
Research Article
Austin J Sleep Disord. 2015;2(1): 1005.
Clinical Efficacy of Walking on Nocturnal Polyuria and Sleep Quality with Benign Prostatic Hyperplasia Patients
Hitoshi Oh-oka*
Department of Urology, Kobe Medical Center, Japan
*Corresponding author: Hitoshi Oh-oka, Department of urology, Kobe Medical Center, 3-1-1, Nishiochiai, Suma-ku, Kobe-shi, Hyogo 654-0155, Japan
Received: December 21, 2014; Accepted: January 27, 2015; Published: January 29, 2015
Abstract
Purpose: We examined the efficacy of walking on nocturnal polyuria and sleep quality with benign prostatic hyperplasia patients as a first treatment intervention.
Patients and Methods: We conducted a prospective evaluation of 20 male patients for symptomatic nocturia (nocturnal polyuria) associated with benign prostatic hyperplasia. I advised to add more 60 min walking before dinner for 4 weeks without other lifestyle modifications. The frequency volume chart, International Prostate Symptom Score, fluid intake, patients’ treatment satisfaction, and Pittsburgh Sleep Quality Index were analyzed before and after treatment.
Results: Mean nighttime urine volume and nocturnal maximum voided volume improved significantly from 793 to 443 ml (p<0.001) and 267 to 315 ml (p=0.002), respectively. Mean nocturnal voids and hours of undisturbed sleep also improved from 2.3 to 1.6 times (p=0.025) and 3.0 to 4.4 hours (p<0.001), respectively. Patients’ treatment satisfaction and Pittsburgh Sleep Quality Index revealed an improvement from 7.2 to 4.3 (p<0.001) and 8.3 to 6.3 (global score; p<0.01). No statistical change was observed regarding water intake.
Conclusions: Walking before dinner was shown to be an effective non invasive treatment modality for patients with nocturnal polyuria with lower urinary tract symptoms associated with benign prostatic hyperplasia. Walking also improves sleep quality and patient satisfaction.
Keywords: Nocturnal polyuria; Lower urinary tract symptoms associated with benign prostatic hyperplasia; Walking; Sleep quality; Treatment satisfaction
Introduction
Nocturia is a common but nevertheless bothersome condition and the impact of nocturia might be substantial, especially in the elderly [1,2]. Nocturnal or global polyuria decreased nocturnal bladder capacity and sleep disorder might be the main cause of nocturia [3]. Other pathological factors which also contribute to nocturia include cardiovascular diseases, diabetes mellitus, autonomic nerve disorders as well as behavioral or environmental conditions [4]. Medical treatment with anticholinergics, desmopressin or diuretics was reported to be effective as management of nocturia (nocturnal polyuria; NP) [5,6], but these medications have the potential risk of adverse effects especially in the elderly. Modifications of lifestyle are sometimes mentioned as treatment modality of NP but their clinical efficacy has not fully been investigated [7,8]. General treatment strategy for nocturia or NP is pharmacological approach including anticholinergics [9], desmopressin [10], hypnosedatives [11], diuretics [12], and alpha-1 blocker (with BPH) [13]. However, these medications have many adverse effects such as; dry mouth, constipation for anticholinergics, hyponatremia for desmopressin [14], risk of falls for hypnosedatives [15], prolonged NP for diuretics [16], orthostatic hypotension for alpha-1 blocker, etc. Lifestyle modifications have also been considered as part of the management of NP associated nocturia [16,17]. Restriction of fluid, especially before retiring to bed seems sensible and recommended NP associated nocturia [18]. Lifestyle modifications like taking a walk in the evening would be cost-effective, is easy to perform with minimum adverse effects. But clinical efficacy of these strategy has not fully been investigated [7,8]. We examined clinical efficacy of walking in the evening or night on nocturia (nocturnal polyuria) and revealed many favorable outcomes on nocturia including improvement of sleep quality.
