatment increases the risk of developing neuroleptic malignant syndrome with hyperthermia, but usually not hypothermia. While antipsychotic induced hypothermia is not uncommon, hypothermia is rare for chlorpromazine. In this case the patient had been taking three antipsychotics for 10 years with no prior history of hypothermia episodes prior to the successive hospital admissions. To the best of our knowledge this is the first case of hypothermia episodes associated with use of chlorpromazine in combination with haloperidol and olanzapine.
Clinicians should be alert to the risk of severe hypothermia when employing chlorpromazine in combination with other antipsychotics having hypothermia potential even following many years without any prior signs of the syndrome.
Keywords: Chlorpromazine; Hypothermia; Multiple antipsychotic treatment
Abbreviations
ED: Emergency Department; ICU: Intensive Care Unit; TRPs: Thermoregulation Apparent Transient Receptor Potential ion channels; D1: Dopamine receptor 1; D2: Dopamine receptor 2; D3: Dopamine receptor 3; 5-HT1: Serotonin receptor 1; 5-HT2: Serotonin receptor 2; H1: Histamine receptor 1; a1: Adrenergic receptors 1; a2: Adrenergic receptors 2; M1: Muscarinic acetylcholine receptors 1; M2: muscarinic acetylcholine receptors 2; 0F: Fahrenheit degrees; 0C: Celsius degrees; BP: Blood pressure in millimeters of mercury (mm Hg); PR: Pulse rate in heart beats per minute (HB/min.); Temp: Core body temperature in degrees Fahrenheit (°F ) and Celsius (°C); RR: Respiratory rate in breaths per minute (B/min.); Weight in pounds (lb.); BMI: Body Mass Index in kilograms/meter squared (kg/m2); SpO2: Peripheral capillary oxygen saturation measure in percent (%)
Case Presentation
A 71 year old African American male with a 44 year history of schizophrenia, moderate neurocognitive disorder, diabetes mellitus and hypertension presented to the Emergency Department (ED) with his first episode of hypothermia. This was followed by three subsequent medical admissions for hypothermia over four months. The admission psychotropic medications included clonazepam 1 mg twice daily, haloperidol 8 mg nightly, chlorpromazine 100 mg twice daily, olanzapine 30 mg daily, oxcarbazepine 1,200 mg twice daily, diphenhydramine 50 mg nightly as needed and lorazepam 1 mg three times a day as needed. Other medications were magnesium hydroxide 15 mL daily, acetaminophen 650 mg every 4 hours as needed, ascorbic acid 500 mg twice daily, aspirin 81 mg daily, ergocalciferol 50,000 units weekly, ferrous sulfate 325 mg twice daily, regular insulin 2 units, 3 times daily, levothyroxine 137.5 mcg daily, omeprazole 20 mg twice daily and tamsulosin 0.4 mg nightly. (Table 1) documents the patient’s core body temperatures at his four ED admissions.
