
Research Article
Austin J Sleep Disord. 2020; 7(1): 1036.
The Impact of Myo-Inositol Supplementation on Sleep Quality in Pregnant Women: A Randomized, Double- Blind, Placebo-Controlled Study
Mashayekh-Amiri S1, Delavar MA2*, Bakouei F2, Faramarzi M2 and Esmaeilzadeh S2
¹Student Committee Research, Babol University of Medical Sciences, Babol, Iran
²Department of Midwifery, Babol University of Medical Sciences, Babol, Iran
*Corresponding author: Mouloud Agajani Delavar, Department of Midwifery, Babol University of Medical Sciences, Babol, Iran
Received: May 29, 2020; Accepted: October 09, 2020; Published: October 16, 2020
Abstract
Myo-inositol, a glial marker which plays a critical role in some brain regions, can supposedly affect sleep processes. The aim of randomized controlled trial was to evaluate the impact of myo-inositol supplementation on sleep quality in pregnant women. This study enrolled 60 women with low risk singleton pregnancies, with a gestational age of at least 14 weeks, in Iran. The participants were block randomized to receive myo-inositol supplementation powder containing 2000 mg of myo-inositol and 200 μg of folic acid or placebo (400μg of folic acid) for 10 weeks from the gestational age of 14 to 24. Sleep quality was measured by the Pittsburgh Sleep Quality Index (PSQI). The primary outcome was the change in sleep quality from the first trimester to the second trimester (24-28 week). The results of ANCOVA boot strapped demonstrated that the mean deference of the total sleep quality score between the two groups was -1.537; 95% CI: -3.050 to -0.024, p=0.047) in the second trimester. Also, there were significant mean differences in subjective sleep quality, sleep duration and habitual sleep efficiency between the two groups at different times (-0.427; 95% CI: -0.725 to -0.128, p=0.006, (-0.427; 95% CI: -0.725 to -0.128; p=0.006 -0.670; 95% CI: -1.240 to -0.101; p=0.022, and 8.066; 95% CI: 1.077 to 15.054; p=0.025, respectively). The research confirmed that myo-inositol supplementation can improve pregnant women’s global sleep quality, subjective sleep quality, and sleep duration. Therefore, these findings applied to minimize the rate of poor sleep quality in pregnant women.
Keywords: Dietary supplements; Iinositol; Pregnant women; Sleep; Sleep hygiene
Abbreviations
PSQI: Pittsburgh Sleep Quality Index; ASSM: The American Academy of Sleep Medicine; SHI: Sleep Hygiene Index; BDI: Beck Depression Inventory; PSS: Perceived Stress Scale
Introduction
It is generally assumed that pregnancy is one of the most important events in a woman’s life. It is a natural process, accompanied by physiological and psychological changes, which can lead to changes in the pattern of sleep [1]. Sleep is presumably a physiological process to restore physical vitality and energy [2]; hence, people spend about one-third of their lives sleeping [3]. Poor sleep patterns in terms of quality and short sleep in terms of duration is the two common phenomena during pregnancy [4]. The quality and duration of sleep are commonly affected by several factors such as hormonal, physical, physiological, and behavioral changes during pregnancy [5]. A study conducted in Iran indicated that 87.2% of pregnant women suffered from sleep-related problems [6].
It is presumed that sleep-related problems could have negative consequences for both mother and fetus during pregnancy. These complications include daily dysfunction, maternal fatigue, obesity, gestational hypertension, preeclampsia, gestational diabetes mellitus, intrauterine growth restriction, low birth weight, and preterm birth. It could also be associated with caesarean section, prolonged labor, and the use of delivery instruments, depression during pregnancy, and postpartum depression [7-11].
It is also thought that pharmacological and non-pharmacological therapies can improve the quality of sleep. Due to the potential risks of drugs on the fetus and the limited number of both pharmacological and non-pharmacological methods, pregnant women are commonly recommended to use other methods such as acupressure, exercise, auricular therapy, massage or relaxation therapy, mindful meditation and yoga, and cognitive behavioral therapy (CBT) to promote the quality of sleep during their pregnancies [12].
