Research Article
Austin J Sleep Disord. 2014;1(1): 5.
Biomimetic Oral Appliance Therapy in Adults with Mild to Moderate Obstructive Sleep Apnea
Singh GD1*, Griffin TM2 and Chandrashekhar R3
1BioModeling Solutions, Inc., Beaverton, USA
2Emerald Coast Dental Sleep Medicine, Panama City Beach, USA
3Sleep Medicine, Ravindra Chandrashekhar Inc., Victorville, USA
*Corresponding author: Prof. Singh GD, BioModeling Solutions, Inc., Cornell Oaks Corporate Center, 15455 NW Greenbrier Parkway, Commons Building, Suite 250, Beaverton, OR 97006, USA
Received: August 29, 2014; Accepted: October 11, 2014; Published: October 16, 2014
Abstract
Introduction: For the management of obstructive sleep apnea (OSA) in adults, some professionals prescribe continuous positive airway pressure (CPAP) while others prefer mandibular advancement devices (MADs). However, both CPAP and MADs represent life-long therapy. In this study, we investigated the use of a biomimetic oral appliance system (the DNA appliance® system) to test the hypothesis that the upper airway can be improved in adults that have been diagnosed with OSA.
Methods and Sample: We recruited 10 consecutive adults for this study who underwent an overnight sleep study, which was interpreted by a sleep physician. Subjects diagnosed with mild to moderate OSA were treated using biomimetic oral appliance therapy (BOAT). Each subject had monthly follow-up visits, including examinations for progress and adjustments of the devices. The mean AHI of the sample was calculated prior to and after BOAT with no appliance in the mouth. The findings were subjected to statistical analysis.
Results: The mean treatment time was 8.7 mos. ± 5.8. Prior to treatment the mean AHI was 13.2 ± 7.2. The mean AHI fell by 65.9% to 4.5 ± 3.6 (p = 0.021) after BOAT with nothing in the mouth when the final overnight sleep study was performed.
Conclusion: This preliminary study suggests that BOAT may be able to reduce the AHI to within normal limits perhaps to the extent that life-long therapy may not potentially be necessary. However, long-term follow up is needed to determine whether these subjects need a maintenance program to retain their initial upper airway improvements.
Keywords: Oral appliance therapy; Biomimetic; Obstructive sleep apnea; Mandibular advancement device
Abbreviations
OSA: Obstructive Sleep Apnea; MAD: Mandibular Advancement Device; CPAP: Continuous Positive Airway Pressure; AHI: Apnea-hypopnea Index; DNA appliance: Daytime-Nighttime Appliance; BOAT: Biomimetic Oral Appliance Therapy; TMJ: Temporo-mandibular Joint; SWS: Slow Wave Sleep; REM: Rapid Eye Movement
Introduction
For the management of obstructive sleep apnea (OSA) in adults, some healthcare professionals prefer to prescribe continuous positive airway pressure (CPAP) masks while others prefer mandibular advancement devices (MADs). White and Shafazand [1] assessed whether MADs had similar health outcomes to CPAP in the short term. In terms of the primary outcomes e.g. improvements in blood pressure, they reported no statistically significant difference between the two types of therapy. However, neither treatment lowered the blood pressure from baseline values in either group after one month of therapy. Thus, both CPAP therapy and MADs may represent lifelong use. But earlier, Aarab et al. [2] investigated the efficacy of both MAD and CPAP use. Their results indicated that while the initial improvements in the AHI remained stable over time within both groups, the AHI improved more in the CPAP group compared to the MAD group. In contrast, more patients withdrew from treatment due to side effects in the CPAP group compared to the MAD group. Nevertheless, while there is a large amount of evidence to support the use of MADs for the management of mild to moderate OSA, and while numerous cases have been treated successfully, there are some concerns about the unwanted side-effects of MADs. For example, Doff et al. [3] reported significant dental changes compared with CPAP use, concluding that MADs should be considered as a lifelong treatment with a risk of dental side effects. Earlier, Doff et al. [4] had found that, compared to CPAP, MADs are associated with increased, transient pain in the temporo-mandibular joint (TMJ) in the initial period of use, which they presumed would remain limited with long-term MAD use.