ED visits
12/30/2013
2/26/2014
3/1/2014
3/8/2014
BP (mm Hg)
87/46
89/51
140/63
144/67
PR (HB/min)
58
74
71
59
Temp °F (°C)
94.0 (34.3)
91.8 (33.2)
92.0 (33.3)
89.0 (31.7)
RR (B/min)
9
17
12
19
Weight (lb)
190
170
170
170
BMI (kg/m2)
28.9
22.4
22.5
22
SpO2
98
98
98
100
Admission Medications
Psychotropic Medications:
clonazepam 1 mg BID
haloperidol 8 mg QD
chlorpromazine 100 mg BID
olanzapine 30 mg QD
oxcarbazepine 1,200 mg BID
Non Psychotropic Medications
magnesium hydroxide 15 mL QD
ascorbic acid 500 mg BID
aspirin 81 mg QD
ergocalciferol 50,000 units weekly,
ferrous sulfate 325 mg BID
regular insulin 2 units TID
levothyroxine 137.5 mcg QD
omeprazole 20 mg BID
tamsulosin 0.4 mg QHS
PRN Medications
Diphenhydramine 50 mg QHS PRN
Lorazepam 1 mg TID PRN,
Acetaminophen 650 mg Q4H PRN,
Chlorpromazine 50 mg BID PRNPsychotropic Medications:
clonazepam 1 mg BID
haloperidol 8 mg QD
chlorpromazine 100 mg BID
olanzapine 30 mg QD
oxcarbazepine 1,200 mg BID
Non Psychotropic Medications
magnesium hydroxide 15 mL QD
ascorbic acid 500 mg BID
aspirin 81 mg QD
ergocalciferol 50,000 units weekly,
ferrous sulfate 325 mg BID
regular insulin 2 units TID
levothyroxine 137.5 mcg QD
omeprazole 20 mg BID
tamsulosin 0.4 mg QHS
PRN Medications
Diphenhydramine 50 mg QHS PRN
Lorazepam 1 mg TID PRN,
Acetaminophen 650 mg Q4H PRN,
Chlorpromazine 50 mg BID PRNPsychotropic Medications:
clonazepam 1 mg BID
haloperidol 8 mg QD
chlorpromazine 100 mg BID
olanzapine 30 mg QD
oxcarbazepine 1,200 mg BID
Non Psychotropic Medications
magnesium hydroxide 15 mL QD
ascorbic acid 500 mg BID
aspirin 81 mg QD
ergocalciferol 50,000 units weekly,
ferrous sulfate 325 mg BID
regular insulin 2 units TID
levothyroxine 137.5 mcg QD
omeprazole 20 mg BID
tamsulosin 0.4 mg QHS
PRN Medications
Diphenhydramine 50 mg QHS PRN
Lorazepam 1 mg TID PRN,
Acetaminophen 650 mg Q4H PRN,
Chlorpromazine 50 mg BID PRNPsychotropic Medications:
clonazepam 1 mg BID
haloperidol 5 mg QD*
chlorpromazine 50 mg BID*
olanzapine 30 mg QD
oxcarbazepine 1,200 mg BID
Non Psychotropic Medications
magnesium hydroxide 15 mL QD
ascorbic acid 500 mg BID
aspirin 81 mg QD
ergocalciferol 50,000 units weekly,
ferrous sulfate 325 mg BID
regular insulin 2 units TID
levothyroxine 137.5 mcg QD
omeprazole 20 mg BID
tamsulosin 0.4 mg QHS
PRN Medications
Diphenhydramine 50 mg QHS PRN
Lorazepam 1 mg TID PRN,
Acetaminophen 650 mg Q4H PRN,
Chlorpromazine 50 mg BID PRN
Table 1: Admission indices at 4 successive emergency department intakes for hypothermia in a patient with schizophrenia who was prescribed 3 antipsychotics.
The patient’s first admission laboratory indices were: sodium131 mmol/L; potassium 4.2 mmol/L; chloride 94 mmol/L; bicarbonate 28 mmol/L; blood urea nitrogen 15 mg/dL; creatinine 0.76 mg/dL; estimated creatinine clearance 9.1 ml/min; white blood cell count 3.9 x109/L; hemoglobin 9.4 gm/dL; mean corpuscular volume 87.7fl; platelets 104 x 109/L; cortisol 9:00 am 6.20-19.4 ug/dL and midnight 2.30-11.9 ug/dL; urine drug screen positive for benzodiazepines (prescribed); negative blood cultures; thyroid stimulating hormone 2.104 mIU/L; and normal liver function test. The head CT and chest x-rays were normal.