The concept of sleep hygiene, applied to regulate sleep problems, was first used by Peter Hauri [13]. The midwives’ role is to maintain the physical and psychological health of pregnant women in prenatal care. One of the important needs of pregnant women is to promote sleep quality through sleep hygiene education and counseling regarding the normal sleep pattern, total sleep time, and sleep alternation during pregnancy [14]. The American Academy of Sleep Medicine (ASSM) [15], and a clinical guideline in the Journal of Clinical Sleep Medicine [16], do not recommend sleep hygiene education as an independent treatment due to the lack of sufficient evidence for sleep problems.
It is assumed that inositol has nine isomers, and that myo-inositol is one of the commonest stereoisomer of C6 sugar alcohol [17,18]. The myo-inositol is one of the secondary messengers in the signal transmission pathways, which can examine the metabolism of fats and sugar in the nerve cells and regulate the secretion of some glands [19,20]. Myo-inositol is also regarded as a glial marker. Generally, the glial brain cell is known as asytrocytes, which can regulate a number of vital functions in the brain [21,22].
In the same vein, animal studies have also found acute changes in sleep variables in animals with the dysfunction of asytrocytes cells, although glial function related to sleep in humans has not been examined yet [23]. The researchers speculate that glial function may have a critical role in brain regions involved with sleep processes [23,24]. Therefore, we hypothesized that myo-inositol, as a glial marker, could have a positive effect on sleep quality in pregnant women. To this end, the aim of this study was to determine the impact of myo-inositol supplementation on sleep quality in pregnant women.
Materials and Methods
Study Design
This study was a randomized, double-blind, placebo-controlled trial attempt to investigate the impact of myo-inositol supplementation on sleep quality. The study was conducted in prenatal clinics of Babol University of Medical Sciences, Babol, Iran from April 2018 to March 2019.
The inclusion criteria were as follows: conscious written consent, pregnant women with singleton pregnancy, age above 18, the gestational age of at least 14 weeks, the absence of chronic diseases such as diabetes, hypertension and cardiovascular diseases, no history of taking prenatal drugs before pregnancy, no history of sleep disorders before pregnancy, employed women without night shifts, no smoking, no alcohol use, no history of psychiatric problems, and no abortion in previous pregnancies. It is worth noting that women with severe depression or severe stress, those with the death experience of one of their first-class relatives during pregnancy, and those with changes in sleep patterns due to travel were excluded from the study.
The primary outcome was to measure sleep quality in the second trimester (24-28 weeks) between the intervention and control groups. The secondary outcome was to measure the score of sleep quality in the third trimester (37-38weeks), the side effects of drugs, and maternal and neonatal conditions at delivery.
Instruments
The tools for the implementation of this research included such socio-demographic characteristics and reproductive history as age, educational level, occupation, family income, monthly cost, the total number of pregnancies, and the total number of birth. We also used Pittsburgh Sleep Quality Index (PSQI), Sleep Hygiene Index (SHI), the Beck Depression Inventory (BDI), and perceived stress scale (PSS) in this study.
PSQI: It is assumed to be one of the most common methods of assessing the sleep status and quality [25]. This questionnaire included 19 items in seven different components including subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, the use of sleeping medication, and daytime dysfunction. The sum of seven component scores gives the total PSQI score. The response of each self-rated question scored between 0 and 3 according to the symptom frequency. The total score has a value in the range of 0-21. High values indicate a poor sleep quality. Having a total score below 5 (0-4 points) indicates that sleep quality is good, and a score of 5 or greater (5-21 points) indicates poor sleep quality. The Cronbach’s alpha of PSQI was reported 0.73 [26]. In Iran, Hossein Abadi et al. (2008), estimated the reliability (r = 0.88) and the reliability of the questionnaire via retest (r = 0.84) [27]. In addition, the Cronbach’s alpha of PSQI was calculated to be 0.75 in our study. The PSQI has a Cronbach’s alpha of 0.83.
SHI: It is a 13-item, self-report questionnaire, which measures sleep hyigene. Each item is graded based on a 5-point Likert’s scale ranging from 0 (never) to 4 (always). The total score ranges from 0–52, with a higher score representing lower sleep hygine. The SHI has been shown to have adequate reliability and validity in sleep hygiene research [28]. In a study by Chehri et al. (2015), the reliability of the Persian version of this questionnaire was established with a Cronbach’s alpha coefficient of 0.83. The Persian version of SHI has been used as a reliable and valid tool to assess sleep hygiene among Iranian women [29]. The a coefficient of sleep hygiene index was 0.66 in our study.
