
Research Article
J Schizophr Res. 2022; 8(1): 1043.
Deep Brain Stimulation for Treatment-Resistant Schizophrenia: A Systematic Review
Saadon JR¹, Wang C¹, Razzaq B¹, Kuhia RL¹, Cleri NA¹, Egnor M¹, Mofakham S1,2 and Mikell CB¹*
1Department of Neurosurgery, Renaissance School of Medicine at Stony Brook University, USA
2Department of Electrical and Computer Engineering, Stony Brook University, USA
*Corresponding author: Charles B. Mikell, Clinical Assistant Professor of Neurological Surgery, Department of Neurological Surgery, Stony Brook University Hospital, 101 Nicolls Road, Stony Brook, NY 11794, USA
Received: July 08, 2022; Accepted: August 15, 2022; Published: August 22, 2022
Abstract
Background: Resistance to treatment characterizes a substantial portion of patients with schizophrenia. Without adequate symptom control from medication, these patients are left with few therapeutic options.
Objective: Given the recent success of Deep Brain Stimulation (DBS) in treatment-refractory neuropsychiatric disorders such as depression, obsessivecompulsive disorder, substance use disorder, and Tourette syndrome, we reviewed the existing evidence for DBS in schizophrenia.
Methods: We searched PubMed and MEDLINE for articles and conducted a systematic review on DBS as a treatment for schizophrenia in line with the PRISMA-P 2020 guidelines.
Results: After the search and screening process, we reviewed a total of three articles with eleven patients implanted. The nucleus accumbens (n = 3), subgenual anterior cingulate cortex (n = 4), habenula (n = 2), and substantia nigra pars reticulata (n = 1) were all targeted for stimulation. Stimulation resulted in symptom reduction on respective outcome measures in nine of ten patients stimulated (one patient was explanted without stimulation). There were ten adverse events across the eleven procedures and five of these were deemed serious adverse events, comparable to other reports of DBS. Three of these events occurred in the same patient, while one event coincided with a patient’s discontinuation of their own antipsychotic regimen. None resulted in permanent harm.
Conclusion: Despite a limited experience, DBS is likely safe, and potentially effective in patients with refractory schizophrenia. Further investigation is necessary to establish ideal targets for stimulation.
Keywords: Deep brain stimulation (DBS); Schizophrenia; Nucleus accumbens; Substantia nigra
Introduction
Schizophrenia is a chronic, debilitating psychiatric disorder affecting over 20 million people worldwide [1]. The diagnosis of schizophrenia requires two or more of the following characteristics: 1) delusions, 2) hallucinations, 3) disorganized speech, 4) disorganized or catatonic behavior, and 5) negative symptoms such as flat affect or avolition, with at least one of the first three characteristics present [2]. Many patients typically exhibit considerable morbidity as their disease can cause cognitive impairment [3], strain interpersonal relationships [4], and is often associated with other psychiatric illnesses [5-8]. Additionally, the rates of all-cause mortality, naturalcause mortality, and suicide are higher in this population [9,10].
Historically, the proposed pathophysiology of schizophrenia has centered around disordered dopamine regulation [11-13]. More specifically, a hyperdopaminergic mesolimbic pathway and hypodopaminergic mesocortical pathway are thought to cause the positive and negative symptoms, respectively [14,15]. First-line treatment for schizophrenia is with antipsychotic medication, often taken chronically to prevent psychotic episodes [16]. The original antipsychotics (typical antipsychotics), are D2 receptor antagonists and treat only the positive symptoms of schizophrenia. Second-generation antipsychotics, also known as atypical, generally demonstrate a decreased affinity for D2 receptors while also affecting serotonergic, cholinergic, and adrenergic synapses [17]. However, pharmacologic treatment is not without its drawbacks as many antipsychotics display metabolic, cardiovascular, endocrine, and neurological side effects that can impact patient adherence or necessitate discontinuation of a medication that effectively treats their disease.
Approximately 10-30% of schizophrenic individuals are deemed treatment-resistant [18-20], meaning they failed two or more trials of an antipsychotic [21]. These patients are often started on clozapine, the most effective antipsychotic drug available. Clozapine is only indicated for refractory schizophrenia because it can cause agranulocytosis, a dangerously low level of white blood cells in the blood [22]. Even so, only about a third of patients will demonstrate a response to clozapine [23,24]. Those who do not respond adequately or cannot tolerate clozapine are then left with few alternatives. Because of the exorbitant societal burden that comes with both controlling patients’ symptoms and managing the psychosocial factors influenced by their disease, there is a continued need to find better methods for controlling schizophrenia symptoms [25].
Since many symptoms are associated with dysregulation of the dopamine system, potential treatments may include targeting of dopaminergic brain regions. Among the more intriguing, nonpharmacologic options for treatment-resistant schizophrenia are neuromodulatory methods such as Deep Brain Stimulation (DBS). Originally indicated for symptomatic improvement in movement disorders such as Parkinson’s Disease and essential tremor, potential therapeutic applications of this technique have grown in recent years to include neuropsychiatric conditions like depression [26], Obsessive-Compulsive Disorder (OCD) [27], substance use disorder [28,29], and Tourette syndrome [30,31].
Aside from its success in other psychiatric disorders, DBS offers an opportunity to directly modulate aberrant circuits in schizophrenia. In animal models of Parkinson’s disease, DBS prevented pathological oscillations associated with the disease and restored normal neuronal activity [32,33]. Multiple lines of evidence have demonstrated aberrant electroencephalographic (EEG) activity in schizophrenia patients across several frequency bands during both cognitive tasks and at rest [34-36]. The most frequently observed differences are in the gamma band, the dominant frequency for local, cortical network communication [37]. Interestingly, task-based studies revealed decreases in gamma power and synchronization in schizophrenia [34,38], whereas investigations of resting-state EEG found increased gamma power [39-41]. Thus, the utility of DBS may lie in its ability to interfere with this aberrant activity and restore more normal oscillatory patterns.
However, few studies have investigated DBS as a therapeutic option for schizophrenia. A single report from the 1950s described intense feelings of rage and fear during stimulation of the amygdala in a patient with schizophrenia [42], yet no further research had been published until the last few years. In this review, we examined the current literature on DBS as a treatment for refractory schizophrenia and emphasize the need for larger, randomized controlled trials to evaluate treatment efficacy. We also summarize existing information on potential targets and suggest the nucleus accumbens (NAc) and substantia nigra pars reticulata (SNr) as structures warranting further investigation.
