
Case Report
J Schizophr Res. 2018; 5(1): 1036.
Serendipitous Improvement of Schizophrenia after Stem Cell Transplant for Hodgkin’s Lymphoma
González-Llano O¹, Saucedo-Uribe E², Martínez- Garza DM¹, Mancías-Guerra C³, Cantú-Rodríguez OG¹ and Mancías-Guerra MC¹*
¹Hematology Service, Autonomous University of Nuevo León, Mexico
²Department of Psychiatry, Autonomous University of Nuevo León, México
³CIT Neuropsique, México
*Corresponding author: Mancías-Guerra MC, Hematology Service, University Hospital “José E. González”. School of Medicine, Autonomous University of Nuevo León, Monterrey, México
Received: September 06, 2018; Accepted: November 01, 2018; Published: November 08, 2018
Abstract
21-year-old male patient with schizophrenia diagnosed at age 15 with a history of poor response to olanzapine and risperidone. He referred auditory hallucinations with a pejorative content about himself. He was very suspicious and socially with drawn, to the extent of dropping out of high school and being unable to leave his home. One year afterward he was diagnosed with Hodgkin’s Lymphoma (HL). He underwent different chemotherapeutic schemes and three Stem Cell Transplants (SCT). Between the second and the third SCT, the patient received therapeutic doses of quetiapine (Positive and Negative Syndrome Scale [PANSS]: 147). In a period of 8 months, after the second SCT, patient’s PANSS dropped 60 points, his hallucinations decreased 90%, and he improved his negative, cognitive, and social symptoms, which allowed him to gradually reincorporate to his usual social and academic life.
Currently, Hematopoietic Stem Cells (HSC) have been proposed as an alternative therapy for many chronic and incurable non-hematologic diseases. In psychiatry, the theory is that these HSC migrate to areas of inflammation in the brain via chemotaxis, and, through immunomodulation and secretion of bioactive molecules enhance neurogenesis, angiogenesis, and remodeling of axonal circuits. Studies on schizophrenic patients have confirmed both, that it is an inflammatory condition (cytokines and interleukins are elevated when compared to controls) and, that the brain is underdeveloped due to deficient neurogenesis; supporting. why HSC assisted in the marked clinical improvement observed in this patient, which would otherwise could not be explained by the natural history of the disease or therapeutic measures alone.
Keywords: Schizophrenia; Stem cell transplant; Hematopoietic stem cells; Neurogenesis; Neuroimmunomodu
Introduction
Psychiatry has fallen behind other medical specialties regarding treatment innovation [1]. It is so, that most of the first line medications are now over 40 years old, and the last drug with an original mechanism of action was introduced almost a decade ago [2]. Nonetheless, psychiatric disorders have a prevalence that may be as high as 26% [3], urging the need to develop novel and precise therapeutic offers [4]. Schizophrenia, with more than 20 million people affected worldwide, currently the third-most disabling condition [5], a lifetime prevalence of 0.7%, and carrying a great burden of human and economic costs [6], is just one of the many examples of mental health diseases demanding new approaches [7-15].
Since their discovery, stem cells have been used as an alternative treatment for many diseases, especially chronic diseases with not few therapeutic options, such as heart and autoimmune disorders [16]. Mental health is not the exception, as psychiatric conditions may be caused by deficiencies in neurogenesis and neurodevelopment, in which stem cells, hypothetically may be able to restore proper psychological function of areas where cells have died or are dysfunctional, or may enhance some degree of neurogenesis [17,18].
Case Report
The patient is a male that started experiencing outbreaks of psychotic symptoms at the age of 15. The family was skeptic about this, so they did not seek for help until after a year when symptoms became more florid. The diagnosis of schizophrenia was made. Olanzapine 10 mg QD and risperidone 4 mg QD were indicated, without satisfactory results, despite complete adherence. Two years later from the start of symptoms the patient sought for a second opinion, being attended by us.
On examination, he appeared disheveled, suspicious, and very anxious. He was very inexpressive with an indifferent and flat affect, avoided eye contact, and was very uncooperative. He had a disorganized thought process and answered to most questions monosyllabically. Patient referred auditory hallucinations; he heard two voices that made fun of him. He had reference delusions about his neighbors and peers making fun of him and claiming that “he was homosexual”. Hence, he became socially withdrawn and eventually dropped out of high school. He did not abandon his home for anything but therapy. Patient slept all day but could not sleep at night because of fear; he sometimes would hide in the walls of his home because he thought people were spying on him. The diagnosis of schizophrenia with predominant negative symptoms was confirmed (positive and negative syndrome scale [PANSS]: 147). He was started on an in crescendo scheme of quetiapine until he reached a maintenance dose of 300mg bid.