At the first ED admission, the patient’s body core temperature was 3.5 degrees Fahrenheit lower than the low-normal body temperature range (97.5°F - 98.8 °F). He was transferred to the Intensive Care Unit (ICU) for further evaluation and treatment. Once admitted to the ICU, all 3 antipsychotics were stopped. His ICU indices were: body core temperature 94°F (34.4°C); blood pressure 87/46 mm Hg; heart rate 58 beats/min; respiratory rate 9 breaths/min; weight 86.2 kg (190 lbs.); body mass index (BMI) 28.9 kg/m2; and peripheral capillary oxygen saturation (SpO2) 98%. He received intravenous fluid therapy 200cc/hr. Antibiotic therapy was initiated and later stopped as no signs of infection were found. During this admission, the patient received supportive treatment. He regained normal temperature after rewarming and he became euthermic with resolution of the leukopenia and thrombocytopenia. He was discharged on the same intake medications. It is notable that the patient began receiving as needed chlorpromazine doses (total mg/day unknown) regularly at his home after discharge.
There were no changes in his psychotropic medications during medicine admissions (Figure 1). At the second and third ED admissions for hypothermia, the patient’s body core temperatures dropped approximately 5.7 degrees Fahrenheit below normal. During the third admission, both haloperidol and chlorpromazine doses were decreased while the olanzapine 30 mg daily remained unchanged (Figure 1).
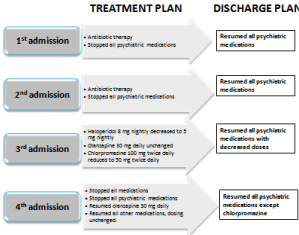
Figure 1: Chlorpromazine.
At the fourth ED admission the patient had altered mental status in addition to a marked hypothermia 8.5 degrees Fahrenheit below normal body temperature (Table 1). The treatment team stopped chlorpromazine, haloperidol and olanzapine and started the patient on supportive therapy. After the patient became euthermic, the team rechallenged him with each antipsychotic individually to gauge their respective effects on body temperature. Rechallenge with haloperidol or olanzapine individually did not reduce body temperature. However, when rechallenged with chlorpromazine, the patient’s temperature dropped to 95°F (35°C). Chlorpromazine was discontinued and the patient’s temperature increased to 97°F. Chlorpromazine was discontinued and the other two antipsychotics were resumed at the doses established during the third admission. All other non-antipsychotic medications remained unaltered from the patient’s first admission. Blood pressure improved (Table 1) following the third and fourth ED admissions, possibly related to the respective antipsychotic dose decreases during these admissions (Figure 1). The patient has been followed for greater than 24 months after his forth hospitalization during which his antipsychotic medications were changed. The patient has not re-experienced any additional hypothermia episodes with the reduced haloperidol (5 mg daily) and the unchanged olanzapine dose (30 mg daily) (Figure 1). The Naranjo Scale score was 9 (definite) [1].
Discussion
The Unbalance theory [2], Vasodilatation Theory [3], Shunting Theory [3] and the function specific protein peptides [3-6] have been proposed to describe some of the underlying mechanisms of corporal temperature dysregulation secondary to medications. The Unbalance Theory suggests that the central nerve system thermoregulation is modulated by antagonism of Dopamine (D1-4) and 5-Hydroxytryptamine-2 (5-HT2) receptors. During euthermia, there is a balance between body temperature reduction (dopamine) and augmentation (5-HT) mechanisms [2]. Consequently, hypothermia is theorized to be precipitated by increased dopamine antagonism by medications such as atypical antipsychotics having a stronger 5-HT2 than D2receptor affinity.
The Vasodilatation Theory involves the role of the sympathetic nervous system in regulating cardiovascular homeostasis through alpha-1 Adrenergic Receptor (a1ARs) mediation of arteriolar smooth muscle constriction and cardiac contraction. It is hypothesized that activation of a1ARs induces vasoconstriction and the shunting of blood away from the skin to maintain core body temperature. In contrast, blockade of these receptors by antipsychotic drugs, such as with atypical antipsychotic medications, promotes, epidermal vasodilation heat loss and when severe, significant contribution to hypothermia [3].