Methods
Search Strategy
This study was performed according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses Protocols (PRISMA-P) 2020 statement standards [43]. Online databases, PubMed and MEDLINE, were searched for relevant studies in early November 2021 utilizing appropriate Boolean modifiers. Our exact search terms were “Deep Brain Stimulation” OR “DBS” AND “Schizophrenia.” Two independent researchers screened and selected relevant articles based on title and abstract. Relevant articles were then screened for eligibility criteria. We also screened several reviews we encountered during our search, namely Mikell et al. [44], Mikell et al. [45], and Agarwal et al. [46].
Eligibility Criteria
We excluded articles on disorders other than schizophrenia, treatments other than DBS, non-human studies, and review articles. All remaining articles were peer-reviewed, original case reports, case series, or randomized trials written in English. No titles were excluded based on the date of publication.
Data Extraction
Two authors compiled a list of citations after screening the titles and abstracts. After discussing and agreeing on the eligibility criteria, the two authors independently reviewed the full-text articles that met the criteria. Information regarding the study design, participant characteristics, location of targets, duration of stimulation and followup, and outcome measures were extracted for comparison. Given the small number of patients across the eligible studies, we were unable to quantitatively assess the strength of evidence.
Three scales were used as primary endpoints for the studies included, namely the Positive and Negative Syndrome Scale (PANSS), Brief Psychiatric Rating Scale (BPRS), and Scale for the Assessment of Negative Symptoms (SANS). The PANSS is a 30-item scale consisting of positive symptoms, negative symptoms, and general psychopathology subscales. Each item is given a rating between one and seven, with higher scores representing more severe symptoms [47]. The BPRS evaluates up to 24 psychotic symptoms, each of which is scored from one to seven based on symptom severity [48]. Finally, the SANS measures negative symptoms on a 25-item scale scored from zero to five for each item [49].
Quality Assessment
Two authors independently assessed the risk of bias for the outcomes of each study in accordance with AHRQ guidelines [50].
Results
Study Selection
Our search of the literature in PubMed and MEDLINE yielded a total of 238 titles (120 and 118, respectively). We found no additional articles from other sources. After removing duplicates, 120 titles remained. Six articles were selected after screening based on their titles and abstracts. One case report [51] contained data from a patient that was also reported in a randomized trial [52], and therefore, was excluded. Another study [53] did not evaluate DBS as a treatment for schizophrenia. Finally, a third article [54] featured a patient with both OCD and schizophrenia, but investigated DBS in the context of their OCD symptoms. Thus, three articles met our eligibility criteria (Figure 1).
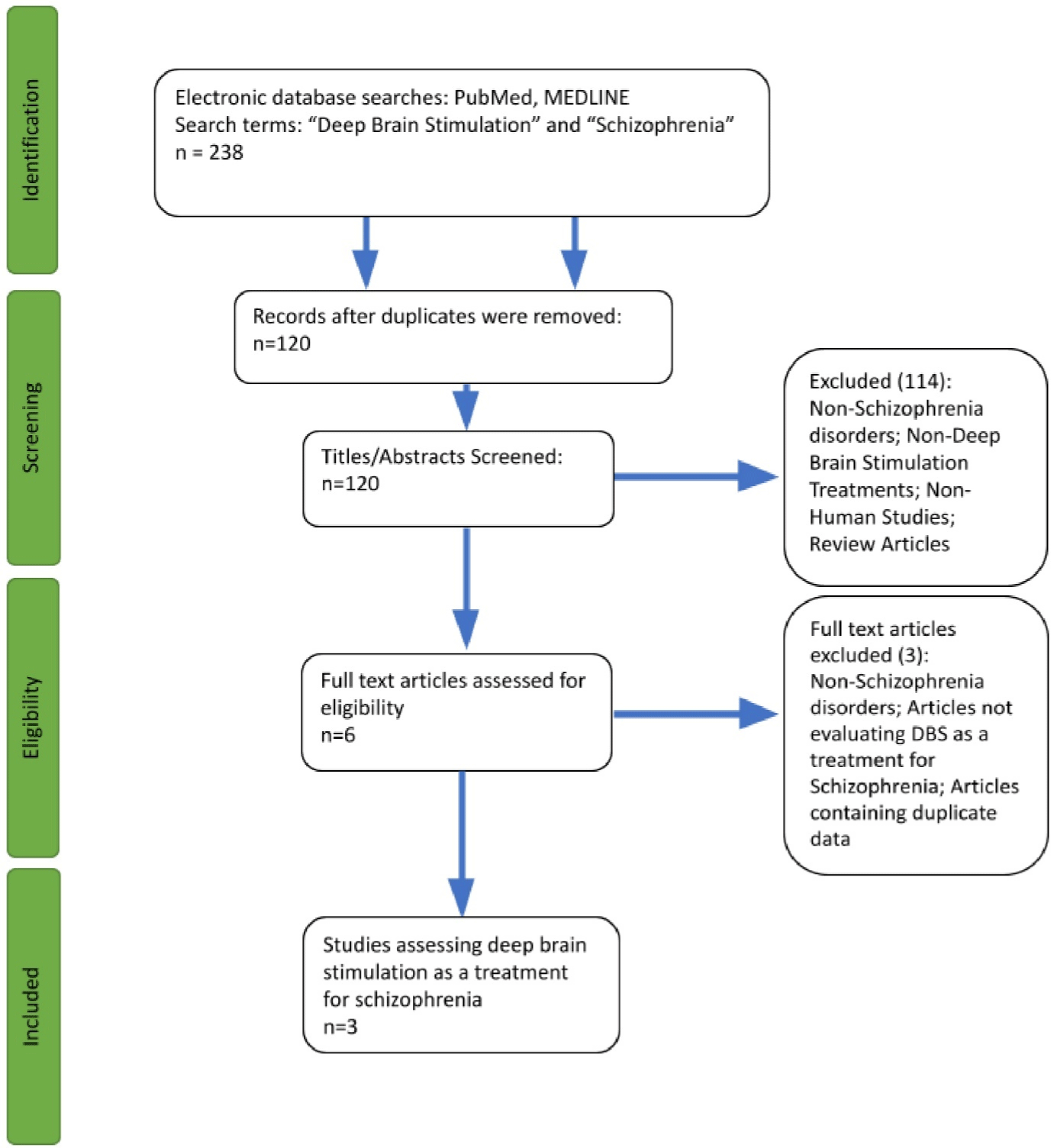
Figure 1: Schematic of literature search and screening process based on PRISMA-P 2020 guidelines [43].