Materials and Methods
20 Japanese male patients with benign prostatic hyperplasia (BPH) who consulted the Department of Urology at our hospital between September 2012 and November 2012 were enrolled with nocturia as a chief complaint (2 times or more voids per night) (age; 69-80, mean 74±3.9 years). Their lower urinary tract symptoms (LUTS) associated with BPH (LUTS/BPH) were not severe within the previous 8 weeks (International Prostate Symptom Score; IPSS [19], were 19 or less), mean prostate volume was 23.2±5.5 (21.0- 35.7) ml, and residual urine volume was under 20 mL on abdominal ultrasound. All of them had no underlying disease and did not take any medication. They did not have liver dysfunction, renal dysfunction, or cardiovascular disease, and also had no neurological abnormalities and no limitation of movement. Their mean body mass index (BMI) was 22.5±3.9 kg/m2, morning systolic/diastolic blood pressure in our hospital was 135±9/82±8 mmHg. Their fluid intake was 25.5±4.7 ml/kg and was avoided in the evening (especially after dinner), and alcohol and caffeine consumption was also small (up to 12-ounces of beer (5-7% alcohol content), 2 cups of coffee, and 3 cups of green tea). They walked everyday in the morning (walking time; 30-58, mean 47±8.5min.) but not in the evening. We advised to add 60 min. extra walking preferably before dinner for 4 weeks without other lifestyle modifications (including fluid restrictions). Patients with bacterial cystitis, bacterial prostatitis, urinary tract cancer, hematuria, or proteinuria were excluded from this study.
The 3 day frequency volume chart (FVC), IPSS, IPSS quality of life (IPSS-QOL 0; delighted, 6; terrible), fluid intake, patients’ treatment satisfaction (PTS), and Pittsburgh Sleep Quality Index (PSQI) were analyzed before and the intervention. Regarding FVC analyses, daytime and nocturnal urine volume, daytime and nocturnal maximum voided volume (MVV), nocturnal voids, hours of undisturbed sleep (HUS), and nocturnal polyuria index (Npi; the ratio of nocturnal urine volume to 24 hour urine volume) were analyzed. PTS was defined as visual analogue scale (0; delighted, 10; terrible). The PSQI [20] is a self-rated questionnaire for evaluating subjective sleep quality. The evaluation consists of a global score and subscores (C1; Subjective sleep quality, C2; Sleep latency, C3; sleep duration, C4; Habitual sleep efficiency, C5; Sleep disturbance, C6; Use of sleeping medication, C7; Daytime dysfunction) and a global score larger than 5 is considered to indicate a sleep disturbance [21]. Approval was obtained from the Ethics Committee of Kobe Medical Center. Before enrollment, the patients were given a detailed explanation of the objectives and methods of the study and gave their consent in writing. Results were expressed as the mean±standard deviation (SD). For statistical analysis, Wilcoxon signed rank test and student’s t-test were used and p<0.05 was considered statistically significant.
Results
All the 20 patients were able to perform an additional evening walk of over 60±9 (50-69) minutes at their own pace with no adverse effects. After 4 weeks walking, their BMI, morning systolic/diastolic blood pressure in our hospital, and alcohol and caffeine consumption did not change significantly compared to before treatment. Mean daytime urine volume (DUV) and daytime MVV (DMVV) decreased significantly (p<0.001). Mean nocturnal urine volume (NUV) and nocturnal MVV (NMVV) also improved significantly (p<0.001, and p=0.002, respectively). Nocturnal voids (NV) reduced (p=0.025), HUS, and Npi improved (p<0.001). On the contrary, fluid intake did not decrease significantly from 25.5±4.7 to 25.0±4.3 ml/kg (Table 1). Regarding IPSS, total IPSS revealed statistically significant improvement (p<0.001). Storage, voiding and post-micturition symptom (sensation of not emptying bladder; IPSS item 1) of IPSS also improved except post-micturition symptom. Storage and voiding symptoms improved from 7.6±1.0 to 5.4±1.0 (p<0.001), and from 4.9±2.4 to 4.2±2.2 (p<0.001), respectively. IPSS-QOL and PTS also revealed marked statistically significant improvement (p<0.001) (Table 2).
DUV
(ml)
NUV
(ml)
DMVV
(ml)
NMVV
(ml)
NV (times)
HUS (hours)
Npi
(%)
Fluid intake (ml/kg)
Before
After
1292±240
1071±169
793±174
443±70
234±46
272±49
267±78
315±77
2.3±1.0
1.6±0.5
3.0±1.5
4.4±1.0
37.0±4.0
29.4±3.0
25.5±4.7
25.0±4.3
p value
<0.001
<0.001
<0.001
<0.002
<0.025
<0.001
<0.001
ns`
ns; not significant
Table 1: Daytime and nocturnal urine volume and maximum voided volume, nocturnal voids, hours of undisturbed sleep, Npi, and mean fluid intake.
