Abstract
Recently, Tendon-Derived Mesenchymal Stem Cells (TMSCs) have been successfully isolated and shown that they possess self-renewal potential and multilineage differentiation capacity which serves them as a promising cell source for tendon tissue engineering.
However, long-term culture in vitro may alter the biology of adult MSCs and result in significant changes on their primitive characteristics. Given that oxygen concentrations in vivo are significantly less at tissue level, it is conceivable that many cells would function more normally in vitro at oxygen concentrations lower than 20%.
To determine the hypoxic effects on rabbit Tendon-Derived Mesenchymal Stem Cells (TMSCs), they were cultured in normoxia (21% 2) vs. hypoxia (5% O2) for up to passage 5 (P5), and their differentiation potential, stem cell marker expression and proliferation rate were compared at early and late passages. We found that TMSCs at 5% O2 significantly increased in proliferation compared to those at 20% O2. Moreover, the expression of two stem cell marker genes, Nanog and Oct-4, was upregulated in the cells cultured at 5% O2. Similarly, more TMSCs expressed three stem cell markers including SSEA-4, nucleostemin and Nanog. The total collagen production released by these stem cells was highly expressed in normoxia group compared to hypoxia group at each passage. In addition to these, higher expression of markers for adipogenesis, osteogenesis and chondrogenesis were observed by qRT-PCR and western blotting.
We conclude that hypoxia conditions have a beneficial effect on TMSCs for their maintenance of stem cell properties and improvement of multidifferentiation potential. Oxygen tension does play a critical role in the niche of TMSCs in vitro.
Keywords: Tendon-derived stem cells; Hypoxia conditions; Normoxia conditions; Self-renewal; Multi-differentiation potential
Abbreviations
TMSCs: Tendon-Derived Mesenchymal Stem Cells; BMSCs: Bone Marrow Stem Cells; MSCs: Mesenchymal Stem Cells; ESCs: Embryonic Stem Cells; IACUC: Institutional Animal use and Care committee; PBS: Phosphate Buffered Saline; DMEM: Dulbecco’s Modified Eagle’s Medium; FBS: Fetal Bovine Serum; PDT: Population Doubling Time; SSEA-4: Stage-Specific Embryonic Antigen-4; GAG: Glycosaminoglycans; FACS: Flow Cytometeryl; FITC: Fluorescein Isothiocyanate; FITC: Fluorescein Isothiocyanate; PE: Phycoerythrin; PPARγ: peroxisome proliferators-activated receptor γ; GAPDH: Glyceraldehyde-3-Phosphate Dehydrogenase; SD: Standard Deviation; ANOVA: Analysis Of Variance; PLSD: Predicted Least- Square Difference
Introduction
Injured or degenerative tendon demonstrates limited capacity for spontaneous repair. The absence of vasculature prevents reparative cells from penetrating the tissue and maintaining its integrity.
Thus the restoration of damaged tendon still remains an ongoing challenge. In recent years, tendon regenerations using stem cell-based therapies and tissue engineering techniques have been attempted, which provide a promising alternative for repair of tendon rupture and tendinopathy. Of particular interest is the use of Bone Marrow- Derived Mesenchymal Stem Cells (BMSCs) to regenerate functional tendons, however, unfortunately, several reports have shown that ectopic bone formation is observed after transplantation [1-3]. Furthermore, it has also been verified that tumor can be induced by undifferentiated BMSCs in some specific circumstances [4]. Recently, a rare cell population from tendons called Tendon-Derived Mesenchymal Stem Cells (TMSCs) has been successfully isolated and confirmed that they possess several universal criteria of stem cell, including clonogenicity, self-renewal and multipotent differentiation capacity, indicating that TMSCs may be an appealing cell source for tendon tissue engineering [5].
Although Mesenchymal Stem Cells (MSCs) show high cell renewal potential, they are also vulnerable to replicative senescence.
The bottleneck of any sources of MSCs for cell therapy is the low survival rate after transplantation of cells. In addition, long-term culture in vitro may alter the biology of adult MSCs and result in significant changes in cell cycle kinetics [6]. There is an increasing body of evidence that MSCs may become senescent during protracted culture as indicated by their decreased differentiation potential, morphologic alterations and reduced telomerase activity [7,8]. More work in these areas needs to be pursued before the clinical application of MSCs.
It is well known that self-renewal and multilineage differentiation are the marked abilities for all kinds of stem cells [9]. These are maintained within a niche composed of various factors, including cytokines, growth factors, adhesion molecules, and extracellular matrix [10]. Apart from these, a hypoxic environment is also denoted as an important regulator which plays a role in the maintenance of multipotency and extension of survival [11,12].
It has been accepted that the oxygen tension in most in vitro settings is considerably higher than that found in most mammalian tissues. These tensions correspond to an oxygen concentration of approximately 4-7% [13,14]. Thus, it is conceivable that many cells would function more normally in vitro at oxygen concentrations lower than 20%. Low O2, therefore, appears to be a niche component for tendon-derived mesenchymal stem cells. As we all know, Oxygen Can Generate Reactive Oxygen Species (ROS), and exposure to aberrant levels of ROS may induce senescence dysfunction in stem cells [15]. Thus, we have sufficient reason to speculate that it is advantageous for TMSCs to localize within a hypoxic niche where the ROS source O2 is limited.