Study Design
Of the three articles that met our criteria, one was a randomized trial [52], one was a case report [55], and one was a case series [56]. Study characteristics are detailed in (Table 1). A total of 11 patients were implanted with electrodes across the three studies. However, one patient, planned for NAc DBS, was never stimulated due to complications from surgery (see Adverse Events). The objective of these three studies was to treat both positive and negative symptoms. Each intended to assess the effects of stimulation over different follow-up periods. Wang and colleagues planned to stimulate for 12 months, however, one of the two patients was only stimulated for 10 months [56]. The other two studies stimulated for a minimum of six months. The single case report includes six months of stimulation data [55], however, this patient is part of an ongoing clinical trial (NCT01725334) that intends to stimulate participants for a total of one year. For the randomized trial, patients received continued stimulation beyond 6-months until their disease was clinically stable. After stabilization, patients were eligible to enter a randomized, double-blind crossover phase if they demonstrated a ≥ 25% reduction in their PANSS that remained stable on 50% of the ensuing assessments. The crossover period consisted of two 12-week blocks, one with and one without stimulation, to which patients were randomized. Four patients met the criteria for the crossover phase, but one declined to participate [52].
Four different loci were stimulated among the ten patients studied; four in the subgenual anterior cingulate cortex (sgACC), three in the NAc, two in the Hb, and one in the SNr. Each of these sites has been implicated in the pathophysiology of schizophrenia (Figure 2). The sgACC is a component of the default mode network (DMN), which governs the resting state of the brain. Some studies have revealed decreased DMN deactivation during task-related experiments in patients with schizophrenia [57,58] (Figure 2A). However, there is continued debate over DMN pathology in schizophrenia [58]. There is more consensus about dopamine dysregulation in schizophrenia. According to one model, aberrant excitatory hippocampal input into the NAc releases the Ventral Tegmental Area (VTA) from ventral pallidal inhibition, resulting in an excess of dopamine release back onto the Nac [59-62] (Figure 2B). There are proposals to target several parts of this system [44]. It is also possible to target the dopamine system directly. The substantia nigra represents a very wellconnected structure in the brain with roles in the dopamine system (pars compacta) as well as extensive projections to the thalamus (pars reticulata) [63]. In schizophrenia, cortical hypofunction leads to decreased striatal inhibition of the SN causing both increased dopamine release back onto the striatum as well as inhibition of the thalamus, further contributing to cortical hypofunction [63,64] (Figure 2C). Finally, the habenula’s inhibitory inputs to both the SN and VTA have led to questions regarding its role in schizophrenia, whereby habenular dysfunction begets excessive dopamine release from both of these structures [65,66] (Figure 2D).
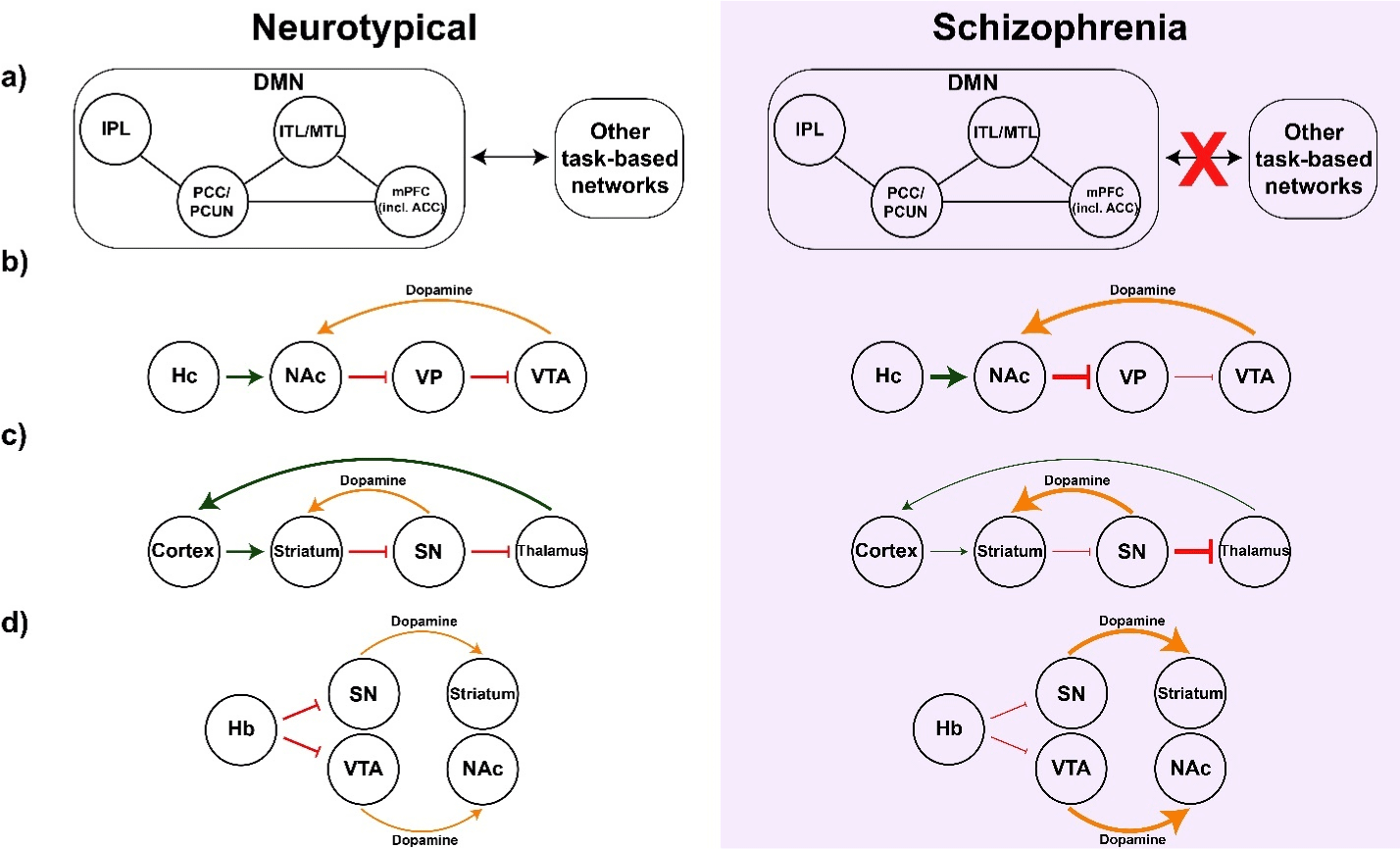
Figure 2: Circuit basis for each of the four DBS targets studied to date. Neurotypical circuits are shown in white and the changes with schizophrenia are shaded
in purple. a) Attending to a task requires switching from the resting state network (DMN) to networks more suitable for goal-directed behavior. Some research
shows that schizophrenia patients display less of this switching (red ‘X’), even when faced with a task. Interrupting the DMN through sgACC-DBS may facilitate
the activation of task-related networks. b) Hippocampal overactivity (bottom) increases NAc inhibition of the VP, thus increasing dopamine release from the VTA
back onto the NAc. c) Decreased cortical excitation of the striatum (bottom) results in excessive SNc dopamine release as well as increased SNr inhibition of
the thalamus, further decreasing cortical activity. d) Hb dysfunction (bottom) allows for unchecked dopamine release from the SNc and VTA. Key: green arrow –
excitation; red bar – inhibition; black, single-headed arrow – dopamine; thick symbols – excess; thin symbols – deficient. Abbreviations: Sz (schizophrenia); DMN
(default mode network); IPL (inferior parietal lobe); PCC (posterior cingulate cortex); PCUN (precuneus); ITL (inferior temporal lobe); MTL (mesial temporal lobe);