IPSS
total score
IPSS
storage symptom subscore
IPSS
voiding symptom subscore
IPSS
post-micturition symptom subscore
IPSS-QOL
score
PTS
Before
After
13.2±3.4
10.0±2.9
7.6±1.0
5.4±1.0
4.9±2.4
4.2±2.2
0.7±0.7
0.4±0.5
4.3±0.5
3.0±0.5
7.2±0.8
4.3±0.5
p value
<0.001
<0.001
<0.001
ns
<0.001
<0.001
PTS; patients’ treatment satisfaction, ns; not significant
IPSS; Wilcoxon signed rank test, patients’ satisfaction (VAS); paired t test
Table 2: IPSS (total score, storage subscore, voiding subscore, post-micturition subscore), IPSS-QOL score, and patients’ treatment satisfaction.
Analyses of PSQI showed significant improvement of the global score (p<0.01). In subscores of PSQI, C1 (Subjective sleep quality), C4 (Habitual sleep efficiency), and C7 (Daytime dysfunction) improved significantly (p<0.01, p<0.05 and p<0.01, respectively) (Figure 1). C2 (Sleep latency) failed to show a significant change, subjective sleep latency improved statistically significant from 42±12 to 29±10 min. (p<0.01).
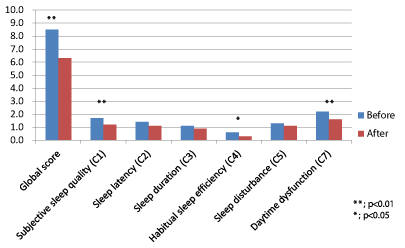
Figure 1: Analysis of Pittsburgh Sleep Quality Index; global score and
subscores.
All patients have indicated that they intend to continue morning and evening (especially evening) walking voluntarily also after the end of this clinical study.
Discussion
This study indicates that walking is effective for the improvement of nocturia (NP) in the elderly with LUTS/BPH. In this study, mean total fluid intake was 25.5±4.7 ml/kg and volume, time distribution of fluid intake was appropriate before treatment, we don’t comment in relation to fluid restriction [22]. After 4 weeks walking, their BMI, blood pressure, and alcohol and caffeine consumption did not change significantly compared to before treatment, their urine volume was reduced significantly compared to before treatment. One of the reasons for this result might be due to an increase in sweating and insensible fluid loss by walking. Sugaya et al. (2007) reported effects of walking exercise on nocturia in the elderly and commented that urinary frequency, blood pressures, body weight, body fat ratio, edema ratio, serum catecholamines, triglycerides, and total cholesterol were also decreased by exercise, suggesting that walking exercise could have an additional preventive effect on lifestyle-related diseases. But main cause regarding decreased nocturia might be the improvement of the sleep quality [23]. It was reported deeper sleep raises the arousal threshold and may increase the bladder capacity during sleep [24,25]. It is possible that decrease of nocturnal voids by walking is believed to be due to the induction of deeper sleep, that is almost the same mechanism of hypnosedative therapy for nocturia. They also commented it is possible that the decrease of catecholamine levels associated with walking exercise was also effective for nocturia by increasing the threshold bladder capacity that induced awaking [23] although we do not examine catecholamine levels. The findings of this study support above mentioned opinion in terms of improved sleep quality, increased daytime and nocturnal MVV, that result in better treatment satisfaction of patients with NP associated nocturia accompanied with LUTS/BPH. Limitation of this study is the selection of patients. They do not have large non-urological complications and they are willing to participate in a clinical trial voluntarily and with a high motivation. Another weakness of this study is the difference of water loss due to climate change.
This study was carried out in autumn, there are possibilities of different results if it was done in the summer or winter. Soda et al. (2010) reported that a daily fluid intake of 2% of body weight is enough for patients of symptomatic nocturia [26]. But fluid intake should be instructed in consideration of the water loss of the individual patient. It is necessary to take into account water loss due to temperature, humidity, and exercise.
Modifications of lifestyle including walking have the possibility to control symptoms and QOL for mild to moderate patients of LUTS/BPH with nocturia (NP). Urologists should analyze frequency of urination, distribution of urine volume, fluid intake and sleep quality more than before and advise lifestyle interventions prior to administration of patients with NP associated nocturia with LUTS/ BPH.
References
- Blanker MH, Bohnen AM, Groeneveld FP, Bernsen RM, Prins A, Ruud Bosch JL. Normal voiding patterns and determinants of increased diurnal and nocturnal voiding frequency in elderly men. J Urol. 2000; 164: 1201-1205.
- Asplund R. Nocturia in relation to sleep, somatic diseases and medical treatment in the elderly. BJU Int. 2002; 90: 533-536.
- Appell RA, Sand PK. Nocturia: etiology, diagnosis, and treatment. Neurourol Urodyn. 2008; 27: 34-39.