In the skin and subcutaneous tissues of human extremities, Arteriovenous Anastomoses (AVA) are connections between arterioles and venules that bypass the nutrient capillary. The AVA have an important role in the regulation as they can incrementally dilate their diameter to 7 times that of capillaries. The greater the formation of AVA and blood flow in shunts, the greater the loss of body temperature and the greater the risk of hypothermia. It is thought that AVA dilation and constriction is regulated by neural and hormonal mechanisms including adrenergic, dopaminergic and serotoninergic receptors. Prior research has demonstrated that increased sympathetic and dopaminergic activation may significantly decrease in AVA flow [7]. Also, 5-HT is involved in augmenting arteriolar blood flow and diminishing AVA blood flow in the skin, fingers, lips, and ears leading to possible hypothermia [8]. In the Shunting Theory, antipsychotic antagonism may alter dopamine, alleles of the 5-HT receptors and a adrenergic receptors resulting in alternations of the arteriovenous shunting capacity contributing to hypothermia through AVA modulation [3,5].
Specific protein peptides found in the brain, such as SN ociceptin/ orphanin FQ (N/OFQ), are participants in causing hypothermia. N/ OFQ is considered to be an endogenous antagonist of dopamine transport either by direct action on dopamine or by GABA inhibition which indirectly alters dopamine levels [9]. It regulates a wide range of biological functions including, but limited to, ociceptin, food intake, cardiovascular and renal functions, in additional to spontaneous locomotor activity to stimulate consummatory behavior and decreased energy expenditure [10,11]. Research data suggest that the 17-amino acid peptide N/OFQ contributes to precipitating hypothermia by binding to the Ociceptin Receptor (NOP) during the complex thermoregulatory system operation of independent hypothalamic thermo effector loops that regulate body temperature [11,12].
Several other neuropeptides are thought to participate in body temperature regulation and hypothermia. Hypo cretins (orexins) are hypothalamic neuropeptides thought to participate in the regulation of sleep, arousal and body temperature control. Szekely et al., [13] reported that Orexin-A decreased basal colon temperature and lipopolysaccharide-induced fever. Also, it is hypothesized that the hypothermic effect of orexins is partially regulated by Neuropeptide Y (NPY). NPY is the most ubiquitous neuropeptide in the central and peripheral nervous systems in mammals. A reduction in hypothalamic NPY is associated with increased uncoupling protein 1 expression in brown adipose tissue, resulting in thermogenesis [14,15]. Based on the finding that NPY inhibits serotonergic neuronal activity via the Y1 receptor in the dorsal raphe nucleus, resulting in suppression of male sexual behavior in low-energy conditions, Inaba et al., theorized that NPY may also participate in hypothermia by inhibiting serotonergic neuronal activity [16].
Based on the theoretical mechanisms of hypothermia, it is appears that taking antipsychotic medications can affect the central nervous system and peripheral thermoregulation systems resulting in hypothermia [17-22]. In general, antipsychotic associated hyperthermia is more frequent than hypothermia. Only a few cases of hypothermia associated with chlorpromazine have been reported over the past 50 years. To the best of our knowledge, there are no reports of hypothermia associated with the concurrent administration of haloperidol, olanzapine and chlorpromazine.
Chlorpromazine, developed in 1950, is employed in the treatment of both acute and chronic schizophrenia, bipolar disorder mania, amphetamine-induced psychoses and the short-term management of severe anxiety and psychotic aggression. It is classified as a lowpotency typical antipsychotic which acts as an antagonist on multiple postsynaptic receptors including Dopamine receptors (D1-4), serotonin receptors (5-HT1,5-HT2), histamine receptors (H1,H2), adrenergic receptors (a1,a2) and muscarinic acetylcholine receptors (M1,M2). Chronic chlorpromazine use may initiate disruption of serotonin and dopamine levels with consequent clinical hyperthermia or hypothermia [19,23]. The a1- and a2-adrenergic receptor antagonist action of chlorpromazine induces vasoconstriction and shunting of blood away from the skin to maintain core body temperature as described by the Vasodilation and Shunting Theories [3,4]. The antagonism of a1- and a2-adrenergic receptors by chlorpromazine may contribute to its hypothermic characteristics, particularly in cool environments.