On the other hand, Tsuda et al. [5] used a questionnaire to study compliance and side effects of non-customized MADs. The majority of the study sample had previously used CPAP therapy. Approx. 80% of non-compliant subjects discontinued MAD use after about 3 months. The most frequent reasons for non-compliance with non-customized MADs were discomfort, dry mouth, excessive salivation and ill-fitting appliances. Thus, long-term or lifelong, non-customized MAD therapy may not be possible in all adults diagnosed with OSA that are CPAP-intolerant. On the other hand, de Almeida et al. [6] quantified compliance and side effects of customized MAD use after approx. 5.5 years in patients diagnosed with OSA. Over 60% of the sample was still complying with MAD therapy but there was no significant difference in the baseline and post-titration respiratory indices used to assess the success of treatment if the appliance was not worn while sleeping. The most frequent reasons why patients discontinued MAD use were discomfort, or the MAD had no effect and the subject used CPAP instead. Other side effects of customized MAD use included; dry mouth, tooth pain, jaw discomfort, and TMJ symptoms. Thus, subjects who were compliant with customized MAD for long periods of use had adequate improvements as long as they continued wearing the MAD.
Cohen-Levy et al. [7] measured forces created in patients wearing MADs. They reported an almost linear relation, with a mean force of approx. 1Nmm-1 of mandibular protrusion, and this level of force is similar to that used during adult orthodontic therapies. Thus, the force values recorded in that study may explain both the dental and skeletal side effects associated with long-term MAD use, in a possible dose-dependent effect. Conversely, biomimetics is a science that uses natural designs or mechanisms to solve human problems. Accordingly, in a manner similar to orthodontic correction, the judicious use of the vectors induced in oral appliance therapy might provide an alternative protocol for the resolution of OSA, with the upper airway being the target in mild to moderate cases. Therefore, the aim of this current study is to test the hypothesis that OSA can be resolved in adults using a novel protocol that utilizes biomimetic oral appliance therapy (BOAT).
Methods and Sample
After obtaining informed consent, 10 consecutive patients were recruited for this study. The rights of the subjects were protected by following the Declaration of Helsinki. Inclusion criteria were: adults aged >21yrs. diagnosed with mild to moderate OSA following an overnight sleep study that had been interpreted by a sleep physician; good oral appliance compliance; no history of hospitalization for craniofacial trauma or surgery; no congenital craniofacial anomalies, and a fully-dentate upper arch. The exclusion criteria included: age <21yrs.; lack of oral appliance compliance; active periodontal disease; tooth loss during treatment; poor oral hygiene, and systemic bisphosphonate therapy. The study protocol (#121310) was reviewed and approved by the institution’s review board.
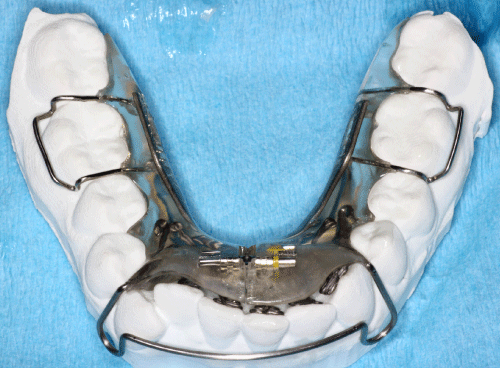
After careful history-taking and craniofacial examination, a bite registration was obtained in the upright-sitting position with corrected jaw posture in the vertical axis specific for each subject. Upper and lower polyvinyl siloxane impressions were also obtained. The upper model was then mounted on an articulator and the lower model was mounted relative to the upper model, using the bite registration captured in the physiologic rest position. Following a diagnosis of mild to moderate OSA, a biomimetic, upper Daytime- Nighttime Appliance (DNA appliance®; Figure 1) was prescribed for each subject. The biomimetic oral appliance therapy (BOAT) is designed to correct maxillo-mandibular hypoplasia in both children and adults [8-16]. The biomimetic oral appliance used in this study had: 6 (patented) anterior 3-D axial springs™, a beaded pharyngeal extension, a midline screw, bilateral occlusal coverage, retentive clasps, and a labial bow (Figure 1a). All subjects were instructed to wear the appliance during the evening and at nighttime (for approx. 12-16hrs. in total), but not during the day time and not while eating, partly in line with the circadian rhythm of tooth eruption [17] although this only occurs in children. Proffit [18] notes that an appliance needs to be worn for at least 8hrs.in the mouth to have a clinical effect. Written and verbal instructions were given to all subjects.