Meanwhile, he was concomitantly diagnosed with HL (Figure 1). He received an induction treatment with an ABVD (adriamycin, bleomycin, vinblastine, and dacarbazine) scheme and received an autologous SCT of peripheral blood as consolidation treatment with etoposide and carmustine as conditioning regimen. Six 3 months later, he relapsed and was started on a second-line scheme that included ifosfamide, gemcitabine, and vinorelbine as the induction therapy, this time he was consolidated with a second autologous SCT with peripheral cryopreserved cells with busulfan and melphalan as conditioning regimen. The patient went into a second complete remission, though, he relapsed for the second time. He underwent a rescue scheme using brentuximab and was now submitted to a haploidentical SCT. Cyclophosphamide, fludarabine, and melphalan were used as conditioning. He reached complete remission, is free of HL and without data of graft versus host disease. Over a period of 18 months the patient received a total of 15×106/kg HSC.
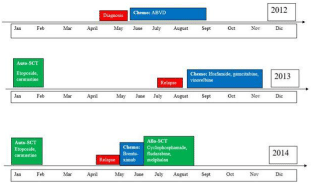
Figure 1: Hodgkin’s Lymphoma evolution.
During this time, the patient dramatically decreased his symptoms and PANSS results (Figure 2). The patient referred hallucinations had decreased 70-90%, and when they happened, he was conscious about them coming from his head and not being real. His negative, social and cognitive symptoms began gradually improving. Soon after the third SCT he could walk and normally interact on the streets and since then has been stable without any relapse. Currently, still on quetiapine, the patient is majoring in arts with academic excellence and with a stable social life. Even though auditory hallucinations persist, they have diminished in frequency, and he now has the ability to neglect them.
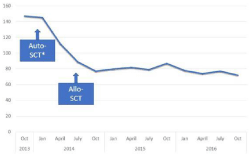
Figure 2: Improvement in PANSS following second and third bone marrow transplant.
*We do not know how PANSS changed between the first and second SCT because we first started seeing the patient after the first SCT.
Discussion
The assumption that the brain cannot be modified after adulthood is long gone. There is evidence of neural stem cells (NSC) that supply the rest of the brain for renovation [19], as they migrate to areas of damage and inflammation [20]. After brain insults that cause neuronal death such as stroke[21,22], schizophrenia, and Alzheimer’s disease [23,24], NSC migrate, differentiate, and incorporate into lost neural circuits [21-25].
They do as such as a result of two things. First, after cell death, many neurogenesis genes activate, such as the ones in the transforming growth factor-beta superfamily [26]. Second and most importantly, because of inflammation, many chemotactic molecules up-regulate (e.g., interleukin 8, monocyte chemoattractant protein-1) [27], attracting not only NSC [28], but also HSC [29], element that becomes the linchpin of how stem cell therapy works in the brain as we will describe later on.
HSC lie on the stroma of the bone marrow and give rise to all the types of blood cells; their phenotype is CD34+ and CD133+ [30-33]. As mentioned before, these are recruited to areas of inflammation in the brain, where they aid in neurorestoration. These cells have limited cellular differentiation potential when compared to other types of stem cells, so their beneficial properties are instead through immunomodulation [34], as they secrete many bioactive molecules such as brain-derived neurotrophic factor, basic fibroblast growth factor, β-nerve growth factor, vascular endothelial growth factor, and angiopoietins 1 and 2, promoting neurogenesis, angiogenesis, axonal growth, and reducing apoptosis in areas of brain damage [35-38]. This means that without any intervention, the natural response of the body to neuronal damage is to create a microenvironment that stimulates neurorestoration through the proliferation and migration of HSC. Therefore, the hypothesis of how stem cells enhance neuronal recovery lies in the fact that exogenously administrating stem cells would multiply and extend the efforts activated physiologically by the body [39-44].