Physiologic hypothermia (< 35°C) may have positive effects on brain physiology such as the protection of the brain after strokes [16]. However, untoward hypothermia is a well-known and potentially life-threatening adverse drug reaction of some antipsychotic medications [5]. Multiple systems are involved in maintaining normal human body temperature (97.5°F - 98.8 °F; 36.4 °C - 37.1°C). The preoptic and dorsomedial hypothalamus are principle elements of the core thermoregulatory center [23]. Also, cardiovascular organs and bioactive peptides have been suggested as playing a key role in temperature adjustment. Thermoregulation apparent Transient Receptor Potential ion channels (TRPs) family members and their receptors have been studied at the cell and molecular levels [6]. In animal studies, chlorpromazine and haloperidol have caused lesions of the preoptic hypothalamus [24-27].
The occurrence of thermoregulatory dysfunction after administration of neuroleptic medications has a multifactorial origin, however, unbalanced dopamine and serotonin levels is accepted as one of the principal mechanisms causing hyper- or hypothermic adverse effects. The signal pathway of thermoregulation in the hypothalamus is mediated by bioactive molecules and neurotransmitters. Dopamine D receptors and serotonergic receptors have been identified as central to the maintenance of body temperature [28-29]. Moreover, the potential pathophysiological mechanisms described by the Vasodilatation and Shunting Theories may also contribute to hypothermia [10]. Although these theories describe some possible pathophysiological mechanisms of hypothermia, they do not decisively explain why the adverse reaction of hyperthermia is significantly more frequent than is hypothermia. Also, these thermoregulation theories do not provide complete understanding of why chronic chlorpromazine use, with concurrent olanzapine and haloperidol as in this case report, resulted in hypothermia after 10 years without any prior hypothermia episodes.
The hypothermic effects of chlorpromazine appear less well known than the hyperthermia adverse reactions. Only 16 cases of chlorpromazine related hypothermia had been reported prior to 2007 [5]. The World health Organization database until 2007 ranks the hypothermia cases related to antipsychotic use as: risperidone (129); clozapine (68); olanzapine (44 cases); haloperidol (32 cases); quetiapine (21); and chlorpromazine (16) [5]. The principal question in this case study is, as olanzapine and haloperidol (76 cases combined) are more frequent causes of hypothermia than chlorpromazine, why was chlorpromazine the single hypothermia precipitating agent? It may be hypothesized that there is a physiologic and/or biochemical mechanism associated with chlorpromazine that varies from the reported hypothermic mechanisms [5,30-32].
The immunologic effects of chlorpromazine may also influence thermoregulation peripherally and centrally at a cellular and molecule level. Recently, Fairchild’s group found that chlorpromazinetreated hypothermic mice had a 2.3-fold and 1.8-fold higher plasma interleukin-6 and interleukin-10 levels respectively, six hours after drug administration when compared with identically treated normothermic mice [33]. This research initiated molecular level studies of the thermoregulation mechanism of chlorpromazine [33].
Risk factors for hypothermia associated with antipsychotic use include: schizophrenia; endocrine comorbidities; concurrent medical disease; organic brain disease; developmental delay; epilepsy; concurrent use of antipsychotics and benzodiazepines; and combinations of antipsychotics and beta blockers [5]. In this case study, the patient had schizophrenia and moderate neurocognitive disease. He was receiving medications for diabetes, hypertension, vitamin D deficiency, iron deficient anemia, hypothyroidism, gastro esophageal reflux and prostate hypertrophy. During the patient’s fourth admission, all psychiatric medications were stopped, followed by single challenges with chlorpromazine, haloperidol or olanzapine. Once chlorpromazine was identified as the single suspected agent, the prior medications for his medical problems were resumed without any return of the hypothermia. This suggests that none of the other medications were involved in the hypothermia episode. It is notable that the patient began receiving as needed chlorpromazine doses regularly at his home for adults after his first, second and third hospital admissions for hypothermia. While the first hypothermia episode began prior to the initiation of as needed chlorpromazine doses, the rapid return to the medical service for the three successive hypothermic episodes requiring hospital admission, suggests that the hypothermia was dose related.