mPFC (medial prefrontal cortex); sgACC (subgenual anterior cingulate cortex); Hc (hippocampus); NAc (nucleus accumbens); VP (ventral pallidum); VTA (ventral
tegmental area); SNr/SNc (substantia nigra pars reticulata/compacta); Hb (habenula).
Outcomes
Length of stimulation ranged from 6-20 months, with a mean of 11.9 ± 3.6 months. Across the ten patients, outcomes were largely positive (Table 1). Nine patients experienced improvement in their total respective outcome measures while one experienced a worsening of symptoms. This patient displayed improvements of 10.8% and 20.3% in their PANSS score at 6- and 7-month followup visits, respectively. However, at 10 months they were withdrawn from the study due to hospitalization for a psychotic episode. Their PANSS score had dropped 9.5% from the pre-stimulation baseline with a 69.2% increase in positive symptoms as well as a mild increase in negative symptoms (4.3%) [56].
Article
N
Age range (% female)
Target(s)
Length of follow-up
Primary outcome measure(s)
Mean score improvement (%)
Corripioet al. [39]
7*
34-54 (57%)
Bilateral NAc (4*), Bilateral sgACC (4)
9-20 months
PANSS
30.8% (NAc), 23.5% (sgACC)
Wang et al. [43]
2
21, 26 (0%)
Bilateral Hb
12, 10 months
PANSS
11.1%
Cascellaet al. [42]
1
35 (100%)
Bilateral SNr
6 months
BPRS, SANS†
52.4% (BPRS)
NAc = nucleus accumbens; sgACC = subgenual anterior cingulate cortex; Hb = habenula; SNr = substantia nigra pars reticulata; PANSS = Positive And Negative Syndrome Scale; BPRS = Brief Psychiatric Rating Scale; SANS = Scale for the Assessment of Negative Symptoms.
*One patient was implanted but did not undergo stimulation secondary to surgical complications.
†The full SANS score for this patient was not reported.
Table 1: Characteristics of articles reviewed.
Among the nine patients who demonstrated lower total outcome measures at the end of follow-up, there were a few who exhibited variable improvements in positive and negative symptoms. One patient implanted in the sgACC and one implanted in the NAc displayed increases in their negative PANSS scores [52]. The patient implanted in the SNr experienced a large reduction in their positive symptoms (Table 1), but only slight reductions in some of their negative symptoms. This patient was also found to have decreased performance (verbal and visuospatial learning/memory) on some aspects of cognitive testing and improved performance (verbal fluency) on others (55).
Only three (1 NAc, 2 sgACC) of the seven patients from the randomized trial entered the crossover phase, where patients received 12 weeks of stimulation followed by 12 weeks of no stimulation or vice versa. They were randomized such that one patient (NAc) began with 12 weeks of stimulation and the other two (sgACC) began with no stimulation. The two patients (sgACC) that entered the crossover phase in the no stimulation group both experienced worsening of symptoms when stimulation was turned off, and had to be withdrawn from the study before completing the crossover. The third patient, who began the crossover phase with stimulation, demonstrated a worsening of negative symptoms when stimulation was turned off, but still completed the trial. However, this change persisted, even after stimulation was turned back on. A fourth patient (NAc) also met symptomatic criteria for the crossover phase but declined to participate. Earlier in the study, it was discovered that this patient’s stimulation had been accidentally turned off for several days and they had displayed a worsening of positive symptoms that improved when stimulation resumed. The four patients who had stimulation turned off all experienced a worsening of symptoms relative to when stimulation was on. This observation, together with the fact that nine of the ten patients across the three studies demonstrated an overall improvement during stimulation, suggest that DBS offers a promising therapeutic alternative in refractory schizophrenia patients
Quality Assessment
Assessment of the risk of bias for each study is detailed in (Table 2).
Selection bias
Performance bias
Attrition bias
Detection bias
Reporting bias
Article
Randomization?
Accounted for confounding?
Accounted for concurrent intervention/unintended exposure?
Fidelity to intervention protocol?
Missing data handled appropriately?
Interventions defined using reliable measures?
Outcomes defined using reliable measures?
Outcome assessors blinded?
Outcomes prespecified?