Figure 1a : The upper acrylic-based Daytime-Nighttime Appliance (DNA appliance®) that was used in this study consisted of: 6 (patented) anterior 3-D axial springs™; a midline jackscrew; bilateral posterior occlusal rests; bilateral retentive clasps, a short labial bow with U loops, and an adjustable, beaded pharyngeal extension.
The BOAT needed to be professionally-adjusted approximately every 4 weeks, and all subjects reported for review each month. At each monthly follow-up, examination for the progress of midfacial development was recorded. Adjustments to the devices were performed to optimize their efficacy. Only gentle pressures were transmitted to the teeth and surrounding tissues and the functionality of the device was checked with the subject activating a mild force on biting. The subjects were encouraged to maintain their treatment regimen as outlined at the outset. Development of the lower arch was implemented using a lower appliance (Figure 1b) to permit arch re-coordination. A lower appliance (Figure 1b) was implemented between 1 to 3 months after the upper appliance, depending on the subject’s progress. Every 3 months, the overnight sleep studies were repeated. The post-treatment sleep tests were done with no appliance in the mouth and were interpreted by a sleep physician. The mean apnea-hypopnea index (AHI) of the study sample was calculated prior to and after BOAT and the findings were subjected to statistical analysis, using paired t-tests.
Figure 1b: The lower acrylic-based Daytime-Nighttime Appliance (DNA appliance®) that was used in this study consisted of: 6 (patented) anterior 3-D axial springs™; a midline jackscrew; bilateral retentive clasps, and a short labial bow with U loops.
Results
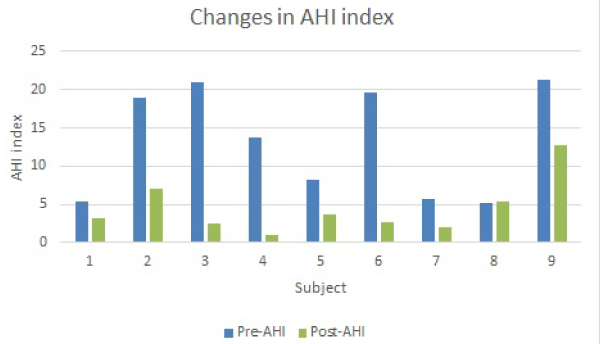
One subject was excluded from the study as the age was <21yrs, leaving a final sample of 6 females and 3 males. The mean age of the sample was approx. 54.5 yrs. and the treatment time of the study sample was 8.7 mos. ± 5.8. Prior to treatment the mean AHI of the sample was 13.2 ± 7.2. The mean AHI fell by 65.9% to 4.5 ± 3.6 (p = 0.021) after BOAT with no appliances in the mouth during sleep when the post-treatment sleep study was undertaken, indicating enhanced upper airway function. These results are summarized in Table 1 and Figure 2.
Figure 2: Graph showing changes in the apnea-hypopnea index (AHI) for the subjects included in this study.
Subject
Pre-treatment AHI
Post-treatment AHI
Treatment time (months)
A
5.4
3.1
16
B
18.9
7.1
19
C
21
2.5
13
D
13.7
1
7
E
8.2
3.7
4
F
19.7
2.7
7
G
5.7
2
4
H
5.1
5.4
4
I
21.3
12.8
4
Mean
13.2
4.5
8.7
Std. dev.
7.2
3.6
5.8
p value
0.021
Table 1: Summary of changes in the AHI after BOAT with no appliances in the mouth during sleep when the post-treatment sleep study was undertaken.