PSS: This scale was developed by Cohen et al. It is a 14-item selfreport questionnaire, which evaluates a person in terms of stress during the past months. It has a good validity, reliability, and stability over time. It is a 5-point Likert’s scale ranging from 0 (never) to 4 (very often). The maximum score in this test is 56, and the least is zero. The scale yielded a single score with high scores indicating higher levels of stress and lower levels indicating lower levels of stress [30]. PSS was translated into Iranian version and validated by [31]. Finally, the internal consistency of the PSS in our study was calculated at 0.86 using Cronbach alpha.
BDI: It is a 13-items self-report questionnaire, which is widely used in numerous studies designed to measure physical, behavioral, and cognitive symptoms of depression. Each item is rated on a fourpoint scale ranging from 0 to 3 point. The sum of 13 items gives the total score ranging from 0 to 39, and sets various degrees of depression from mild to severe (no=0-4, mild=5-7, moderate=8-15, severe=16-39) [32]. Rajabi et al. has shown that this questionnaire is a reliable and valid scale for screening depression among samples with psychiatric outpatients in Iran [33]. The a coefficient of the questionnaire in the current study was calculated to be 0.75.
Participants
Around 63 healthy pregnant women that met the inclusion criteria were asked to sign the written informed consent forms. At first, the women were instructed to complete the Beck Depression Inventory (BDI) and Perceived Stress Scale (PSS). The participants with severe depression (scored greater than 15) and severe stress (scored greater than 28) were excluded from the study.
Finally, 60 pregnant women with low risk singleton pregnancies with a gestational age of at least 14 weeks were requested to complete the socio-demographic questionnaire, PSQI, and SHI according to the instructions provided. Then, all participants received a sleep hygiene education, which was delivered to them through training and a booklet. The evidence regarding sleep hygiene education was collected through multiple databases and search engines. The content of the sleep hygiene, which was taken from academic search engine and several library databases, included such areas as maintaining regular hours of bedtime and arising, keeping the bedroom cool, dark, and quite, maintaining a regular exercise schedule, having a light dinner before bedtime, not drinking tea, coffee or caffeine consumption before sleep, and not using alcohol, cigarette, etc.
The participants were then randomly assigned into intervention and control groups. Randomization was accomplished through blocked randomization with a block size of 4 for the two groups (A, B). At first, blocks containing 4 of 2 treatments (myo-inositol and placebo) were written with different possible combinations, and each one was assigned a number. The sachets for the two groups, which were identical in appearance, were prepared by a single laboratory. The envelops were sealed and a number was assigned to each. Then, using a random number table, after the coding procedure (A or B), 70 sachets were placed in a box for each participant. The sachets were then given to the researcher and the randomization page was hidden. The researcher was unaware of the contents of the sachets, and the identities of the sachets were available only to the pharmacist. It should be noted that both the researchers and participants were blinded to the whole process (double-blind). Breaking blinding was possible in two cases: after the delivery and in case of severe side effects encountered. The women in the intervention group received a myo-inositol supplementation powder (the Inofulic commercial name, LO.LI. Pharma, Rome, Italy) containing 2000 mg of myoinositol and 200 μg of folic acid once daily before sleep per night (one hour a sachet is dissolved in a glass of water) for 10 weeks from the gestational age of 14 to 24 weeks. The control group received placebo containing 2000 mg of wheat flour and 200 μg of folic acid, identical in color, flavor and texture to the myo-inositol powder to be taken in the same dose and for the same duration of time. According to the World Health Organization protocol, all samples should receive one tablet containing 60 mg elemental iron (as 300 mg ferrous sulphate) from 14-16th week of gestation and should continue it until birth. Text messages or telephone calls were considered by the researcher to ensure the use of supplements during the study. Participants were also requested to bring the supplements box at each visit.