However, the degree and duration of hypoxia described in the literature vary greatly and may result in opposite effects on the proliferation and differentiation capacities of MSCs [16-18]. So far only one study described the effect of low O2 tension (2%) on the in vitro expansion and maintenance of undifferentiated stem cell characteristics of human TMSCs and showed higher clonogenicity, cell proliferation but lower differentiation potential [19]. In the present study we investigated the effect of reduced oxygen (5%) in vitro on rabbit patella Tendon-Derived Mesenchymal Stem Cells (TMSCs), this oxygen tension approximates that of tendon in vivo, as described above. Rabbit TMSCs were cultured in normoxia (21% O2) versus hypoxia (5% O2) for up to passage 5 (P5) and their differentiation potential, stem cell marker expression and proliferation rate were compared at early and late passages. We observed that hypoxia was conducive to maintenance of the stem-cell characteristics and enhancement of the multi-differentiation capabilities during their expansion in vitro.
Materials and Methods
Control of hypoxic and normoxic culture conditions
We used a tri-gas incubator to achieve hypoxic culture conditions (Thermo Scientific Heracell 150i, Thermo Scientific, Pittsburgh, PA). In the tri-gas incubator, the concentration of oxygen was precisely controlled by two gas controllers and one oxygen sensor. The supply of nitrogen and carbon dioxide gases was achieved by using a nitrogen gas controller and a carbon dioxide gas controller which were connected to two nitrogen tanks and two carbon dioxide tanks, respectively. The gas tank could be automatically switched to another when the gas in one tank ran out. To avoid extra air was brought into the incubator by opening the door, the incubator was separated into three isolation chambers and each chamber was sealed by double doors. The oxygen in the incubator was further controlled by an oxygen sensor. With these control devices in place, the oxygen concentration in the incubator was kept at the constant level of 5% during our cell culture experiments.
For normoxic culture conditions, a regular tissue culture incubator (Thermo Scientific) was used, where 95% air and 5% carbon dioxide were fed into the incubator and as a result, a 20% O2 concentration inside the incubator was achieved.
Isolation of rabbit TMSCs
Five female New Zealand white rabbits (8-10 week-old, 3.0-4.0 kg) were used in all experiments. The protocol for use of the rabbits was approved by the IACUC of University of Pittsburgh. TMSCs were isolated from rabbit patellar tendons. The procedures for isolation of TMSCs were similar to our previously published protocol [20].
Cell culture
Rabbit TMSCs were seeded in 6-well plate at a density of 1.5×104/ well and cultured with 3 ml of 20% FBS-DMEM/well in the tri-gas incubator as described above to achieve a 5% 2 culture condition, or in the regular incubator to realize a 20% 2 culture condition. When changed medium for the cells cultured in the tri-gas incubator, we placed the replacement medium inside the tri-gas incubator for 30 min before being used. The medium was changed every 3 days under both hypoxic and normaxic conditions. The cell proliferation was determined by cell counting at each time point according to the method published previously [21].
Cellular production of total collagen
Rabbit TMSCs were seeded in 6-well plates at a density of 4.5×104 per well and grown in growth medium in the tri-gas or regular incubator for 5 days. The assay of collagen production was performed when the cell culture became confluent at 80%. After the cell-conditioned medium was collected, cells were detached by trypsinization. Cell numbers were then counted using auto cellometer (Nexcelom Bioscience LLC). To measure total soluble collagen in cell-conditioned media, we used a Sircol collagen assay (Biodye Science, Biocolor Ltd, Carrickfergus, Northern Ireland and UK). Briefly, the cell-conditioned medium was mixed with Sircol dye reagent on an orbital shaker for 30 minutes. This solution was then centrifuged to obtain a collagen-dye complex pellet, which was solubilized with an alkali reagent. A microplate reader (Spectra MAX 190, Molecular Devices, Sunnyvale, California) was used to measure absorbance of the samples at a wavelength of 540 nm. A standard curve for calculating collagen concentration was obtained using a manufacturer-supplied acid soluble type I collagen calibration standard solution. Finally, to compare the hypoxia group with the normoxia group at different passages, we normalized the amounts of collagen with the total cell number produced by each group.
Expression of stem cell markers
Immunocytochemical assay was used for the expression of the following stem cell markers: nucleostemin, Nanog, stagespecific embryonic antigen-4 (SSEA-4) on TMSCs. To perform immunostaining, the cells were seeded in two 12-well plates at a density of 1.5×104/well with 1.5 ml medium and cultured in either 5% O2 or 20% O2 conditions for 3 days. All of them were fixed with 4% paraformaldehyde in PBS for 30 min at room temperature and treated with 0.1% Triton X-100 for 30 min for Nanog and nucleostemin staining. After washing the cells with PBS, either mouse anti-Nanog (1:350, Santa Cruz Biotechnology, Inc., cat. # sc-33759, Santa Cruz, CA) or goat anti-nucleostemin (1:400, Neuromics, Cat. # GT15050, Edina, MN) was applied for 2 h at room temperature. The cells were washed with PBS for three times, and either Cy-3-conjugated goat anti-mouse IgG antibodies (1:500 for Nanog, Millipore, Cat. # AP124C, Billerica MA) or Cy3-conjugated donkey anti-goat IgG antibodies (1:500 for nucleostemin, Millipore, Cat. # AP18°C, Billerica, MA) was applied for 1 h at room temperature. In order to stain for SSEA-4, fixed cells were incubated with mouse anti-human SSEA-4 antibodies (1:500, Invitrogen, Cat. # 414000, Frederick, MD) for 2 hours at room temperature. After washing the cells with PBS, cells were treated with Cy3-conjugated goat anti-mouse IgG antibodies (1:500, Millipore, Cat. # AP124C, Billerica MA) for 1h at room temperature. To quantify the expression of stem cell markers, the stained samples were examined using an inverted fluorescence microscope and images were taken with a 20 × objective using a CCD camera.