Discussion
Both CPAP therapy and MADs represent lifelong use for the treatment of OSA, but the upper airway is a complex, adaptive system, which can undergo remodeling in pathologic conditions [19]. Similarly, pneumatization following bone remodeling is a well-known craniofacial phenomenon [20-21], but none of these mechanisms have been applied to upper airway correction in patients with OSA. For example, Gindre et al. [22] investigated dose-effect, long-term use and tolerance of MADs used for OSA. When MAD therapy was started at 80% of maximum mandibular protrusion, the final titrated position resulted in a 70% decrease in AHI but 17% of the subjects showed no response. After 17 months of treatment, approx. 80% of patients were still using a MAD on almost all nights but the side effects reported, such as occlusal changes, were frequent. Chen et al. [23] investigated occlusal changes that occur after long-term MAD use. They found that a variety of occlusal alterations occurred with long-term MAD therapy, and that these changes could be regarded as adverse or beneficial, depending upon the particular case. For example, the mandibular arch width increased more than maxillary arch in some cases; crowding decreased in both arches in other cases; eruption occurred in the premolar area in others, while the lower posterior segment moved forward in relation to the maxillary arch in some patients. In addition, there were instances of decreased overbite (bite opening) in some cases with decreases in the overjet in others. Thus, rather than ignore or overlook these adaptive changes, in this present study we utilized a clinical protocol that putatively harnesses the corrective mechanisms of the craniofacial system, similar to orthodontic treatments. Indeed, increases in 3D midfacial bone volume after BOAT have been reported in adults [15] and initial studies confirming increased nasal cavity volumes have also been found after BOAT in adults [16]. Thus, the target of correction in this study is the upper airway, and the intention of this study was to determine whether BOAT might be advantageous as an alternative to MADs and CPAP in the management of patients with mild to moderate OSA.
Upper airway correction is associated with improved sleep architecture. For example, patients treated with CPAP for OSA have been reported with a rebound of slow wave sleep (SWS) and rapid eye movement (REM) sleep rebound, which results in an improvement in sleep quality [24]. Indeed, it appears that REM rebound, but not SWS rebound, is associated with CPAP compliance [25]. Although a 20% increase in REM sleep has been proposed as a threshold to identify REM rebound, one study reported >70% REM sleep of the total sleep time. However, the large REM rebound in that case could have been due to additive effects of CPAP therapy and suspension of anti-depressive treatment [26]. Nevertheless, while rebound of SWS and REM is observed in patients who are on CPAP therapy for OSA, neither has been objectively defined. But, rebound SWS and rebound REM can be predicted by abnormal sleep architecture/sleep fragmentation prior to the commencement of CPAP treatment [27]. Thus, it is possible that the results of our current study simply reflect the rebound phenomenon. Despite this contention, it should be noted that improvements in sleep quality in the absence of CPAP or MADs in patients diagnosed with OSA have never been reported in the literature to the best of our knowledge. Therefore, our preliminary results might represent an alternative to CPAP and MADs for the resolution of OSA.
It is known that patients report various degrees of compliance with CPAP and MADs. Almeida et al. [28] assessed patients’ preferences regarding treatment with either CPAP or MADs for OSA. The parameters assessed included: expectations and benefits of treatment, side effects, and other factors impacting treatment choice. Patient expectations included: improved overall health and sleep, elimination of OSA/reduced snoring, and reduced daytime fatigue. But, previous studies have shown that without continued MAD use, the underlying etiology of OSA is typically neither addressed nor corrected. For example, Gong et al. [29] investigated the length of treatment, long-term efficacy and safety of MADs in the treatment of OSA in Chinese subjects. About 15% had been treated for >10yrs. The longest treatment time was >12yrs., with a median of approx. 6yrs. Side effects were reported to be transient and included tooth soreness, dry mouth, occlusal changes and excessive salivation. In the long term, MAD therapy remained effective as long as the appliance was worn in the mouth while sleeping. For example, the AHI remained elevated at 25.5events/hr. without the appliance in the mouth after approx. 6yrs. of continuous therapy (although it was reduced to 4.2 with the appliance in the mouth while sleeping). In contrast, the results of our present study support the contention that the upper airway can be improved in adults to the extent that relatively short-term BOAT may potentially be successful in reducing the AHI to within normal limits, since no appliance was in the mouth when the post-treatment study was performed. If so, BOAT might represent an alternative to CPAP and MADs with the potential for maximum medical improvement in cases of mild to moderate OSA in adults.