- Weiss JP, Blaivas JG, Stember DS, Chaikin DC. Evaluation of the etiology of nocturia in men: the nocturia and nocturnal bladder capacity indices. Neurourol Urodyn. 1999; 18: 559-565.
- Rackley R, Weiss JP, Rovner ES, Wang JT, Guan Z; 037 STUDY GROUP. Nighttime dosing with tolterodine reduces overactive bladder-related nocturnal micturitions in patients with overactive bladder and nocturia. Urology. 2006; 67: 731-736.
- Moon DG, Jin MH, Lee JG, Kim JJ, Kim MG, Cha DR. Antidiuretic hormone in elderly male patients with severe nocturia: a circadian study. BJU Int. 2004; 94: 571-575.
- Weiss JP, Blaivas JG. Nocturia. J Urol. 2000; 163: 5-12.
- Marinkovic SP, Gillen LM, Stanton SL. Managing nocturia. BMJ. 2004; 328: 1063-1066.
- Rackley R, Weiss JP, Rovner ES, Wang JT, Guan Z; 037 STUDY GROUP. Nighttime dosing with tolterodine reduces overactive bladder-related nocturnal micturitions in patients with overactive bladder and nocturia. Urology. 2006; 67: 731-736.
- Mattiasson A, Abrams P, Van Kerrebroeck P, Walter S, Weiss J. Efficacy of desmopressin in the treatment of nocturia: a double-blind placebo-controlled study in men. BJU Int. 2002; 89: 855-862.
- Marschall-Kehrel D. Update on nocturia: the best of rest is sleep. Urology. 2004; 64: 21-24.
- Pedersen PA1, Johansen PB. Prophylactic treatment of adult nocturia with bumetanide. Br J Urol. 1988; 62: 145-147.
- Paick JS, Ku JH, Shin JW, Yang JH, Kim SW. alpha-blocker monotherapy in the treatment of nocturia in men with lower urinary tract symptoms: a prospective study of response prediction. BJU Int. 2006; 97: 1017-1023.
- Weatherall M. The risk of hyponatremia in older adults using desmopressin for nocturia: a systematic review and meta-analysis. Neurourol Urodyn. 2004; 23: 302-305.
- Leipzig RM, Cumming RG, Tinetti ME. Drugs and falls in older people: a systematic review and meta-analysis: I. Psychotropic drugs. J Am Geriatr Soc. 1999; 47: 30-39.
- Marinkovic SP, Gillen LM, Stanton SL. Managing nocturia. BMJ. 2004; 328: 1063-1066.
- Weatherall M, Arnold T; New Zealand Nocturia Guideline Committee. Nocturia in adults: draft New Zealand guidelines for its assessment and management in primary care. N Z Med J. 2006; 119: U1976.
- Reynard J. Fluid balance therapy of nocturia in women. Int Urogynecol J Pelvic Floor Dysfunct. 1999; 10: 43-48.
- Homma Y, Tsukamoto T, Yasuda K, Ozono S, Yoshida M, Shinji M. Linguistic validation of Japanese version of International Prostate Symptom Score and BPH impact index. Nihon Hinyokika Gakkai Zasshi. 2002; 93: 669-680.
- Doi Y, Minowa M, Uchiyama M, Okawa M, Kim K, Shibui K, et al. Psychometric assessment of subjective sleep quality using the Japanese version of the Pittsburgh Sleep Quality Index (PSQI-J) in psychiatric disordered and control subjects. Psychiatry Res. 2000; 97: 165-172.
- Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989; 28: 193-213.
- Matthiesen TB, Rittig S, Nørgaard JP, Pedersen EB, Djurhuus JC. Nocturnal polyuria and natriuresis in male patients with nocturia and lower urinary tract symptoms. J Urol. 1996; 156: 1292-1299.
- Sugaya K, Nishijima S, Owan T, Oda M, Miyazato M, Ogawa Y. Effects of walking exercise on nocturia in the elderly. Biomed Res. 2007; 28: 101-105.
- Fujikawa K, Kasahara M, Matsui Y, Takeuchi H. Human atrial natriuretic peptide is a useful criterion in treatment of nocturia. Scand J Urol Nephrol. 2001; 35: 310-313.
- Takami N, Okada A. Triazolam and nitrazepam use in elderly outpatients. Ann Pharmacother. 1993; 27: 506-509.
- Soda T, Masui K, Okuno H, Terai A, Ogawa O, Yoshimura K. Efficacy of nondrug lifestyle measures for the treatment of nocturia. J Urol. 2010; 184: 1000-1004.