Corripioet al. [39]
Yes
No
No
Yes
No
Yes
Yes
No
Yes
Wang et al. [43]
N/A
No
No
Yes
No
Yes
Yes
No
No
Cascellaet al. [42]
N/A
No
No
Yes
N/A
Yes
Yes
No
No
Table 2: Risk of bias assessment.
Adverse Events
Adverse Events (AE) from the three studies are reported in (Table 3). In total, there were ten AEs across the eleven procedures. However, all ten AEs occurred in six patients. We classified five of the AEs as serious. All but one of the serious AEs (SAEs) occurred in the randomized trial [52], and three of them were in a single patient. This patient was planned for NAc DBS, but never received stimulation secondary to postoperative complications. They experienced a right internal capsule hemorrhage after surgery and subsequently developed an infection in the device, requiring complete hardware removal three months after surgery [52]. The patient did not suffer any permanent neurological deficits, however, they did have seizures that were controllable with antiepileptic medication. Another SAE,mood instability with occasional suicidal ideation, coincided with the patient’s discontinuation of their own antipsychotic regimen despite previously demonstrating symptom reduction with a combination of antipsychotics and DBS [52].
Transient AEs
Serious AEs
Article
No. of procedures
Event
No. of events
Time to resolution
Event
No. of events
Corripioet al. [52]
8
Akathisia
1
Not specified
Perioperative internal capsule hemorrhage
1
Positional dysesthesia
1
Not specified
Device infection
1
Confusion
1
4 days
Seizures requiring AEDs
1
Mood instability requiring hospitalization with occasional SI
1*
Wang et al. [56]
2
Aggravated psychotic episode requiring hospitalization
1
Cascellaet al. [55]
1
Increased appetite causing significant weight gain
1
3 months
Decreased performance on tests of verbal and visuospatial learning/memory
1
>6 months†
AE = adverse event; AED = antiepileptic drug; SI = suicidal ideation.
*This patient was hospitalized only once while having persistent mood instability. The number of episodes of SI was not specified. The authors note that this patient discontinued their antipsychotic medication immediately prior to displaying mood instability.
†This patient demonstrated decreased performance throughout the follow-up period but the authors remained uncertain as to whether this would continue.
Table 3: Adverse events reported in each study.
Discussion
This review explores the current literature on DBS in patients with schizophrenia to evaluate its safety and efficacy as an alternative treatment for individuals whose symptoms cannot be controlled with antipsychotic medication alone. Little progress has been made in this area despite widespread use of DBS in the realm of movement disorders for decades [67,68]. Although research evaluating DBS in psychiatric disorders lagged behind considerably, there has been an explosion of studies investigating the technique in multiple illnesses including depression [26], Obsessive Compulsive Disorder (OCD) [27], substance use disorder [28,29], and Tourette syndrome [30,31]. Studies on DBS as a treatment for schizophrenia have only just recently begun to publish their data, with the three articles reviewed in this work all published within the last two years [52,55,56]. The most recent of these [55], is part of a clinical trial currently underway (NCT02361554). Additionally, another clinical trial (NCT01725334) began in 2012 to treat the negative symptoms of schizophrenia, targeting both the nucleus accumbens/ventral striatum as well as the VTA. This trial was unfortunately discontinued due to an inability to enroll participants. It is not clear why this group was unable to enroll any patients. One possible contributor is that they sought patients with predominantly negative symptoms, which might decrease their desire to participate in the study of an unproven therapy. Additionally, Corripio and colleagues report that the most common reason patients were excluded from their trial was because they did not believe they required treatment. The failure of the prior trial underscores another major challenge that accompanies enrolling schizophrenic patients in research. There are inherent ethical issues associated with consenting patients with psychosis, which is further muddied by neurosurgery’s checkered past in treating psychiatric illnesses [69,70].
Despite existing hurdles and a lack of data, the early results are promising. Nine of the ten patients studied demonstrated sustained improvement in their respective outcome measures at the end of the follow-up period. The final patient had deteriorated at 10 months to where he was withdrawn from the study, but still displayed an improvement in symptoms at earlier time points [56]. The overall safety of the procedure itself mirrored that of studies assessing DBS for other indications [71] and in all but one case, stimulation was associated with an improvement in psychotic symptoms.
Further studies are necessary to determine the ideal target for stimulation. However, given the heterogeneity of the disease, it is certainly possible that different structures may be suitable depending on symptomatology. From the available data, we believe the NAc to be the most encouraging. Patients showed greater improvement with NAc versus sgACC stimulation in a side-by-side comparison, albeit in a small sample [52]. This is consistent with the current views on the circuitry involved in the pathophysiology of the disease where overactivity of the hippocampus increases the inhibitory tone from the NAc to the ventral pallidum, thus removing inhibition on the VTA. The lack of VTA inhibition results in increased dopamine release back onto the NAc and the positive symptoms of the disease [59-62].
Although there is a report of exacerbating psychotic symptoms at higher amplitudes when NAc stimulation was used to treat severe depression [72], the three patients from the randomized trial did not display worsening of symptoms despite receiving higher stimulation parameters in the Nac [52]. This also corroborates evidence from an earlier report of NAc stimulation for treatment of OCD in an individual with a history of psychosis [54]. The stimulation amplitude was again higher than in Graatet al. [72] and the patient did not display a worsening of psychotic symptoms.
The SNr presents another intriguing target. With GABAergic projections to the mediodorsal nucleus of the thalamus (MD) and thus, widespread connections to the prefrontal cortex, it is hypothesized to play a role in the pathophysiology of schizophrenia [73-77]. Results from the one patient who underwent SNr implantation appear to support this hypothesis, however, a larger sample and longer followup is necessary to investigate the cognitive deficits that accompanied treatment in this patient [55]. More data should be available within the next few years as the patient participated in an ongoing clinical trial (NCT02361554).
Chief among limitations of our study is the scarcity of existing evidence, making the prospect of a meta-analysis futile. In line with this, seven out of the ten patients we report are from the same trial which undoubtedly introduces bias into our results. Our review demonstrates both the promise of DBS for yet another psychiatric indication and also emphasizes the need for larger, randomized trials in order to facilitate target-specific analyses. Importantly, the authors support the use of DBS in conjunction with antipsychotics and cognitive behavioral therapy as these remain the cornerstones of treatment. All patients reported in this study were maintained on an antipsychotic regimen during DBS treatment, and to the best of our knowledge, there are no reports of treatment with DBS alone for schizophrenia. Finally, we believe that future studies will reveal multiple reasonably safe and effective targets for patients with schizophrenia and location selection will focus on the individual symptoms and goals of treatment for each patient.