Conclusion
The risks of antipsychotic combinations remain poorly understood. The chronic concurrent administration of multiple antipsychotics associated with hypothermia is a significant risk factor for precipitating hypothermia. This case study of a hypothermia syndrome involving three antipsychotics, each with a history of precipitating hypothermia, is rare and not previously reported. The addition of chlorpromazine on an as needed basis daily after the first hypothermia episode and the rapidity of the successive hospitalizations, suggests that chlorpromazine hypothermia increased with the cumulative effect of additional chlorpromazine as needed dosing.
Combination antipsychotic treatment increases the risk of adverse side effects including hypothermia. Early recognition and rapid intervention likely contributed to a favorable outcome in this case of hypothermia. In this case with three principal risk factors, stopping all medications and performing serial challenges with each medication individually was an effective strategy for resolving the hypothermia syndrome. Clinicians should be alert to the risk of hypothermia in psychiatry patients who are receiving multiple antipsychotics that are associated with a risk of hypothermia in combination with other comorbid hypothermia risk factors.
References
- Brevik A, Farver D. Atypical antipsychotic induced mild hypothermia. S D J Med. 2003; 56: 67-70.
- Young DM. Risk factors for hypothermia in psychiatric patients. Ann Clin Psychiatry. 1996; 8: 93-97.
- Schwaninger M, Weisbrod M, Schwab S, Schröder M, Hacke W. Hypothermia induced by atypical neuroleptics. Clin Neuropharmacol. 1998; 21: 344-346.
- Sethi R, Kavuru B. Hypothermia secondary to ziprasidone use in a man with schizophrenia. Prim Care Companion CNS Disord. 2012; 14.
- Rasnayake LR, Wimalarathne H, Jayapala RK, Gamage CD, Dassanayake DL, Ratnayake SL, et al. An unusual case of hypothermia associated with therapeutic doses of olanzapine: a case report. J Med Case Rep. 2011; 5: 189.
- Kreuzer P, Landgrebe M, Wittmann M, Schecklmann M, Poeppl TB, Hajak G, et al. Hypothermia associated with antipsychotic drug use: a clinical case series and review of current literature. J Clin Pharmacol. 2012; 52: 1090-1097.
- Yamawaki S, Lai H, Horita A. Dopaminergic and serotonergic mechanisms of thermoregulation: mediation of thermal effects of apomorphine and dopamine. J Pharmacol Exp Ther. 1983; 227: 383-388.
- Gibbons GM, Wein DA, Paula R. Profound hypothermia secondary to normal ziprasidone use. Am J Emerg Med. 2008; 26: 737.
- Gillman PK. Neuroleptic malignant syndrome: mechanisms, interactions, and causality. Mov Disord. 2010; 25: 1780-1790.
- van Marum RJ, Wegewijs MA, Loonen AJ, Beers E. Hypothermia following antipsychotic drug use. Eur J Clin Pharmacol. 2007; 63: 627-631.
- Caterina MJ. Transient receptor potential ion channels as participants in thermosensation and thermoregulation. Am J Physiol Regul Integr Comp Physiol. 2007; 292: R64-76.
- Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981; 30: 239-245.
- Endean ED, Mukhopadhyay SK, Fung LK, Cronenwett JL, Stanley JC, Lindenauer SM. Adrenergic and dopaminergic control of the canine paw microcirculation. J Surg Res. 1986; 40: 49-54.
- Saxena PR, Verdouw PD. Redistribution by 5-hydroxytryptamine of carotid arterial blood at the expense of arteriovenous anastomotic blood flow. J Physiol. 1982; 332: 501-520.