Sleep quality was evaluated through Pittsburgh Sleep Quality Index (PSQI) in the second trimester, in weeks [24-28], and in the third trimester, in weeks. In addition, the pregnancy outcomes and the occurrence of side effects caused by treatments such as the presence of nausea/vomiting, diarrhea, headache, uterine contractions, tiredness and flatulence were assessed during the follow-up visits. Maternal outcomes included: weight gain during pregnancy (expressed as kilogram based on cutoff 12), gestational age at delivery (expressed as the number of patients who delivered before ‘37 weeks’ and ‘37- 42 weeks’ of gestation), the mode of delivery (expressed as vaginal delivery and caesarean section incidence), gestational hypertension (defined as a systolic pressure of at least 140 mm Hg or a diastolic pressure of at least 90 mm Hg, and preterm delivery (expressed as number of participants delivered before 37 weeks’ gestation completed). Fetal and neonatal outcomes included: the birth weight expressed by grams and percentile, macrosomia (birth weight >4 kg), admission to Neonatal Intensive Care Unit (NICU), and shoulder dystocia. The participant drop-out rate was 4 (3 participants in myoinositol group and 1 in palacebo group) (Figure 1).
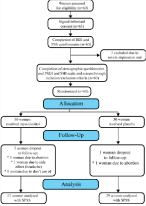
Figure 1:
Statistical Analysis
The analysis was done by IBM SPSS Statistics for Windows, version 21.0 (IBM Corp., Armonk, N.Y., USA). The normality of measured variables was tested by Kolmogorov-Smirnov test. The normality test has shown that the data were not normally distributed. Therefore bootstrap method for reporting P-value, and CI differences was used. The ANCOVA was performed to estimate the differences between groups on the outcome after adjusting for covariate variables such as sleep hygiene, perceived stress, depression, and sleep quality in the first trimester. Also, repeated measures analysis was used for comparing the follow-up variables between groups. p<0.05 was accepted as statistically significant. To minimize attrition bias, Intention to Treat Analysis (ITT) was used. The poor sleep quality (score ≥5) in the second trimester of pregnancy was compared between the two groups for the calculation of the number Needed-to- Screen (NNS). The NNT was calculated using Graphpad Quickcalcs (http://graphpad.com/quickcalcs/nnt1.cfm).
Results
In this double-blind clinical trial, 56 pregnant women (27 in myo-inositol group and 29 in the placebo group) completed both the treatment and follow up. The mean ± SD age for both the myoinositol group and the placebo group were 27.8 ± 4.1 and 29.4 ± 4.4, respectively. The two groups were homogenous with respect to age, educational status, occupation, monthly cost, family income, and gravidity (Table 1).
Characteristic
Myo-inositol group (N=27) N (%) or Mean (SD)
Placebo group (N=29) N (%) or Mean (SD)
Age, years
19-29
16(59.3)
13(44.8)
=30
11(40.7)
16(55.2)
Education
Under diploma
1(3.7)
1(3.4)
Diploma
8(29.6)
14(48.3)
Over diploma
18(66.7)
14(48.3)
Occupation
In work
22(81.5)
26(89.7)
Out work
5(18.5)
3(10.3)
Family income (IRR*)
<10000000
9(33.3)
10(34.5)
=10000000
18(66.7)
19(65.5)
Monthly cost (IRR*)
<10000000
7(25.9)
9(31.0)
=10000000
20(74.1)
20(69.0)
Total number pregnancy
1
15(55.6)
16(55.2)
3-Feb
12(44.4)
13(44.8)
Beck depression inventory (BDI)
No depression (0-4)
17(63.0)
23(79.3)
Mild (5-7)
4(14.8)
5(17.2)
Moderate (8-15)
6(22.2)
1(3.4)
Perceived stress scale (PSS)
21.1 ±4.7
20.8 ±9.7
Sleep hygiene index SHI)
9.7 ±5.6
8.7 ±4.7
*IRR = 0.00002 USD
Table 1: Socio-demographic characteristics, reproductive history of pregnant women in Babol city (n=60).
The results of ANCOVA boot strapped with covariates in the model demonstrated that the mean difference of the global sleep quality score between the two groups (myo-inositol group – placebo group) was -1.537; 95% CI: -3.050 to -0.024, p=0.047). In addition, there were significant mean differences in subjective sleep quality, sleep duration and habitual sleep efficiency between the two groups at different times (-0.427; 95% CI: -0.725 to -0.128, p=0.006, -0.670; 95% CI: -1.240 to -0.101, p=0.022, and 8.066; 95% CI: 1.077 to 15.054, p=0.025, respectively) (Table 2).