Multilineage differentiation potential
Multilineage differentiation potential was tested in vitro for adipogenesis, chondrogenesis, and osteogenesis, respectively. The cells at passage 2 were seeded in 6-well plates at a density of 2.4 × 105 cells/well in basic growth medium (DMEM plus 10% FBS) and cultured either under 5% O2 or 20% O2 tension. To test adipogenic potential, cells were cultured in adipogenic induction medium (Millipore, Billerica, MA) consisting of basic growth medium added with dexamethasone (1 m?), insulin (10 mg/ml), indomethacin (100 m?), and isobutylmethylxanthine (0.5 m?). As a test of chondrogenic potential, they were cultured in basic growth medium supplemented with proline (40 mg/ml), dexamethasone (39 ng/ml), TGF-β3 (10 ng/ml), ascorbate 2-phosphate (50 mg/ml), sodium pyruvate (100 mg/ml), and insulintransferrin-selenious acid mix (50 mg/ml) (BD Bioscience, Bedford, MA). Finally, the osteogenic potential was tested by culturing cells in osteogenic induction medium (Millipore, Billerica, MA) consisting of basic growth medium supplemented with dexamethasone (0.1 mM), ascorbic 2-phosphate (0.2 mM), and glycerol 2-phosphate (10 mM). After culturing for 21 days, Oil red O assay, Safranin O assay, and Alizarin red S assay, as descried previously [21], were used to assess adipogenesis, chondrogenesis, and osteogenesis of TMSCs when grown in 5% O2 and 20% O2 culture conditions.
Semi-quantification of the extent of cell differentiation
The stained samples were examined using an inverted microscope and images were taken with a 20 × objective using a CCD camera. A total number of eight views from each well were randomly chosen. The areas of positive staining were identified manually and computed by a SPOT imaging software (Diagnostic Instruments, Inc., Sterling Heights, MI). The ratio of positive staining was calculated by dividing the stained area by the view area. The values of all views from three duplicate wells (24 views in total) were averaged to obtain the percentage of positive staining, which represented the extent of cell differentiation in the respective induction medium.
Quantitative real-time PCR (qRT-PCR)
To measure the stemness and multipotential of TMSCs under hypoxic and normaxic culture conditions, we performed qRT-PCR. Total RNA was extracted using an RNeasy Mini-Kit with an oncolumn DNase I digest (Qiagen). First-strand cDNA was synthesized in a 20 μl reaction of 1μg total RNA through reverse transcription with Super-Script II (Invitrogen). The conditions for the cDNA synthesis were: 65°C for 5 min and cooling for 1 min at 4°C, then 42°C for 50 min, and finally 72°C for 15 min. The qRTPCR was carried out using QIAGEN QuantiTect SYBR Green PCR Kit (Qiagen) [22]. In a 50 μl PCR reaction mixture, 2 μl cDNA (total 100 ng RNA) were amplified in a Chromo 4 Detector (MJ Research). Rabbit-specific primers were used for stem cell genes expression, including Oct- 4 and Nanog. For differentiated TMSCs, rabbit-specific primers were used for collagen type II, peroxisome proliferators-activated receptor γ (PPAR γ), Sox9, osteocalcin, and Runx2. Glyceraldehyde- 3-Phosphate Dehydrogenase (GAPDH) was used as an internal control. The forward and reverse primer sequences and the resultant products were designed according to published methods, and are listed in (Table 1) [23-26]. All primers were synthesized by Invitrogen (Carlsbad, CA). The relative gene expression levels were calculated from 2-?CT, where ?CT was determined by the formula: ?CT = (Cttarget-CTGAPDH) differentiation-(Cttarget- CTGAPDH) control. In the formula, CTtarget and CTGAPDH are the cycle thresholds of target gene and GAPDH gene, respectively, for each RNA sample. The Standard Deviation (SD) of the ?CT was determined from at least three parallel tests.
Gene
Size(bp)
Primers
Type
Tm
PPAR?
200
5’-TGG GGA TGT CTC ATA ATG CCA-3’
Forward
59oc
5’-TTC CTG TCA AGA TCG CCC TCG-3’
Reverse
Collagen II
84
5’-TGG GTG TTC TAT TTA TTT ATT GTC TTC CT-3’
Forward
63oc
5’-GCG TTG GAC TCA CAC CAG TTA GT-3’
Reverse
Sox9
79
5’-AGT ACC CGC ACC TGC ACA AC-3’
Forward
59oc
5’-CGC TTC TCG CTC TCG TTC AG-3’
Reverse
Runx2
70
5’-TGA TGA CAC TGC CAC CTC TGA-3’
Forward
58oc
5’-GCA CCT GCC TGG CTC TTC T-3’
Reverse
GAPDH
107
5’-ACT TTG TGA AGC TCA TTT CCT GGT A-3’
Forward
63oc
5’-GTG GTT TGA GGG CTC TTA CTC CTT-3’
Reverse
Nanog
382
5’-CCCAGCTGTGTGTGCTCAA-3’
Forward
52oc
5’-CCAGGCTTGGGAGTACCAGG-3’
Reverse
Oct4
575
5’-CTCGGCGCAGCGCACGCCCTGGAG-3’
Forward
66oc
5’-CAGCTGGTCGCGCAGCGGGCCCAG-3’
Reverse
osteocalcin
70
5’-GAAGCCCAGCGGTGCA-3’
Forward
59oc
5’-CACTACCTCGCTGCCCTCC-3’
Reverse
Table 1: Primers used for qRT-PCR analysis.