Acknowledgments
The authors would like to thank Dr. Anissa Abi-Dargham, Dr. Raphael Davis, and Susan Fiore for their helpful discussion and feedback. We also thank the Department of Neurosurgery at Stony Brook University Hospital for supporting this research.
Statement of Ethics
An ethics statement is not applicable because this study is based exclusively on published literature.
Conflict of Interest Statement
The authors do not report any conflicts of interest.
Funding Sources
This work was funded by the Growing Convergence Research program (NSF Award 2021002), a FUSION-TRO award (63845) from the Renaissance School of Medicine at Stony Brook University, as well as SEED grant funding from the Office of the Vice President for Research at Stony Brook University.
Credit Author Statement
Jordan R. Saadon: Conceptualization, Investigation, Original draft writing, Review and editing, Visualization, Project administration. Cassie Wang: Investigation, Original draft writing, Review and editing, Visualization. Bayan Razzaq: Original draft writing, Review and editing. Regina Leilani Kuhia: Investigation, Original draft writing, Review and editing. Nathaniel A. Cleri Review and Editing. Michael Egnor: Review and editing, Supervision. Sima Mofakham: Conceptualization, Review and editing, Supervision, Project administration, Funding acquisition. Charles B. Mikell: Conceptualization, Review and editing, Supervision, Project administration, Funding acquisition.
Data Availability Statement
All data used in this study were obtained from previously published literature.
References
- Vos T, Abajobir AA, Abbafati C, Abbas K, Abate KH, Abd-Allah F, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet (London, England). 2017; 390: 1211-1259.
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders, fifth edition. 2013.
- Bowie CR, Harvey PD. Cognitive deficits and functional outcome in schizophrenia. Neuropsychiatric Disease and Treatment. 2006; 2: 531-536.
- Addington J, Addington D. Neurocognitive and social functioning in schizophrenia: a 2.5 year follow-up study. Schizophrenia Research. 2000; 44: 47-56.
- Roth S. The seemingly ubiquitous depression following acute schizophrenic episodes, a neglected area of clinical discussion. The American journal of psychiatry. 1970; 127: 51-58.
- Goodwin R, Lyons JS, McNally RJ. Panic attacks in schizophrenia. Schizophrenia Research. 2002; 58: 213-220.
- Buckley PF. Prevalence and consequences of the dual diagnosis of substance abuse and severe mental illness. The Journal of clinical psychiatry. 2006; 67: 5-9.
- Pokos V, Castle DJ. Prevalence of Comorbid Anxiety Disorders in Schizophrenia Spectrum Disorders: A Literature Review. Curr Psychiatry Rev. 2006; 2: 285–307.
- Palmer BA, Shane Pankratz V, Bostwick JM. The Lifetime Risk of Suicide in Schizophrenia. Archives of General Psychiatry. 2005; 62: 247.
- Olfson M, Gerhard T, Huang C, Crystal S, Stroup TS. Premature Mortality Among Adults With Schizophrenia in the United States. JAMA psychiatry. 2015; 72: 1172.
- CARLSSON A, LINDQVIST M. EFFECT OF CHLORPROMAZINE OR HALOPERIDOL ON FORMATION OF 3METHOXYTYRAMINE AND NORMETANEPHRINE IN MOUSE BRAIN. Acta pharmacologica et toxicologica. 1963; 20: 140-144.
- Laruelle M, Abi-Dargham A, Dyck CHV, Gil R, D’Souza CD, Erdos J, et al. Single photon emission computerized tomography imaging of amphetamineinduced dopamine release in drug-free schizophrenic subjects. Proceedings of the National Academy of Sciences of the United States of America. 1996; 93: 9235-9240.
- Abi-Dargham A, Rodenhiser J, Printz D, Zea-Ponce Y, Gil R, Kegeles LS, et al. Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proceedings of the National Academy of Sciences of the United States of America. 2000; 97: 8104-8109.
- Davis KL, Kahn RS, Ko G, Davidson M. Dopamine in schizophrenia: a review and reconceptualization. The American journal of psychiatry. 1991; 148: 1474-1486.
- Kapur S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. The American journal of psychiatry. 2003; 160: 13-23.
- American Psychiatric Association. The American Psychiatric Association Practice Guideline for the Treatment of Patients with Schizophrenia, Third Edition. American Psychiatric Pub; 2020. 336.
- Miyamoto S, Miyake N, Jarskog LF, Fleischhacker WW, Lieberman JA. Pharmacological treatment of schizophrenia: a critical review of the pharmacology and clinical effects of current and future therapeutic agents. Molecular Psychiatry. 2012; 17: 1206-1227.
- Lehman AF, Lieberman JA, Dixon LB, McGlashan TH, Miller AL, Perkins DO, et al. Practice guideline for the treatment of patients with schizophrenia, second edition. The American journal of psychiatry. 2004; 154: 1-63.
- Lally J, Ajnakina O, Forti MD, Trotta A, Demjaha A, Kolliakou A, et al. Two distinct patterns of treatment resistance: clinical predictors of treatment resistance in first-episode schizophrenia spectrum psychoses. Psychological Medicine. 2016; 46: 3231-3240.
- Demjaha A, Lappin JM, Stahl D, Patel MX, MacCabe JH, Howes OD, et al. Antipsychotic treatment resistance in first-episode psychosis: prevalence, subtypes and predictors. Psychological Medicine. 2017; 47: 1981-1989.
- Howes OD, McCutcheon R, Agid O, Bartolomeis AD, Beveren NJMV, Birnbaum ML, et al. Treatment-Resistant Schizophrenia: Treatment Response and Resistance in Psychosis (TRRIP) Working Group Consensus Guidelines on Diagnosis and Terminology. The American journal of psychiatry. 2017; 174: 216-229.
- Alvir JM, Lieberman JA, Safferman AZ, Schwimmer JL, Schaaf JA. Clozapineinduced agranulocytosis. Incidence and risk factors in the United States. The New England journal of medicine. 1993; 329: 162-167.