Sleep components
Time
(1)Myo-inositol group N=27 Mean ± SD
(2)Placebo group N=29 Mean ± SD
Mean Difference (1-2)
95% CI
P-value
Subjective sleep quality (component1)
Before intervention
1.15±0.46
1.00±0.38
-0.427
-0.725,-0.128
0.006
After intervention
0.96±0.52
1.31±0.66
Sleep latency
Before intervention
1.85±1.66
1.93±1.75
0.038
-0.388, 0.464
0.859
(component2)
After intervention
1.30±0.86
1.31±1.03
Sleep duration (component3)
Before intervention
0.55±0.89
0.38±0.62
-0.67
-1.240,-0.101
0.022
After intervention
0.74±0.98
1.24±1.15
Habitual sleep efficiency (component4)
Before intervention
88.40±9.55
85.20±9.49
8.066
1.077, 15.054
0.025
After intervention
87.25±10.44
78.75±14.87
Sleep disturbances (component5)
Before intervention
5.81±4.21
5.93±3.71
-0.827
-2.599, 0.944
0.353
After intervention
6.89±4.11
7.00±4.23
Use of sleeping medication (component6)
Before intervention
0.00±0.000
0.17±0.66
-0.063
-0.132, 0.007
0.075
After intervention
0.00±0.000
0.03±0.19
Daytime dysfunction (component7)
Before intervention
1.00±0.92
0.45±0.57
0.078
-0.492, 0.648
0.785
After intervention
0.93±1.14
0.59±0.90
Global sleep quality
Before intervention
6.07±3.14
5.55±3.19
-1.537
-3.050, -0.024
0.047
After intervention
5.67±3.10
6.72±3.62
Maximum score: global sleep quality= 21 and each components sleep quality=3
Covariate variables: sleep hygiene, perceived stress, depression, and sleep quality in first trimester
Bootstrap results are based on 1000 bootstrap sample
Table 2: Comparisons of sleep quality components in pregnant women based on Pittsburgh Sleep Quality Index (PSQI) before and after intervention in both groups in second trimester.
The scores for sleep quality before the intervention (14 weeks), the first follow-up (24-28 weeks) and the second follow-up (37-38 weeks) are demonstrated in Figure 2. In the myo-inositol group, the global PSQI decreased by 0.40 points from the first to the second trimester of pregnancy, while the corresponding rise in the placebo group was 1.7 points. The follow-up data group in the third trimester (37-38 weeks) revealed that the mean score of sleep quality was lower in myo-inositol group compared with that of the placebo.

Figure 2:
The secondary outcomes (maternal and neonatal outcomes) among pregnant women are shown in Table 3. There were not significant differences between the two groups for the occurrence of maternal and neonatal outcomes.
Secondary outcomes
Myo-inositol group N=27 N (%)
Placebo group N=29 N (%)
P-value
Maternal outcomes
Weight gain during pregnancy
=12
12 (44.4)
20 (69.0)
0.064
>12
15 (55.6)
9 (31.0)
Gestational age at delivery
<37
2 (7.4)
2 (6.9)
0.064
37- 42
25 (92.6)
27 (93.1)
Mode of delivery
Vaginal
10 (37.0)
10 (34.5)
0.842
C/S
17 (63.0)
19 (65.5)
Gestational hypertension
Yes
0 (0.0)
1 (3.4)
0.518
No
27 (100.0)
28 (96.6)
Preterm delivery
2 (7.4)
2 (6.9)
Neonatal outcomes
Birth weight
=4000
25 (92.6)
27 (93.1)
0.667
>4000
2 (7.4)
2 (6.9)
Admission to neonatal intensive care unit (NICU)
Yes
2 (7.4)
5 (17.2)
0.242
No
25 (92.6)
24 (82.8)
Shoulder dystocia
Yes
0 (0.0)
2 (6.9)
0.264
No
27 (100.0)
27 (93.1)
Table 3: Secondary outcomes (maternal and neonatal outcomes) among pregnant women (N=60).
No serious side effects were reported for both groups. Out of 56 subjects, ten of them experienced such minor side effects as nausea (just one participant in placebo group) and flatulence (3 participants in placebo group and six participants in myo-inositol group). The differences between the two groups were not statistically significant, however. Besides, it should be stated that one participant had to be excluded from the study due to a severe side effect (headache).