Western blot
The cells at passage 2 were seeded in 6-well plates at a density of 6×104 per well and cultured separately with adipogenic, osteogenic and chondrogenic induction media under different oxygen tension for 21 days. Then they were lysed using a mammalian protein extraction reagent cocktail (Pierce, Rockford, Illinois) containing 1.5% protease inhibitors (Sigma-Aldrich). After centrifugation at 12,000 rpm for 10 minutes, the protein concentrations of the supernatants were determined using a BCA Protein Assay (Pierce). Equal amounts of total protein were run on 12% SDS polyacrylamide gels (Bio-Rad) at a constant voltage of 100 V for 60 minutes. Proteins were blotted to a nitrocellulose membrane using a Semi-Dry transfer module (Bio- Rad) at 200 mA for 90 minutes. The membrane was blocked in a 5% dry milk/TBS-Tween 20 solution for 1 hour at room temperature and then probed for 5 hours with a mouse monoclonal anti-human adiponectin antibody (Millipore; Cat # MAB3604) at a dilution of 1:1000; mouse monoclonal anti-human osteocalcin (Abcam; Cat # ab13418) at a dilution of 1:1000 for 5 hours; and mouse anti-human collagen II (Millipore; Cat # MAB8887) at a dilution of 1:500 in a 1% dry milk/PBS-Tween 20 solution. Incubation with the primary antibody was followed by a horseradish peroxidase-conjugated goat anti-mouse antibody (Millipore; Cat #12-349) at a dilution of 1:2000 in a 1% dry milk/PBS solution. The targeted protein bands were detected using an ECL (enhanced luminol-based chemiluminescence) detection kit (Amersham Biosciences, Piscataway, New Jersey), followed by exposure of the membrane to x-ray film. Membranes were also re-probed for mouse anti- glyceraldehyde-3-phosphate dehydrogenase (Santa Cruz Biotechnology; Cat # sc-66163) to verify equal protein loading in the gels. The quantification of protein bands were performed by the Image J software (https://rsb.info.nih.gov/ nih-image/) and normalized by respect GAPDH using the following formulae:
Protein Expression (target-hypoxia) = Intensity (target-hypoxia) /Intensity (GAPDH-hypoxia)
Protein Expression (target-normoxia) = Intensity (targetnormoxia) /Intensity (GAPDH-normoxia)
Statistical analysis
Data is presented as mean plus and minus Standard Deviation (SD). At least three replicates for each experimental condition were performed, and the presented results are representative of these replications. One-way Analysis Of Variance (ANOVA), followed by either Fisher’s Predicted Least-Square Difference (PLSD) for multiple comparisons or two tailed student t-test wherever applicable, were used for statistical analysis. Differences between two groups were considered significant when the p-value was less than 0.05.
Results and Discussion
The proliferation of rabbit TMSCs was investigated by Population Doubling Time (PDT) calculated through cell number counting using cytometry. Our data showed that PDT of TMSCs cultured at 20% O2 was much longer than that at 5% O2 at each passage, demonstrating that the latter proliferated much faster than former. Great difference was found between two groups at each passage (Figure 1, p<0.01). Also, the amount of collagenous proteins provided by TMSCs which was pre-cultured under different oxygen tension was measured. The total collagen production released by these stem cells was lower in hypoxia group compared to that in normoxia group at each passage. Moreover, with the passage increasing, the discrepancy between two groups became more and more apparent (Figure 2, p<0.01). Immunocytochemical staining of these cells showed that both hypoxia group and normoxia group in culture for more than one month could express SSEA-4, nucleostemin and Nanog despite their decreasing numbers compared to passage 1 (Figure 3). Although semi-quantification measurement of immunostaining results showed that cells cultured at 5% O2 tension expressed higher levels for these three stem cell markers than that at 20% O2 tension, there was no great difference between the two groups at P1 and P2. However, from the beginning of passage 3, the percentages of positive staining for the three markers decreased sharply in the normoxia group and significant difference exhibited (Figure 4, p<0.05 for SSEA4 at passage-3; P<0.01 for Oct-4 and Nanog at passages 3 to 5). Similar situation occurred in gene analysis. Our results demonstrated that the expression levels of both Nanog and Oct-4 genes were significantly up-regulated at 5% O2 tension than they were at 20% O2 tension after passage-3 (Figure 5, p<0.05 for passage-3, p<0.01 for passages 4 and 5), whereas non significantly higher expressions of the two stem cell genes displayed between the two groups at P1 and P2 (Figure 5).