Studies have shown increased levels of proinflammatory cytokines (e.g., tumor necrosis factor alpha, interleukin-6, and interleukin-2) in schizophrenia [45,46]. This is critical because it confirms that psychiatric disorders constitute a state of active and chronic inflammation, which would justify the migration of peripheral blood stem cells to the brain to execute their paracrine effects of up-regulating and augmenting both neurogenesis and angiogenesis. Ergo, linking the clinical improvements with a SCT.Even though many trials advocate for intra-organ stem cell transplant (in this case the brain), it could be sufficient to allocate them intravenously, given that they are capable of crossing the blood-brain barrier (BBB) [47], and finding their way to reach damaged areas, even within the brain [48].
This is not the first psychiatric disease in which the neurogenesis theory has been tested on. For instance, it has been demonstrated that lower levels of 5-hydroxytryptamine decrease the rate of neurogenesis in the hippocampus [49], and that the chronic administration of serotonin selective reuptake inhibitors, currently the first-line therapy for depression, increase neurogenesis [50] and leads to the upregulation of Brain-Derived Neurotrophic Factor (BDNF) messenger ribonucleic acid [51]. Another example is bipolar disorder, in which it has been proven that neurogenesis is diminished while proper pharmacotherapy augments it [52-54].
Even though clinical improvements were seen in our patient at the same time quetiapine was being used at high doses, this would not justify the marked improvement (58-point drop in PANSS) in just eight months, after a stable course. Even though positive symptoms tend to respond well to medication [55-61], negative symptoms, such as flat affect and asociality are far less responsive to antipsychotics [62]. Therefore, knowing how refractory negative and cognitive symptoms are [62], their notable amelioration cannot be explained by quetiapine alone.
Moreover, this is not the natural history of schizophrenia; positive symptoms do tend to lessen with age. However, negative and cognitive symptoms continue to worsen as the patient gets older [63], contributing to a poor quality of life [64,65]. Some patients achieve do recover and function well, though this is the minority [66]. In fact, three of every four patients end up having some type of disability with multiple relapses [62], and up to 85% end up unemployed [67].
One of the limitations of this report is the fact that we first saw the patient after receiving the first SCT, and we do not know the severity of the disease before the transplant and how it might have been altered by it. Nonetheless, given the history and presentation, it is clear that his schizophrenia was a refractory one.
Conclusion
The organic approach shift that mental health is experiencing nowadays is supported by the volume of research and new discoveries of how the brain is modified during a psychiatric illness. This is not the first time that by serendipity patients submitted to SCT improved in concomitant diseases. For example, renal and cardiac function improvements after SCT have been reported as well [68,69]. Given the disabling character of schizophrenia, paired with the high prevalence of it, we must not ignore any hint of mending the disease. We must encourage further research to clarify this matter, aiming to provide hope of removing, or at least lighten the great burden of this condition.
References
- Harrison PJ, Cader MZ, Geddes JR. Reprogramming psychiatry: Stem cells and bipolar disorder. Lancet [Internet]. 2016; 387: 823-825.
- Krystal JH, State MW. Psychiatric disorders: Diagnosis to therapy. Cell [Internet]. 2014; 157: 201-214.
- Barrett JE. The Prevalence of Psychiatric Disorders in a Primary Care Practice. Arch Gen Psychiatry [Internet]. 1988; 45: 1100-1106.
- Kaiser T, Feng G. Modeling psychiatric disorders for developing effective treatments. Nat Med [Internet]. 2015; 21: 979-988.
- Ustün TB, Rehm J, Chatterji S, Saxena S, Trotter R, Room R, et al. Multipleinformant ranking of the disabling effects of different health conditions in 14 countries. WHO/NIH Joint Project CAR Study Group. Lancet (London, England). 1999; 354: 111-1115.
- Van Os J, Kapur S. Schizophrenia. Lancet. 2009; 374: 635-645.
- Bremner JD, Narayan M, Anderson ER, Staib LH, Miller HL, Charney DS. Hippocampal volume reduction in major depression. Am J Psychiatry. 2000; 157: 115-118.
- Sawyer K, Corsentino E, Sachs-Ericsson N, Steffens DC. Depression, hippocampal volume changes, and cognitive decline in a clinical sample of older depressed outpatients and non-depressed controls. Aging Ment Health [Internet]. 2012; 16: 753-762.
- Benes FM, Kwok EW, Vincent SL, Todtenkopf MS. A reduction of nonpyramidal cells in sector CA2 of schizophrenics and manic depressives. Biol Psychiatry. 1998; 44: 88-97.
- Wise T, Radua J, Via E, Cardoner N, Abe O, Adams TM, et al. Common and distinct patterns of grey-matter volume alteration in major depression and bipolar disorder: evidence from voxel-based meta-analysis. Mol Psychiatry [Internet]. 2016; 22: 1455-1463.