Discussion
A quick look at the existing literature reveals that there is no randomized clinical trial to assess the effect of myo-inositol supplementation on sleep quality, although myo-inositol supplementation has been extensively used to treat psychological disorders, polycystic ovarian syndromes and gestational diabetes mellitus [34-37]. This research study confirmed that myo-inositol supplementation CAN improve pregnant women’s global sleep quality, subjective sleep quality, and sleep duration. The results also demonstrated that myo-inositol was associated with poor habitual sleep efficiency. This finding is in line with the results of the previous studies claiming that myo-inositol in brain glial cells is a marker of astroglia and microglia activation [38,39], which can play a critical role in brain regions to improve sleep [23,24]. However, Nathan et al. conducted a research on 43 healthy older people and reported that higher levels of hippocampal myo-inositol was associated with greater sleep complaints, specifically poor sleep quality, and poorer sleep efficiency [24].
In addition, other research studies in obstructive sleep apnea (OSA) patient showed that higher level of myo-inositol in midbrain was associated with sleep disorder [40-42]. However, Singh et al. revealed that the administration of Ebselen (inositol monophosphatase inhibitor) to the participants led to decreased slow-wave sleep, while it did not affect both the total sleep time and the total sleep quality [43]. Also, the analysis of nineteen non-medicated adolescent boys, performed by Urrila et al. (2017), found that the frontal cortical myoinositol may thus be linked to the incidence of depression and sleep symptoms among maturing adolescents. Daytime sleepiness and short sleep duration may be associated with the frontal cortex myoinositol independently from depression [44]. In contrast, the result of another study in obstructive sleep apnea (OSA) patient indicated that glial activation is likely a global, rather than region-specific phenomenon in sleep problems. Also, higher level of myo-inositol was associated with glial activation [42]. Increased myo-inositol in OSA patients was similarly reported in the limbic regions of the insular cortex, thalamus, putamen and the temporal and frontal brain regions by earlier spectroscopy studies [41,45].
In fact, it is worth mentioning that this study was the first double blind randomized clinical trial that tried to assess the impact of myoinositol supplementation on sleep quality among Iranian pregnant women, which could be regarded as strength for this study. The pregnant women accepted the supplementation intervention without hesitation.
Limitation
As for the limitations, this study had several limitations. First, the study used a self-report questionnaire to determine the sleep quality, and did not consider which participant was getting serious sleep hygiene at home, which may have induced response bias. Second, convenience sampling was used in this study, which could not represent the entire population. Block randomization was, nonetheless, used between the two groups, which was appropriate for clinical trials. Third, the small sample size could be another limitation for this study. Future studies could provide further insights into the etiological link between myo-inositol supplementation and sleep quality in pregnant women by larger sample size and an increased dose of myo-inositol in pregnancy.
Conclusions
In conclusion, after the intervention, 51.9% and 44.8 % of the pregnant women in myo-inositol and placebo groups had good sleep quality (a total score < 5), respectively. According to good sleep quality in the second trimester of pregnancy, the Number Needed to Treat (NNT) was 11 and 15 with no intention and with intention, respectively. Therefore, findings from this can be useful in order to increase the rate of good sleep quality in pregnant women.
Funding
This research was supported by Babol University, Iran (grant numbers 4729).
Ethical Approval
Ethical approval for this study was obtained from Babol University of Medical Science (Ethic ID: IR. MUBABOL.HRI.REC. 1397. 032).
Acknowledgements
The authors acknowledge the assistance and cooperation of Iranian pregnant women for their participation in this study. We also truly appreciate the financial support of Babol University of Medical Sciences.
References
- Fung AM, Wilson DL, Lappas M, Howard M, Barnes M, O’Donoghue F, et al. Effects of maternal obstructive sleep apnoea on fetal growth: a prospective cohort study. PLoS One. 2013; 8: e68057.
- Dijk DJ, von Schantz M. Timing and consolidation of human sleep, wakefulness, and performance by a symphony of oscillators. J Biol Rhythms. 2005; 20: 279-90.
- Murali NS, Svatikova A, Somers VK. Cardiovascular physiology and sleep. Front Biosci. 2003; 8: s636-52.
- Palagini L, Gemignani A, Banti S, Manconi M, Mauri M, Riemann D. Chronic sleep loss during pregnancy as a determinant of stress: impact on pregnancy outcome. Sleep Med. 2014; 15: 853-9.
- Mindell JA, Jacobson BJ. Sleep disturbances during pregnancy. J Obstet Gynecol Neonatal Nurs. 2000; 29: 590-7.