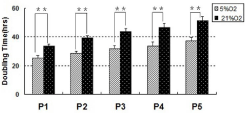
Figure 1: Proliferation of TMSCs cultured under hypoxic and normaxic
conditions. Rabbit TMSCs were cultured either in 5% 2 or 20% 2 for 5
passages during total 35 days. The medium in each condition was changed
every three days. Cell proliferation was assessed by measuring the Population
Doubling Time (PDT) of the TMSCs in each culture. As indicated, the PDT
of TMSCs grown in 20% oxygen tension was much longer than that in 5%
oxygen tension at each passage, demonstrating that the latter proliferated
faster than former (**P<0.01).
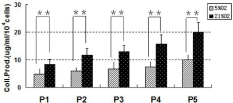
Figure 2: Collagen production in the media of TMSCs cultured in hypoxic and
normxic conditions. After 5 days culture, the collagen production in the media
produced by rabbit TMSCs grown under hypoxic condition was markedly
decreased when compared to those grown under normoxic condition. These
findings indicated that hypoxia prevent TMSCs from differentiation by default
(* *P<0.01).
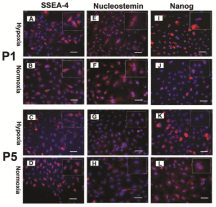
Figure 3: The expression of stem cell markers of TMSCs under hypoxic
(A, E, I, C, G, K) and normaxic (B, F, J, D, H, L) culture conditions. Rabbit
TMSCs were cultured either in hypoxic or normaxic conditions for 3 days at
each passage. The hypoxia effect on the stemness of rabbit TMSCs was
tested by immunostaining at passage 1 (A, B, E, F, I, J) and passage 5 (C,
D, G, H, K, L). There was no significant difference on the expression of three
stem cell markers including SSEA4 (A, B), Nucleostemin (E, F) and Nanog
(I, J) between two culture conditions of TMSCs at passage 1. However, more
TMSCs cultured under hypoxic condition at passage 5 were still positively
stained by these three stem cell markers (C, G, K) than those grown under
normoxic condition (D, H, L). (A-D): SSEA4; (E-H): Nucleostemin; (I-L):
Nanog. Insets showed enlarged view of positive staining. Scale bars: 50 μm.
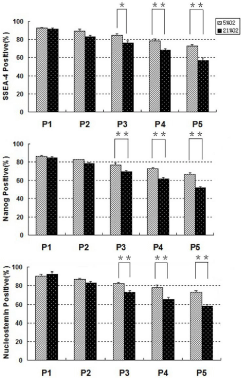
Figure 4: Semi-quantification of staining results for three stem cell markers
on TMSCs cultured under hypoxic and normoxic conditions. Rabbit TMSCs
specifically stained for SSEA4, Nanog and nucleostemin by immunostaining
at each passage in each culture condition were analyzed by semiquantification.
There was no significant difference on the expression of these
three stem cell markers between two culture conditions of TMSCs at earlier
passages. However, from passage-3, the expression of these three stem cell
markers was decreased in the TMSCs cultured under normoxic condition (*P
< 0.05). As indicated, significantly higher percentages of TMSCs at passages
4 and 5 cultured under 5% O2 conditions expressed the stem cell markers
(SSEA-4, nucleostemin and Nanog) compared to those cultured under 20%
O2 conditions (**P<0.01).
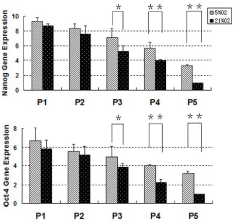
Figure 5: Stem cell marker gene expression by rabbit TMSCs cultured in
hypoxic and normoxic conditions. Rabbit TMSCs cultured either in hypoxic or
normoxic conditions for 7 days at each passage were used for gene analysis
by qRT-PCR. Total RNA was extracted from these cells and tested by rabbit
specific primers to Nanog and Oct-4. There was no significant difference on
the expression of these two stem cell marker genes between two culture
conditions of TMSCs at earlier passages. However, from passage-3, the
expression of these two stem cell marker genes was decreased in the TMSCs
cultured under normoxic condition (*P < 0.05). Both Nanog and Oct-4 genes
were all expressed at significantly high levels by TMSCs at higher passages
under hypoxia conditions in contrast with normoxia conditions (**P<0.01).
Note that for real time RT-PCR analysis, the gene expression levels were
normalized to GAPDH, obtained from at least three independent experiments.
After 21 days in respective induction media, the multidifferentiation potential of rabbit TMSCs towards adipogenesis, osteogenesis and chondrogenesis in different oxygen conditions were determined through special staining and qRT-PCR. The data showed that the degree of adipogenesis, chondrogenesis, and osteogenesis of rabbit TMSCs were all more extensive at 5% 2 conditions compared to that at 20% 2 conditions (Figure 6). Regarding adipogenesis, both groups exhibited lipid droplets, an indicator of adipogenesis which can be detected by Oil red O staining. Semi-quantification, by calculating stained area, showed that more than 48% of cells were positively stained at 5% oxygen tension whereas only 32% were stained positively at 20% oxygen tension (Figure 6 G, p<0.05). Similarly, the gene expression level of PPAR? in hypoxia group was upregulated 1.75 times compared to that in normoxia group (Figure 7, p<0.05). For osteogenesis, more than 51% positive areas were found by Alizarin Red S assay in hypoxia group compared to 36% in normoxia group (Figure 6 G, p<0.05). Meanwhile, the gene expression of osteogenic markers osteocalcin and Runx-2 were all significantly higher in hypoxia group than that in normoxia group (Figure 7, p<0.05). For chondrogenesis, Safranin O assay showed that 82% positive staining under hypoxia condition appeared while only 43% under normoxia condition (Figure 6 G, p<0.01). Equally, the expression levels of collagen type II and Sox9 were upregulated 3.82 and 3.94 times at 5% tension in comparison to 20% tension (Figure 7, p<0.01). Western blotting was performed to quantify the level of specific protein expression in the two groups. It was evident that adiponectin (1.3 times, p=0.021), osteocalcin (1.79 times, p=0.046) and collagen type II (3.49 times, p=0.028) were expressed higher in rabbit TMSCs grown at 5% tension than those at 20% tension (Figure 8).