- Bremner JD, Randall PR, Scott TM, Bronen RA, Delaney RC, Seibyl JP, et al. MRI-based measurement of hippocampal volume in posttraumatic stress disorder. Am J Psychiatry. 1995; 152: 973-978.
- Morris SA, Eaves DW, Smith AR, Nixon K. Alcohol inhibition of neurogenesis: A mechanism of 8hippocampal neurodegeneration in an adolescent alcohol abuse model. Hippocampus. 2010; 20: 596-607.
- Xu C, Loh HH, Law PY. Effects of addictive drugs on adult neural stem/ progenitor cells. Cell Mol Life Sci. 2016; 73: 327-348.
- Jablonski M, Mazur JK, Tarnowski M, Dolegowska B, Pedziwiatr D, Kubis E, et al. Mobilization of Peripheral Blood Stem Cells and Changes in the Concentration of Plasma Factors Influencing their Movement in Patients with Panic Disorder. Stem Cell Rev Reports. 2016; 13: 217-225.
- Ecklund K, Vajapeyam S, Feldman HA, Buzney CD, Mulkern R V, Kleinman PK, et al. Bone marrow changes in adolescent girls with anorexia nervosa. J Bone Miner Res [Internet]. 2010; 25: 298-304.
- Trounson A, Thakar RG, Lomax G, Gibbons D. Clinical trials for stem cell therapies. BMC Med. 2011; 9: 52.
- Benninghoff J. Stem cell approaches in psychiatry--challenges and opportunities. Dialogues Clin Neurosci [Internet]. 2009; 11: 397-404.
- Buffo A, Rolando C, Ceruti S. Astrocytes in the damaged brain: Molecular and cellular insights into their reactive response and healing potential. Biochem Pharmacol. 2010; 79: 77-89.
- Lin R, Iacovitti L. Classic and novel stem cell niches in brain homeostasis and repair. Brain Res [Internet]. 2015; 1628: 327-342.
- Iskander A, Knight RA, Zhang ZG, Ewing JR, Shankar A, Varma NRS, et al. Intravenous administration of human umbilical cord blood-derived AC133+ endothelial progenitor cells in rat stroke model reduces infarct volume: magnetic resonance imaging and histological findings. Stem Cells Transl Med. 2013; 703-714.
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002; 8: 963-970.
- Parent JM, Vexler ZS, Gong C, Derugin N, Ferriero DM. Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Ann Neurol. 2002; 52: 802-813.
- Reif A, Fritzen S, Finger M, Strobel A, Lauer M, Schmitt A, et al. Neural stem cell proliferation is decreased in schizophrenia, but not in depression. Mol Psychiatry [Internet]. 2006; 11: 514-522.
- Jin K, Peel AL, Mao XO, Xie L, Cottrell BA, Henshall DC, et al. Increased hippocampal neurogenesis in Alzheimer’s disease. Proc Natl Acad Sci USA [Internet]. 2004; 101: 343-347.
- Hou SW, Wang YQ, Xu M, Shen DH, Wang JJ, Huang F, et al. Functional integration of newly generated neurons into striatum after cerebral ischemia in the adult rat brain. Stroke. 2008; 39: 2837-2844.
- Liu XS, Zhang ZG, Zhang RL, Gregg S, Morris DC, Wang Y, et al. Stroke induces gene profile changes associated with neurogenesis and angiogenesis in adult subventricular zone progenitor cells. J Cereb Blood Flow Metab [Internet]. 2007; 27: 564-574.
- Minami M, Katayama T, Satoh M. Brain cytokines and chemokines: Roles in ischemic injury and pain. J Pharmacol Sci. 2006; 100: 461-470.
- Bajetto A, Bonavia R, Barbero S, Schettini G. Chemokines and their receptors in the central nervous system. J Neurochem. 2001; 22: 1311-1329.
- Wang Y, Deng Y, Zhou GQ. SDF-1a/CXCR4-mediated migration of systemically transplanted 10bone marrow stromal cells towards ischemic brain lesion in a rat model. Brain Res. 2008; 1195: 104-112.
- Fernandez-Egea E, Bruna A, Garcia-Rizo C, Bernardo M, Kirkpatrick B. Stem cell signaling in newly diagnosed, antipsychotic-naive subjects with nonaffective psychosis. Mol Psychiatry [Internet]. 2009; 14: 989-991.