- Jahdi F, Rezaie E, Behboodi Moghadam Z, Hagani H. Prevalence of sleep disorders in the pregnant women. 2013.
- Zafarghandi N, Hadavand S, Davati A, Mohseni SM, Kimiaiimoghadam F, Torkestani F. The effects of sleep quality and duration in late pregnancy on labor and fetal outcome. J Matern Fetal Neonatal Med. 2012; 25: 535-7.
- Warland J, Dorrian J, Morrison JL, O’Brien LM. Maternal sleep during pregnancy and poor fetal outcomes: A scoping review of the literature with meta-analysis. Sleep Med Rev. 2018; 41: 197-219.
- Zhong C, Chen R, Zhou X, Xu S, Li Q, Cui W, et al. Poor sleep during early pregnancy increases subsequent risk of gestational diabetes mellitus. Sleep Med. 2018; 46: 20-5.
- Izci-Balserak B, Pien GW. The relationship and potential mechanistic pathways between sleep disturbances and maternal hyperglycemia. Curr Diab Rep. 2014; 14: 459.
- Qiu C, Frederick IO, Sorensen TK, Enquobahrie DA, Williams MA. Sleep duration and plasma leptin concentrations in early pregnancy among lean and overweight/obese women: a cross sectional study. BMC Res Notes. 2014; 7: 20.
- Hollenbach D, Broker R, Herlehy S, Stuber K. Non-pharmacological interventions for sleep quality and insomnia during pregnancy: A systematic review. J Can Chiropr Assoc. 2013; 57: 260-70.
- Hauri PJ. Sleep hygiene, relaxation therapy, and cognitive interventions. Case studies in insomnia: Springer. 1991; 65-84.
- Bat-Pitault F, Deruelle C, Flori S, Porcher-Guinet V, Stagnara C, Guyon A, et al. Sleep pattern during pregnancy and maternal depression: study of Aube cohort. J Sleep Disord Manag. 2015; 1.
- Morgenthaler T, Kramer M, Alessi C, Friedman L, Boehlecke B, Brown T, et al. Practice parameters for the psychological and behavioral treatment of insomnia: an update. An american academy of sleep medicine report. Sleep. 2006; 29: 1415-9.
- Schutte-Rodin S, Broch L, Buysse D, Dorsey C, Sateia M. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008; 4: 487-504.
- Corrado F, D’Anna R, Di Vieste G, Giordano D, Pintaudi B, Santamaria A, et al. The effect of myoinositol supplementation on insulin resistance in patients with gestational diabetes. Diabet Med. 2011; 28: 972-5.
- Bizzarri M, Fuso A, Dinicola S, Cucina A, Bevilacqua A. Pharmacodynamics and pharmacokinetics of inositol(s) in health and disease. Expert Opin Drug Metab Toxicol. 2016; 12: 1181-96.
- Granata R, Settanni F, Biancone L, Trovato L, Nano R, Bertuzzi F, et al. Acylated and unacylated ghrelin promote proliferation and inhibit apoptosis of pancreatic beta-cells and human islets: involvement of 3’,5’-cyclic adenosine monophosphate/protein kinase A, extracellular signal-regulated kinase 1/2, and phosphatidyl inositol 3-Kinase/Akt signaling. Endocrinology. 2007; 148: 512-29.
- Brusco GF, Mariani M. Inositol: effects on oocyte quality in patients undergoing ICSI. An open study. Eur Rev Med Pharmacol Sci. 2013; 17: 3095-102.
- Giaume C, Koulakoff A, Roux L, Holcman D, Rouach N. Astroglial networks: a step further in neuroglial and gliovascular interactions. Nat Rev Neurosci. 2010; 11: 87-99.
- Halassa MM, Haydon PG. Integrated brain circuits: astrocytic networks modulate neuronal activity and behavior. Annu Rev Physiol. 2010; 72: 335- 55.
- Frank MG. Beyond the neuron: astroglial regulation of mammalian sleep. Curr Top Med Chem. 2011; 11: 2452-6.
- Cross NE, Lagopoulos J, Duffy SL, Cockayne NL, Hickie IB, Lewis SJ, et al. Sleep quality in healthy older people: Relationship with ¹H magnetic resonance spectroscopy markers of glial and neuronal integrity. Behavioral neuroscience. 2013; 127: 803.