- McEvoy JP, Lieberman JA, Stroup TS, Davis SM, Meltzer HY, Rosenheck RA, et al. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. The American journal of psychiatry. 2006; 163: 600-10.
- Kahn RS, Rossum IWV, Leucht S, McGuire P, Lewis SW, Leboyer M, et al. Amisulpride and olanzapine followed by open-label treatment with clozapine in first-episode schizophrenia and schizophreniform disorder (OPTiMiSE): a three-phase switching study. The lancet. Psychiatry. 2018; 5: 797-807.
- Desai P, Lawson KA, Barner JC, Rascati KL. Estimating the direct and indirect costs for community-dwelling patients with schizophrenia. Journal of Pharmaceutical Health Services Research. 2013; 4: 187-194.
- Dandekar MP, Fenoy AJ, Carvalho AF, Soares JC, Quevedo J. Deep brain stimulation for treatment-resistant depression: an integrative review of preclinical and clinical findings and translational implications. Molecular Psychiatry. 2018; 23: 1094-1112.
- Rapinesi C, Kotzalidis GD, Ferracuti S, Sani G, Girardi P, Casale AD. Brain Stimulation in Obsessive-Compulsive Disorder (OCD): A Systematic Review. Current Neuropharmacology. 2019; 17: 787-807.
- Coles AS, Kozak K, George TP. A review of brain stimulation methods to treat substance use disorders. The American Journal on Addictions. 2018; 27: 71-91.
- Hassan O, Phan S, Wiecks N, Joaquin C, Bondarenko V. Outcomes of deep brain stimulation surgery for substance use disorder: a systematic review. Neurosurgical Review. 2020; 44: 1967-1976.
- Baldermann JC, Schüller T, Huys D, Becker I, Timmermann L, Jessen F, et al. Deep Brain Stimulation for Tourette-Syndrome: A Systematic Review and Meta-Analysis. Brain Stimulation. 2016; 9: 296-304.
- Wehmeyer L, Schüller T, Kiess J, Heiden P, Visser-Vandewalle V, Baldermann JC, et al. Target-Specific Effects of Deep Brain Stimulation for Tourette Syndrome: A Systematic Review and Meta-Analysis. Frontiers in Neurology. 2021; 12.
- Garcia L, Audin J, D’Alessandro G, Bioulac B, Hammond C. Dual Effect of High-Frequency Stimulation on Subthalamic Neuron Activity. The Journal of Neuroscience. 2003; 23: 8743-8751.
- McConnell GC, So RQ, Hilliard JD, Lopomo P, Grill WM. Effective Deep Brain Stimulation Suppresses Low-Frequency Network Oscillations in the Basal Ganglia by Regularizing Neural Firing Patterns. The Journal of Neuroscience. 2012; 32: 15657-15668.
- Spencer KM, Nestor PG, Niznikiewicz MA, Salisbury DF, Shenton ME, McCarley RW. Abnormal Neural Synchrony in Schizophrenia. The Journal of Neuroscience. 2003; 23: 7407-7411.
- Uhlhaas PJ, Singer W. High-frequency oscillations and the neurobiology of schizophrenia. Dialogues in Clinical Neuroscience. 2013; 15: 301-313.
- Tanaka-Koshiyama K, Koshiyama D, Miyakoshi M, Joshi YB, Molina JL, Sprock J, et al. Abnormal Spontaneous Gamma Power Is Associated With Verbal Learning and Memory Dysfunction in Schizophrenia. Frontiers in Psychiatry. 2020; 11.
- von Stein A, Sarnthein J. Different frequencies for different scales of cortical integration: from local gamma to long range alpha/theta synchronization. Int J Psychophysiol. 2000; 38: 301–13.
- Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nature Reviews Neuroscience. 2010; 11: 100-113.
- Tikka SK, Yadav S, Nizamie SH, Das B, Tikka DL, Goyal N. Schneiderian First Rank Symptoms and Gamma Oscillatory Activity in Neuroleptic Naïve First Episode Schizophrenia: A 192 Channel EEG Study. Psychiatry Investigation. 2014; 11: 467.
- Baradits M, Kakuszi B, Bálint S, Fullajtár M, Mód L, Bitter I, et al. Alterations in resting-state gamma activity in patients with schizophrenia: a high-density EEG study. European Archives of Psychiatry and Clinical Neuroscience. 2018; 269: 429-437.
- Koshiyama D, Miyakoshi M, Tanaka-Koshiyama K, Joshi YB, Sprock J, Braff DL, et al. Abnormal phase discontinuity of alpha- and theta-frequency oscillations in schizophrenia. Schizophrenia Research. 2021; 231: 73-81.
- HEATH RG, MONROE RR, MICKLE WA. Stimulation of the amygdaloid nucleus in a schizophrenic patient. The American journal of psychiatry. 1955; 111: 862-863.
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. The BMJ. 2021; 372: n71.
- Mikell CB, McKhann GM, Segal S, McGovern RA, Wallenstein MB, Moore H. The Hippocampus and Nucleus Accumbens as Potential Therapeutic Targets for Neurosurgical Intervention in Schizophrenia. Stereotactic and Functional Neurosurgery. 2009; 87: 256-265.
- Mikell CB, Sinha S, Sheth SA. Neurosurgery for schizophrenia: an update on pathophysiology and a novel therapeutic target. Journal of neurosurgery. 2016; 124: 917-928.
- Agarwal P, Sarris CE, Herschman Y, Agarwal N, Mammis A. Schizophrenia and neurosurgery: A dark past with hope of a brighter future. Journal of Clinical Neuroscience. 2016; 34: 53-58.
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia bulletin. 1987; 13: 261-276.
- Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychol Rep. 1962; 10: 799–812.
- Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS): Conceptual and Theoretical Foundations. British Journal of Psychiatry. 1989; 155: 49-52.
- Viswanathan M, Patnode CD, Berkman ND, Bass EB, Chang S, Hartling L, et al. Assessing the Risk of Bias in Systematic Reviews of Health Care Interventions. In: Methods Guide for Effectiveness and Comparative Effectiveness Reviews. Rockville (MD): Agency for Healthcare Research and Quality (US); 2017.