- Boyajyan A, Khoyetsyan A, Chavushyan A. Alternative complement pathway in Schizophrenia. Neurochem Res. 2010; 35: 894-898.
- Hakobyan S, Boyajyan A, Sim RB. Classical pathway complement activity in schizophrenia. Neurosci Lett. 2005; 374: 35-37.
- Birbrair A, Frenette PS. Niche heterogeneity in the bone marrow. Ann NY Acad Sci. 2016; 1370: 82-96.
- Caplan A. Why are MSCs therapeutic? New data: new insight. J Pathol. 2009; 217: 318-324.
- Moniche F, Gonzalez A, Gonzalez-Marcos JR, Carmona M, Pinero P, Espigado I, et al. Intra-arterial bone marrow mononuclear cells in ischemic stroke: a pilot clinical trial. Stroke [Internet]. 2012; 43: 2242-2244.
- Chen J, Li Y, Katakowski M, Chen X, Wang L, Lu D, et al. Intravenous bone marrow stromal cell therapy reduces apoptosis and promotes endogenous cell proliferation after stroke in female rat. J Neurosci Res. 2003; 73: 778-786.
- Shen LH, Li Y, Gao Q, Savant-Bhonsale S, Chopp M. Down-regulation of neurocan expression in reactive astrocytes promotes axonal regeneration and facilitates the neurorestorative effects of bone marrow stromal cells in the ischemic rat brain. Glia. 2008; 56: 1747-1754.
- Chen J, Zhang ZG, Li Y, Wang L, Xu YX, Gautam SC, et al. Intravenous administration of human bone marrow boundary zone after stroke in rats. Circ Res. 2003; 92: 692-699.
- Martínez-Garza DM, Cantú-Rodríguez OG, Jaime-Pérez JC, Gutiérrez- Aguirre CH, Góngora-Rivera JF, Gómez-Almaguer D. Current state and perspectives of stem cell therapy for stroke [Internet]. Medicina Universitaria. 2016.
- Mura G, Donatella Rita Petretto, Krishna M Bhat, Mauro Giovanni Carta. Schizophrenia: from Epidemiology to Rehabilitation. Clin Pract Epidemiol Ment Heal [Internet]. 2012; 8: 52-66.
- Schurov IL, Handford EJ, Brandon NJ, Whiting PJ. Expression of disrupted in schizophrenia 1 (DISC1) protein in the adult and developing mouse brain indicates its role in neurodevelopment. Mol Psychiatry [Internet]. 2004; 9: 1100-1110.
- Schumacher J, Laje G, Jamra RA, Becker T, Mühleisen TW, Vasilescu C, et al. The DISC locus and schizophrenia: Evidence from an association study in a central European sample and from a meta-analysis across different European populations. Hum Mol Genet. 2009; 18: 2719-2727.
- Kapczinski F, Dal-Pizzol F, Teixeira AL, Magalhaes PVS, Kauer-Sant’Anna M, Klamt F, et al. Peripheral biomarkers and illness activity in bipolar disorder. J Psychiatr Res [Internet]. 2011; 45: 156-161.
- Patas K, Penninx BWJH, Bus BAA, Vogelzangs N, Molendijk ML, Elzinga BM, et al. Association between serum brain-derived neurotrophic factor and plasma interleukin-6 in major depressive disorder with melancholic features. Brain Behav Immun [Internet]. 2014; 36: 71-79.
- Asevedo E, Gadelha A, Noto C, Mansur RB, Zugman A, Belangero SIN, et al. Impact of 12peripheral levels of chemokines, BDNF and oxidative markers on cognition in individuals with schizophrenia. J Psychiatr Res [Internet]. 2013; 47: 1376-1382.
- Kunz M, Ceresér KM, Goi PD, Fries GR, Teixeira AL, Fernandes BS, et al. Serum levels of IL-6, IL-10 and TNF-a in patients with bipolar disorder and schizophrenia: differences in pro- and anti-inflammatory balance. Rev Bras Psiquiatr [Internet]. 2011; 33: 268-274.
- Liu L, Eckert M a, Riazifar H, Kang DK, Agalliu D, Zhao W. From blood to the brain: Can systemically transplanted mesenchymal stem cells cross the blood-brain barrier? Stem Cells Int. 2013; 2013: 1-7.