- Liu Z, Wang Y, Zhang J, Ding J, Guo L, Cui D, et al. Orphanin FQ: an endogenous antagonist of rat brain dopamine transporter. Neuroreport. 2001; 12: 699-702.
- Baiula M, Bedini A, Spampinato SM. Role of nociceptin/orphanin FQ in thermoregulation. Neuropeptides. 2015; 50: 51-56.
- Szekely M, Petervari E, Balasko M. Thermoregulation, energy balance, regulatory peptides: recent developments. Front Biosci (Schol Ed). 2010; 2: 1009-1046.
- Chen X, McClatchy DB, Geller EB, Liu-Chen L, Tallarida RJ, Adler MW. Possible mechanism of hypothermia induced by intracerebroventricular injection of orphanin FQ/nociceptin. Brain Res. 2001; 904: 252-258.
- Székely M, Pétervári E, Balaskó M, Hernádi I, Uzsoki B. Effects of orexins on energy balance and thermoregulation. Regul Pept. 2002; 104: 47-53.
- Jászberényi M, Bujdosó E, Kiss E, Pataki I, Telegdy G. The role of NPY in the mediation of orexin-induced hypothermia. Regul Pept. 2002; 104: 55-59.
- Balaskó M, Szelényi Z, Székely M. Central thermoregulatory effects of neuropeptide Y and orexin A in rats. Acta Physiol Hung. 1999; 86: 219-222.
- Inaba A, Komori Y, Muroi Y, Kinoshita K, Ishii T. Neuropeptide Y signaling in the dorsal raphe nucleus inhibits male sexual behavior in mice. Neuroscience. 2016; 320: 140-148.
- Imoto Y, Kado H, Masuda M, Yasui H. Effects of chlorpromazine as a systemic vasodilator during cardiopulmonary bypass in neonates. Jpn J Thorac Cardiovasc Surg. 2002; 50: 241-245.
- Chai CY, Lin MT. The enhancement of chlorpromazine-induced hypothermia by lesions in the anterior hypothalamus. Br J Pharmacol. 1977; 61: 77-82.
- Joseph C, Buga AM, Vintilescu R, Balseanu AT, Moldovan M, Junker H, et al. Prolonged gaseous hypothermia prevents the upregulation of phagocytosis-specific protein annexin 1 and causes low-amplitude EEG activity in the aged rat brain after cerebral ischemia. J Cereb Blood Flow Metab. 2012; 32: 1632-1642.
- Dimicco JA, Zaretsky DV. The dorsomedial hypothalamus: a new player in thermoregulation. Am J Physiol Regul Integr Comp Physiol. 2007; 292: 47-63.
- de la Cruz F. Hypothermia elicited by haloperidol in rats with hypothalamic lesions. Exp Neurol. 1988; 99: 234-238.
- Salmi P, Ahlenius S. Further evidence for clozapine as a dopamine D1 receptor agonist. Eur J Pharmacol. 1996; 307: 27-31.
- Salmi P, Karlsson T, Ahlenius S. Antagonism by SCH 23390 of clozapine-induced hypothermia in the rat. Eur J Pharmacol. 1994; 253: 67-73.
- Ben-Shachar D, Pinhassi B, Youdim MB. Prevention of neuroleptic-induced dopamine D2 receptor supersensitivity by chronic iron salt treatment. Eur J Pharmacol. 1991; 202: 177-183.
- Morris E, Green D, Graudins A. Neuroleptic malignant syndrome developing after acute overdose with olanzapine and chlorpromazine. J Med Toxicol. 2009; 5: 27-31.
- Fick LG, Fuller A, Mitchell D. Thermoregulatory, motor, behavioural, and nociceptive responses of rats to 3 long-acting neuroleptics. Can J Physiol Pharmacol. 2005; 83: 517-527.
- Stewart CR, Landseadel JP, Gurka MJ, Fairchild KD. Hypothermia increases interleukin-6 and interleukin-10 in juvenile endotoxemic mice. Pediatr Crit Care Med. 2010; 11: 109-116.