- Corripio I, Sarró S, McKenna PJ, Molet J, álvarez E, Pomarol-Clotet E, et al. Clinical Improvement in a Treatment-Resistant Patient With Schizophrenia Treated With Deep Brain Stimulation. Biological Psychiatry. 2016; 80: e69-e70.
- Corripio I, Roldán A, Sarró S, McKenna PJ, Alonso-Solís A, Rabella M, et al. Deep brain stimulation in treatment resistant schizophrenia: A pilot randomized cross-over clinical trial. EBioMedicine. 2020; 51: 102568.
- Roldán A, Portella MJ, Sampedro F, Alonso-Solís A, Sarró S, Rabella M, et al. Brain metabolic changes in patients with treatment resistant schizophrenia treated with deep brain stimulation: A series of cases. Journal of psychiatric research. 2020; 127: 57-61.
- Plewnia C, Schober F, Rilk A, Buchkremer G, Reimold M, Wächter T, et al. Sustained improvement of obsessive-compulsive disorder by deep brain stimulation in a woman with residual schizophrenia. The international journal of neuropsychopharmacology. 2008; 11: 1181.
- Cascella N, Butala AA, Mills K, Kim MJ, Salimpour Y, Wojtasievicz T, et al. Deep Brain Stimulation of the Substantia Nigra Pars Reticulata for Treatment- Resistant Schizophrenia: A Case Report. Biological Psychiatry. 2021; 90: e57-e59.
- Wang Y, Zhang C, Zhang Y, Gong H, Li J, Jin H, et al. Habenula deep brain stimulation for intractable schizophrenia: a pilot study. Neurosurgical focus. 2020; 49: E9.
- Pomarol-Clotet E, Salvador R, Sarró S, Gomar J, Vila F, Martínez A, et al. Failure to deactivate in the prefrontal cortex in schizophrenia: dysfunction of the default mode network?. Psychological Medicine. 2008; 38: 1185-1193.
- Hu M, Zong X, Mann JJ, Zheng J, Liao Y, Li Z, et al. A Review of the Functional and Anatomical Default Mode Network in Schizophrenia. Neuroscience Bulletin. 2016; 33: 73-84.
- Grace AA. Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: A hypothesis for the etiology of schizophrenia. Neuroscience. 1991; 41: 1-24.
- Floresco SB, Todd CL, Grace AA. Glutamatergic Afferents from the Hippocampus to the Nucleus Accumbens Regulate Activity of Ventral Tegmental Area Dopamine Neurons. The Journal of Neuroscience. 2001; 21: 4915-4922.
- Lodge DJ, Grace AA. Aberrant Hippocampal Activity Underlies the Dopamine Dysregulation in an Animal Model of Schizophrenia. The Journal of Neuroscience. 2007; 27: 11424-11430.
- Schobel SA, Lewandowski NM, Corcoran CM, Moore H, Brown T, Malaspina D, et al. Differential targeting of the CA1 subfield of the hippocampal formation by schizophrenia and related psychotic disorders. Archives of general psychiatry. 2009; 66: 938.
- Macpherson T, Hikida T. Role of basal ganglia neurocircuitry in the pathology of psychiatric disorders. Psychiatry and Clinical Neurosciences. 2019; 73: 289-301.
- Yoon JH, Minzenberg MJ, Raouf S, D’Esposito M, Carter CS. Impaired Prefrontal-Basal Ganglia Functional Connectivity and Substantia Nigra Hyperactivity in Schizophrenia. Biological Psychiatry. 2013; 74: 122-129.
- Sandyk R. Relevance of the habenular complex to neuropsychiatry: a review and hypothesis. The International journal of neuroscience. 1991; 61: 189- 219.
- Fakhoury M. The habenula in psychiatric disorders: More than three decades of translational investigation. Neuroscience & Biobehavioral Reviews. 2017; 83: 721-735.
- Benabid AL, Pollak P, Hoffmann D, Gervason C, Hommel M, Perret JE, et al. Long-term suppression of tremor by chronic stimulation of the ventral intermediate thalamic nucleus. The Lancet. 1991; 337: 403-406.
- Larson PS. Deep Brain Stimulation for Movement Disorders. Neurotherapeutics. 2014; 11: 465-474.
- El-Hai J. The lobotomist: A Maverick medical genius and his tragic quest to rid the world of mental illness [Internet]. 2005.
- Cleary DR, Ozpinar A, Raslan AM, Ko AL. Deep brain stimulation for psychiatric disorders: where we are now. Neurosurgical focus. 2015; 38: E2.
- Youngerman BE, Chan AK, Mikell CB, McKhann GM, Sheth SA. A decade of emerging indications: deep brain stimulation in the United States. Journal of neurosurgery. 2016; 125: 461-471.
- Graat I, Bergfeld IO, Koning PD, Vulink N, Schuurman PR, Denys D, et al. Delusions following deep brain stimulation of the nucleus accumbens. Brain Stimulation. 2019; 12: 770-771.
- Miyamoto Y, Jinnai K. The inhibitory input from the substantia nigra to the mediodorsal nucleus neurons projecting to the prefrontal cortex in the cat. Brain Research. 1994; 649: 313-318.
- Deniau JM, Mailly P, Maurice N, Charpier S. The pars reticulata of the substantia nigra: a window to basal ganglia output. Progress in brain research. 2007; 160: 151-172.
- Yoon JH, Minzenberg MJ, Ursu S, Walter BSR, Wendelken C, Ragland JD, et al. Association of dorsolateral prefrontal cortex dysfunction with disrupted coordinated brain activity in schizophrenia: relationship with impaired cognition, behavioral disorganization, and global function. The American journal of psychiatry. 2008; 165: 1006-1014.
- Tanibuchi I, Kitano H, Jinnai K. Substantia nigra output to prefrontal cortex via thalamus in monkeys. I. Electrophysiological identification of thalamic relay neurons. Journal of neurophysiology. 2009; 102: 2933-2945.
- Antal M, Beneduce BM, Regehr WG. The Substantia Nigra Conveys Target- Dependent Excitatory and Inhibitory Outputs from the Basal Ganglia to the Thalamus. The Journal of Neuroscience. 2014; 34: 8032-8042.