- Bourjeily G, Raker C, Chalhoub M, Miller M. Excessive daytime sleepiness in late pregnancy may not always be normal: results from a cross-sectional study. Sleep Breath. 2013; 17: 735-40.
- Skouteris H, Wertheim EH, Germano C, Paxton SJ, Milgrom J. Assessing sleep during pregnancy: a study across two time points examining the Pittsburgh Sleep Quality Index and associations with depressive symptoms. Womens Health Issues. 2009; 19: 45-51.
- Hossein A, Nowrouzi K, Pouresmaili R, Karimloo M, Maddah B. Acupoint massage in improving sleep quality of older adults. Archives of Rehabilitation. 2008; 9: 8-14.
- Mastin DF, Bryson J, Corwyn R. Assessment of sleep hygiene using the Sleep Hygiene Index. J Behav Med. 2006; 29: 223-7.
- Chehri A, Kiamanesh A, Ahadi H, Khazaie H. Psychometric Properties of the Persian Version of Sleep Hygiene Index in the General Population. Iran J Psychiatry Behav Sci. 2016; 10: e5268.
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983; 24: 385-96.
- Salehi Ghadri J. Efficacy of multiplicity of roles and satisfaction with it on the self esteem and stress in female teachers: MA Thesis, Tarbiat Modares University. 1994.
- Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Pers Assess. 1996; 67: 588-97.
- Rajabi GR. Psychometric properties of Beck depression inventory short form items (BDI-13). Journal of Iranian Psychologists. 2005; 1: 291-8.
- Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics. 2005; 115: e290-6.
- Zeng L, Yang K. Effectiveness of myoinositol for polycystic ovary syndrome: a systematic review and meta-analysis. Endocrine. 2018; 59: 30-8.
- Pintaudi B, Di Vieste G, Corrado F, Lucisano G, Giunta L, D’Anna R, et al. Effects of myo-inositol on glucose variability in women with gestational diabetes. Eur Rev Med Pharmacol Sci. 2018; 22: 6567-72.
- Shirayama Y, Takahashi M, Osone F, Hara A, Okubo T. Myo-inositol, Glutamate, and Glutamine in the Prefrontal Cortex, Hippocampus, and Amygdala in Major Depression. Biol Psychiatry Cogn Neurosci Neuroimaging. 2017; 2: 196-204.
- Bitsch A, Bruhn H, Vougioukas V, Stringaris A, Lassmann H, Frahm J, et al. Inflammatory CNS demyelination: histopathologic correlation with in vivo quantitative proton MR spectroscopy. AJNR Am J Neuroradiol. 1999; 20:1619-27.
- Chang L, Ernst T, Osborn D, Seltzer W, Leonido-Yee M, Poland RE. Proton spectroscopy in myotonic dystrophy: correlations with CTG repeats. Arch Neurol. 1998; 55: 305-11.
- Kang J, Tian Z, Li M. Changes in insular cortex metabolites in patients with obstructive sleep apnea syndrome. Neuroreport. 2018; 29: 981-6.
- Yadav SK, Kumar R, Macey PM, Woo MA, Yan-Go FL, Harper RM. Insular cortex metabolite changes in obstructive sleep apnea. Sleep. 2014; 37: 951- 8.
- Macey PM, Sarma MK, Prasad JP, Ogren JA, Aysola R, Harper RM, et al. Obstructive sleep apnea is associated with altered midbrain chemical concentrations. Neuroscience. 2017; 363: 76-86.
- Singh N, Sharpley AL, Emir UE, Masaki C, Herzallah MM, Gluck MA, et al. Effect of the Putative Lithium Mimetic Ebselen on Brain Myo-Inositol, Sleep, and Emotional Processing in Humans. Neuropsychopharmacology. 2016; 41: 1768-78.
- Urrila AS, Hakkarainen A, Castaneda A, Paunio T, Marttunen M, Lundbom N. Frontal Cortex Myo-Inositol Is Associated with Sleep and Depression in Adolescents: A Proton Magnetic Resonance Spectroscopy Study. Neuropsychobiology. 2017; 75: 21-31.
- Sarma MK, Macey PM, Nagarajan R, Aysola R, Harper RM, Thomas MA. Accelerated Echo Planer J-resolved Spectroscopic Imaging of Putamen and Thalamus in Obstructive Sleep Apnea. Sci Rep. 2016; 6: 31747.