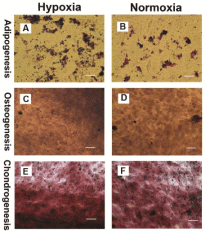
Figure 6: Histochemical staining of differentiated cells. Multi-differentiation
capacity of rabbit TMSCs cultured separately in adipogenic, chondrogenic
and osteogenic induction media under different oxygen conditions for 21
days was tested by histochemical staining using Oil red O for adipogenesis,
Alizarin red S for osteogenesis and Safranin O for chondrogenesis. Rabbit
TMSCs cultured either at 5% O2 or 20% O2 were able to differentiate into
adipocytes (A, B), osteocytes (C, D) and chondrocytes (E, F), as shown by
the accumulation of lipid droplets, calcium deposits and proteoglycans on cell
surfaces. However, the extent of differentiation under hypoxia conditions was
greater than that under normoxia conditions. Scale bars: 50 μm.
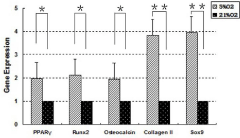
Figure 7: The qRT-PCR analysis of the expression for adipogenic,
osteogenic and chondrogenic marker genes. Multi-differentiation capacity
of rabbit TMSCs cultured separately in adipogenic, chondrogenic and
osteogenic induction media under different oxygen conditions for 21 days
was also tested by real time RT-PCR using specific primers: PPAR? for
adipogenesis, Runx-2 and osteocalcin for osteogenesis, and collagen II
and Sox-9 for chondrogenesis. Compared to normoxia group, these marker
genes were all highly up-regulated by the cells in hypoxia group. Note that
the gene expression levels were normalized to GAPDH, and obtained from at
least three independent experiments (*P < 0.05, **P<0.01).
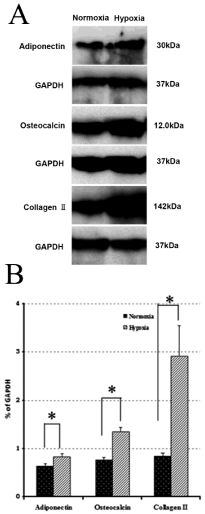
Figure 8: Representative western blots of TMSCs cultured in respective
induction media at different oxygen tensions. Multi-differentiation capacity
of rabbit TMSCs cultured separately in adipogenic, chondrogenic and
osteogenic induction media under different oxygen conditions for 21 days
was further tested by western blot using specific antibodies: adiponectin
for adipogenesis, osteocalcin for osteogenesis, and collagen II for
chondrogenesis. (A) Band images for western blot of the proteins isolated
from rabbit TMSCs. (B) Quantification of protein bands using densitometry. It
was apparent that adiponectin (1.3 times, *p=0.021), osteocalcin (1.79 times,
*p=0.046) and collagen type II (3.49 times, *p=0.028) were all markedly
increased by the cells under reduced oxygen tension.
Tendon is a dynamic tissue which transmits force from muscle to bone, thus allowing limb and joint movement possible. Tendon is frequently target of injury from trauma in sports and aging. Damaged tendon tissue heals very slowly and rarely attains the structural integrity and mechanical strength of normal tendon [27]. Recently, a tissue engineering approach has been sought to restore the damaged tendon using MSCs due to their capability for self-renewal and multilineage differentiation potential [28-30]. In addition to these, the discovery of tendon-derived stem cells that possess regenerative capabilities opens new possibilities for treating damaged tendon tissue. Therefore, TMSCs may be an optimal cell source for effective tissue engineering of injured tendons.
Unfortunately, therapeutic potential of MSCs is always hampered by an incomplete understanding of in vitro culture parameters that can maintain their stem-cell phenotype and multi-differentiation capabilities during expansion. It is well known that TMSCs tend to differentiate and lose their stemness quickly under regular culture conditions that use 95% air and 5% C2. It has been shown that MSCs and other stem cell populations continuously grown in vitro for 10 passages are subject to the replicative senescence known as Hayflick limit [7,31]. In addition, traditional cell-culture techniques can facilitate billion-fold expansion of MSCs, but result in a gradual loss of their primitive characteristics and self-renewal properties [32]. Furthermore, MSCs can undergo spontaneous transformation to malignant cells during extended culture in vitro [33]. Hence the application of MSCs therapy for tendon regeneration remains a great challenge because the role and niche of Mesenchymal Stem Cells (MSCs) in tendon have not been firmly established [34].
In order to obtain sufficient numbers of tendon stem cells for cell therapy of injured tendons, simpler and faster approaches to improve the survival of stem cells, maintain their self-renewal and promote their multi-lineage potential need to be pursued.