To understand our results more precisely, other biochemical and polysomnographic data other than AHI ought to be included. For example, although the complete pathogenesis of OSA is not fully understood, the role of OSA in atherosclerosis development is important. Indeed, Ciccone et al. [30] found an increased carotid artery intima-media thickness in patients with long-standing OSA, which predisposed them to a higher risk of atherosclerosis. Similarly, Ciccone et al. [31] reported a correlation between intima-media thickness and inflammatory markers, such as C-reactive protein (CRP), interleukin (IL)-6, tumor necrosis factor (TNF)-a and pentraxin (PTX)-3, in the plasma of patients with OSA. More importantly perhaps, Brunetti et al. [32] demonstrated that OSA can impair endothelial function and thus worsen cardiovascular risk children. On the other hand, while CPAP therapy is able to improve endothelial function in patients with OSA [33], the role of MADs and BOAT on endothelial function and atherosclerosis remains unknown. Therefore, our current results need to be viewed with some caution as BOAT is a technique-sensitive protocol for upper airway correction. The lack of a control group is another limitation of this study (although there are no studies in the literature comparing patients with OSA on CPAP therapy with untreated controls). But our initial results are encouraging despite the limited sample size of this preliminary study, so BOAT may be a useful method of managing a selection of adults diagnosed with OSA. Nevertheless, long-term follow up is needed to confirm these initial findings, as well as assessments of craniofacial and upper airway modifications to determine the stability of the changes achieved.
References
- White DP, Shafazand S. Mandibular advancement device vs. CPAP in the treatment of obstructive sleep apnea: are they equally effective in Short term health outcomes? J Clin Sleep Med. 2013; 9: 971-972.
- Aarab G, Lobbezoo F, Heymans MW, Hamburger HL, Naeije M. Long-term follow-up of a randomized controlled trial of oral appliance therapy in obstructive sleep apnea. Respiration. 2011; 82: 162-168.
- Doff MH, Finnema KJ, Hoekema A, Wijkstra PJ, de Bont LG, Stegenga B. Long-term oral appliance therapy in obstructive sleep apnea syndrome: a controlled study on dental side effects. Clin Oral Investig. 2013; 17: 475-482.
- Doff MH, Veldhuis SK, Hoekema A, Slater JJ, Wijkstra PJ, de Bont LG, et al. Long-term oral appliance therapy in obstructive sleep apnea syndrome: a controlled study on temporomandibular side effects. Clin Oral Investig. 2012; 16: 689-697.
- Tsuda H, Almeida FR, Masumi S, Lowe AA. Side effects of boil and bite type oral appliance therapy in sleep apnea patients. Sleep Breath. 2010; 14: 227-232.
- de Almeida FR, Lowe AA, Tsuiki S, Otsuka R, Wong M, Fastlicht S, et al. Long-term compliance and side effects of oral appliances used for the treatment of snoring and obstructive sleep apnea syndrome. J Clin Sleep Med. 2005; 1: 143-152.
- Cohen-Levy J, Pételle B, Pinguet J, Limerat E, Fleury B. Forces created by mandibular advancement devices in OSAS patients: a pilot study during sleep. Sleep Breath. 2013; 17: 781-789.
- Singh GD, Lipka G. Case Report: Introducing the wireframe DNA applianceTM. J Am Acad Gnathol Orthop. 2009; 26:8-11.
- Singh GD, Wendling S, Chandrashekhar R. Midfacial development in adult obstructive sleep apnea. Dent Today. 2011; 30: 124-127.
- Singh GD, Utama J. Effect of the DNA ApplianceTM on migraine headache: case report. Int J Orthod Milwaukee. 2013; 24: 45-49.
- Singh GD, Cress SE. Craniofacial enhancement using a biomimetic oral appliance. Dent Today. 2013; 32: 92-94.
- Singh GD, Callister JD. Effect of a maxillary appliance in an adult with obstructive sleep apnea: a case report. Cranio. 2013; 31: 171-175.
- Singh GD, Ataii P. Combined DNA applianceTM and InvisalignTM therapy without interproximal reduction: A preliminary case series. J Clin Case Rep. 2013; 3: 1-3.
- Harris WG, Singh GD. Resolution of ‘gummy smile’ and anterior open bite using the DNA applianceTM: Case Report. J Amer Orthod Soc. 2013: 30-34.
- Singh GD, Heit T, Preble D. Changes in 3D midfacial parameters after biomimetic oral appliance therapy in adults. J Ind Orthod Soc. 2014; 48: 104-108.