- Gritti F, Guiochon G. Adsorption mechanism of acids and bases in reversedphase liquid chromatography in weak buffered mobile phases designed for liquid chromatography/mass spectrometry. J Chromatogr A. 2009; 1216: 1776-1788.
- Brezun JM, Daszuta A. Depletion in serotonin decreases neurogenesis in the dentate gyrus and the subventricular zone of adult rats. Neuroscience. 1999; 89: 999-1002.
- Santarelli L, Saxe M, Gross C, Surget A, Dulawa S, Weisstaub N, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2017; 301: 805-809.
- Nibuya M, Morinobu S, Duman RS. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci. 1995; 15: 7539-7547.
- Shirayama Y, Chen AC-H, Nakagawa S, Russell DS, Duman RS. Brainderived neurotrophic factor produces antidepressant effects in behavioral models of depression. J Neurosci [Internet]. 2002; 22: 3251-3261.
- Tfilin M, Sudai E, Merenlender A, Gispan I, Yadid G, Turgeman G. Mesenchymal stem cells increase hippocampal neurogenesis and counteract depressive-like behavior. Mol Psychiatry [Internet]. 2010; 15: 1164-1175.
- Schloesser RJ, Chen G, Manji HK. Neurogenesis and Neuroenhancement in the Pathophysiology and Treatment of Bipolar Disorder. Int Rev Neurobiol [Internet]. 2007; 77: 143-178.
- Chen G, Rajkowska G, Du F, Seraji-Bozorgzad N, Manji HK. Enhancement of hippocampal neurogenesis by lithium. J Neurochem. 2000; 75: 1729-1734.
- Chen RW, Chuang DM. Long term lithium treatment suppresses p53 and Bax expression but increases Bcl-2 expression. A prominent role in neuroprotection against excitotoxicity. J Biol Chem. 1999; 274: 6039-6042.
- Chen DF, Schneider GE, Martinou JC, Tonegawa S. Bcl-2 promotes regeneration of severed axons in mammalian CNS. [Internet]. Nature. 1997; 385: 434-439.
- Gould E, McEwen BSS, Tanapat P, Galea LAM a, Fuchs E. Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J Neurosci. 1997; 17: 2492-2498.
- Lucassen PJ, Meerlo P, Naylor AS, van Dam AM, Dayer AG, Fuchs E, et al. Regulation of adult neurogenesis by stress, sleep disruption, exercise and inflammation: Implications for depression and antidepressant action. Eur Neuropsychopharmacol [Internet]. 2010; 20: 1-17.
- Ataka K, Asakawa A, Nagaishi K, Kaimoto K, Sawada A, Hayakawa Y, et al. Bone marrow-derived microglia infiltrate into the paraventricular nucleus of chronic psychological stress-loaded mice. PLoS One. 2013; 8: 1-14.
- Eroglu S, Toprak S, Urgan O, Ozge E, Onur, Arzu Denizbasi, et al. DSM-IV Diagnostic and Statistical Manual of Mental Disorder. American Psychiatric Organization. 2012; 33: 1-915.
- Smith T, Weston C, Lieberman J. Schizophrenia ( Maintenance Treatment ). 2010; 16: 1007.
- Karim S, Overshott R, Burns A. Older people with chronic schizophrenia. Aging Ment Health [Internet]. 2005; 9: 315-324.
- Velligan DI, Mahurin RK, Diamond PL, Hazleton BC, Eckert SL, Miller AL. The functional significance of symptomatology and cognitive function in schizophrenia. Schizophr Res. 1997; 25: 21-31.
- Galuppi A, Turola M, Nanni M, Mazzoni P, Grassi L. Schizophrenia and quality of life: how important are symptoms and functioning? Int J Ment Health Syst [Internet]. 2010; 4: 31.
- Warner R. Recovery from schizophrenia and the recovery model. Curr Opin Psychiatry [Internet]. 2009; 22: 374-380.
- Owen MJ, Sawa A, Mortensen PB. Schizophrenia. Lancet. 2016; 388: 86-97.
- Davani S, Deschaseaux F, Chalmers D, Tiberghien P, Kantelip JP. Can stem cells mend a broken heart? Cardiovasc Res. 2005; 65: 305-316.
- Bruno S, Tapparo M, Collino F, Chiabotto G, Deregibus MC, Lindoso RS, et al. Renal Regenerative Potential of Different Extracellular Vesicle Populations Derived from Bone Marrow Mesenchymal Stromal Cells. Tissue Eng [Internet]. 2017; 23: 1262-1273.