It is well known that oxygen concentrations in vivo at tissue level are significantly less than that supplied (20%) in normal cell culture. In this study we focused on hypoxia preconditioning as a powerful tool through which the stemness of TMSCs can be maintained and their multipotency can be enhanced in expansion. This is the first time to investigate the effect of reduced oxygen tension on Tendon-Derived Stem Cells (TMSCs) cultured at different passages during expansion in vitro. The results showed that with the passages increasing, both hypoxia and normoxia groups exhibited descenting trend in proliferative potential, however, the cells cultured at 5% oxygen conditions grew much more quickly compared to the cells at 20% oxygen conditions.
Because there is no specific stem cell marker for TMSCs, we used general stem cell markers (SSEA-4, Nanog and nucleostemin) to characterize the stemness of TMSCs under both hypoxic and normaxic conditions. It was noteworthy that the cells from rabbit tendons cultured at different oxygen tensions all expressed high levels of these characteristic stem cell markers at the beginning, indicating that they possessed property of stem cells. However, from the passage 3, many cells grown in normoxic conditions lose their “stemness” as evidenced by immunostaining and RT-PCR. As a contrast, higher percentage of TMSCs grown under reduced oxygen conditions even at passages 4 and 5 were still positively stained by these three stem cell markers than those grown in normoxic conditions at the same passages. Consistent with this result, qRT-PCR analysis showed that the expression of the Nanog and Oct-4 genes were all significantly higher in hypoxia group than that in normoxia group after passage 3. These findings indicated that significantly more TMSCs at 5% 2 tension remained undifferentiated state and preserved self-renewal capability compared with those at 20% 2 tension during expansion.
Also, as tendon-specific stem cells, TMSCs are always prone to differentiate into tenocytes by default [21], detection of total collagen production in cell-conditioned media can elucidate whether TMSCs undergo differentiation during expansion. Less collagen production in TMSCs at 5% 2 culture conditions indicated that more tendon stem cells remained undifferentiated state. Namely, hypoxia conditions could maintain the “stemness” of TMSCs better than normoxia conditions.
Finally, we demonstrated TMSCs at hypoxia and normoxia conditions differentiated into adipocytes, chondrocytes, and osteocytes in the respective induction media, evidenced by relevant histochemical staining, qRT-PCR and western blotting. Expression of markers for adipogenesis, osteogenesis and chondrogenesis were all elevated in cultures which had been in low oxygen throughout their cultivation time. Collectively these data suggest that reduced oxygen tension exerted a beneficial influence on promoting multidifferentiation potential of tendon-derived stem cells.
In this study, we found that hypoxic condition enhanced proliferation of tendon stem cells, although the mechanism is largely unknown, one of possible reasons is that hypoxic condition provides a mimic in vivo niche for tendon stem cell growth. Compared to vascular-rich organs and tissues, such as lungs, heart, liver and kidneys, the oxygen levels in tendons tissues in vivo are very low due to very few blood vessels in tendons. That means tendon stem cells prefer hypoxic conditions. It has been reported that low oxygen tensions in stem cell niches offers a selective advantage that is well suited to their particular biological roles [35].
Although our study provides clear evidence that hypoxia can maintain the TMSCs undifferentiated and in parallel enhance their pluripotency, a few limitations still exist. First, we only cultured rabbit TMSCs under reduced oxygen conditions for five passages. It still remains indeterminate in the long-term culture with more than five passages. Second, oxygen concentration we chose was based on the literatures which have been published, however, which type of oxygen gradient is optimal in keeping properties of TMSCs needs to be further investigated. Finally, the molecular mechanisms that are responsible for enhanced “stemness” in rabbit TMSCs as shown in this study remain unknown. It has been accepted that the transcription factor complex hypoxia-inducible factor 1 (HIF1) is a key mediator of adaptive responses to changes in cellular oxygen level [36]. Under hypoxic conditions, HIF-1 is stabilized and permits the activation of genes essential to cellular adaptation to low oxygen conditions [36]. Therefore, HIF-1 deserves much attention in the future.
Conclusion
In conclusion, this study has illustrated the dramatic influence of oxygen concentration as a potent regulator of in vitro stem cell physiology. Our data indicate that low oxygen tension (5%) provided a mimic in vivo environment for tendon cells. Hypoxia condition can significantly remain the TMSCs at an undifferentiated state, increase their proliferation rate and enhance their multi-differentiation potential. Oxygen tension does, therefore, appear to be critical for establishing the tendon-derived stem cell niche in vitro.
References
- Chong AK, Ang AD, Goh JC, Hui JH, Lim AY, Lee EH, et al. Bone marrow-derived mesenchymal stem cells influence early tendon-healing in a rabbit achilles tendon model. J Bone Joint Surg Am. 2007; 89: 74-81.
- Ouyang HW, Goh JC, Thambyah A, Teoh SH, Lee EH. Knitted poly-lactide-co-glycolide scaffold loaded with bone marrow stromal cells in repair and regeneration of rabbit Achilles tendon. Tissue Eng. 2003; 9: 431-439.
- Harris MT, Butler DL, Boivin GP, Florer JB, Schantz EJ, Wenstrup RJ. Mesenchymal stem cells used for rabbit tendon repair can form ectopic bone and express alkaline phosphatase activity in constructs. J Orthop Res. 2004; 22: 998-1003.