- Singh GD, Heit T, Preble D. Chandrashekhar R. Changes in 3D nasal volume after biomimetic oral appliance therapy in adults. Sleep (Abstract Suppl.). 2014; 37: A150.
- Proffit WR, Frazier-Bowers SA. Mechanism and control of tooth eruption: overview and clinical implications. Orthod Craniofac Res. 2009; 12: 59-66.
- Proffit WR, Fields HW Jr., Sarver DM. In: Contemporary Orthodontics, 4th edn. Philadelphia: Elsevier. 2006.
- Gupta S, Hartley R, Khan UT, Singapuri A, Hargadon B, Monteiro W, et al. Quantitative computed tomography-derived clusters: redefining airway remodeling in asthmatic patients. J Allergy Clin Immunol. 2014; 133: 729-738.
- Savi de Carvalho R, Consolaro A, Francischone CE Jr, de Macedo Carvalho AP. Sinus augmentation by orthodontic movement as an alternative to a surgical sinus lift: A clinical report. J Prosthet Dent. 2014; 22. [Epub ahead of print].
- Saglam M, Akman S, Malkoç S, Hakki SS. Modification of maxillary sinus floor with orthodontic treatment and implant therapy: A case letter. J Oral Implantol. 2012. [Epub ahead of print].
- Gindre L, Gagnadoux F, Meslier N, Gustin JM, Racineux JL. Mandibular advancement for obstructive sleep apnea: dose effect on apnea, long-term use and tolerance. Respiration. 2008; 76: 386-392.
- Chen H, Lowe AA, de Almeida FR, Fleetham JA, Wang B. Three-dimensional computer-assisted study model analysis of long-term oral-appliance wear. Part 2. Side effects of oral appliances in obstructive sleep apnea patients. Am J Orthod Dentofacial Orthop. 2008; 134: 408-417.
- Verma A, Radtke RA, Van Landingham KE, King JH, Husain AM. Slow wave sleep rebound and REM rebound following the first night of treatment with CPAP for sleep apnea: correlation with subjective improvement in sleep quality. Sleep Med. 2001; 2: 215-223.
- Koo BB, Wiggins R, Molina C. REM rebound and CPAP compliance. Sleep Med. 2012; 13: 864-868.
- Lo Bue A, Salvaggio A, Insalaco G, Marrone O. Extreme REM Rebound during Continuous Positive Airway Pressure Titration for Obstructive Sleep Apnea in a Depressed Patient. Case Rep Med. 2014; 2014: 292181.
- Brillante R, Cossa G, Liu PY, Laks L. Rapid eye movement and slow-wave sleep rebound after one night of continuous positive airway pressure for obstructive sleep apnoea. Respirology. 2012; 17: 547-553.
- Almeida FR, Henrich N, Marra C, Lynd LD, Lowe AA, Tsuda H, et al. Patient preferences and experiences of CPAP and oral appliances for the treatment of obstructive sleep apnea: a qualitative analysis. Sleep Breath. 2013; 17: 659-666.
- Gong X, Zhang J, Zhao Y, Gao X. Long-term therapeutic efficacy of oral appliances in treatment of obstructive sleep apnea-hypopnea syndrome. Angle Orthod. 2013; 83: 653-658.
- Ciccone MM, Scicchitano P, Mitacchione G, Zito A, Gesualdo M, Caputo P, et al. Is there a correlation between OSAS duration/severity and carotid intima-media thickness? Respir Med. 2012; 106: 740-746.
- Ciccone MM, Scicchitano P, Zito A, Cortese F, Boninfante B, Falcone VA, et al. Correlation between inflammatory markers of atherosclerosis and carotid intima-media thickness in Obstructive Sleep Apnea. Molecules. 2014; 19: 1651-1662.
- Brunetti L, Francavilla R, Scicchitano P, Tranchino V, Loscialpo M, Gesualdo M, et al. Impact of sleep respiratory disorders on endothelial function in children. ScientificWorldJournal. 2013; 2013: 719456.
- Ciccone MM, Favale S, Scicchitano P, Mangini F, Mitacchione G, Gadaleta F, et al. Reversibility of the endothelial dysfunction after CPAP therapy in OSAS patients. Int J Cardiol. 2012; 158: 383-386.