- Tasso R, Augello A, Carida M, Postiglione F, Tibiletti MG, Bernasconi B, et al. Development of sarcomas in mice implanted with mesenchymal stem cells seeded onto bioscaffolds. Carcinogenesis. 2009; 30: 150-157.
- Bi Y, Ehirchiou D, Kilts TM, Inkson CA, Embree MC, Sonoyama W, et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med. 2007; 13: 1219-1227.
- Izadpanah R, Kaushal D, Kriedt C, Tsien F, Patel B, Dufour J, et al. Long-term in vitro expansion alters the biology of adult mesenchymal stem cells. Cancer Res. 2008; 68: 4229-4238.
- Izadpanah R, Trygg C, Patel B, Kriedt C, Dufour J, Gimble JM, et al. Biologic properties of mesenchymal stem cells derived from bone marrow and adipose tissue. J Cell Biochem. 2006; 99: 1285-1297.
- Bonab MM, Alimoghaddam K, Talebian F, Ghaffari SH, Ghavamzadeh A, Nikbin B. Aging of mesenchymal stem cell in vitro. BMC Cell Biol. 2006; 7: 14.
- Seita J, Weissman IL. Hematopoietic stem cell: self-renewal versus differentiation. Wiley Interdiscip Rev Syst Biol Med. 2010; 2: 640-653.
- Scadden DT. The stem-cell niche as an entity of action. Nature. 2006; 441: 1075-1079.
- Ding H, Chen S, Yin JH, Xie XT, Zhu ZH, Gao YS, et al. Continuous hypoxia regulates the osteogenic potential of mesenchymal stem cells in a time-dependent manner. Mol Med Rep. 2014; 10: 2184-2190.
- Ma T, Grayson WL, Frohlich M, Vunjak-Novakovic G. Hypoxia and stem cell-based engineering of mesenchymal tissues. Biotechnol Prog. 2009; 25: 32-42.
- Rich IN. A role for the macrophage in normal hemopoiesis I. Functional capacity of bone-marrow-derived macrophages to release hemopoietic growth factors. Experimental hematology. 1986; 14: 738-745.
- Rich IN, Kubanek B. The effect of reduced oxygen tension on colony formation of erythropoietic cells in vitro. Br J Haematol. 1982; 52: 579-588.
- Jang YY, Sharkis SJ. A low level of reactive oxygen species selects for primitive hematopoietic stem cells that may reside in the low-oxygenic niche. Blood. 2007; 110: 3056-3063.
- Salim A, Nacamuli RP, Morgan EF, Giaccia AJ, Longaker MT. Transient changes in oxygen tension inhibit osteogenic differentiation and Runx2 expression in osteoblasts. The Journal of biological chemistry. 2004; 279: 40007-4016.
- Lennon DP, Edmison JM, Caplan AI. Cultivation of rat marrow-derived mesenchymal stem cells in reduced oxygen tension: effects on in vitro and in vivo osteochondrogenesis. Journal of cellular physiology. 2001; 187: 345-355.
- Malladi P, Xu Y, Chiou M, Giaccia AJ, Longaker MT. Effect of reduced oxygen tension on chondrogenesis and osteogenesis in adipose-derived mesenchymal cells. Am J Physiol Cell Physiol. 2006; 290: 1139-1146.
- Lee WY, Lui PP, Rui YF. Hypoxia-mediated efficient expansion of human tendon-derived stem cells in vitro. Tissue Eng Part A. 2012; 18: 484-498.
- Zhang J, Wang JH. Mechanobiological response of tendon stem cells: implications of tendon homeostasis and pathogenesis of tendinopathy. Mons J Orthop Res. 2010; 28: 639-643.
- Zhang J, Wang JH. Characterization of differential properties of rabbit tendon stem cells and tenocytes. BMC Musculoskelet Disord. 2010; 11: 10.
- Claus R, Lacorn M, Welter H, Lekhkota O, Messe N, Wagner A, et al. Expression of 11beta-hydroxysteroid-dehydrogenase 2 in Sertoli cells of boar testes. Mol Cell Endocrinol. 2007; 272: 86-92.
- Zhao SP, Dong SZ. Effect of tumor necrosis factor alpha on cholesterol efflux in adipocytes. Clinica chimica acta; international journal of clinical chemistry. 2008; 389: 67-71.
- Emans PJ, Spaapen F, Surtel DA, Reilly KM, Cremers A, Van Rhijn LW, et al. A novel in vivo model to study endochondral bone formation; HIF-1alpha activation and BMP expression. Bone. 2007; 40: 409-418.
- Intawicha P, Ou YW, Lo NW, Zhang SC, Chen YZ, Lin TA, et al. Characterization of embryonic stem cell lines derived from New Zealand white rabbit embryos. Cloning Stem Cells. 2009; 11: 27-38.
- Martins A, Pinho ED, Correlo VM, Faria S, Marques AP, Reis RL, et al. Biodegradable nanofibers-reinforced microfibrous composite scaffolds for bone tissue engineering. Tissue Eng Part A. 2010; 16: 3599-3609.
- Moshaverinia A, Xu X, Chen C, Ansari S, Zadeh HH, Snead ML, et al. Application of stem cells derived from the periodontal ligament or gingival tissue sources for tendon tissue regeneration. Biomaterials. 2014; 35: 2642-2650.
- Ramdass B, Koka PS. Ligament and tendon repair through regeneration using mesenchymal stem cells. Curr Stem Cell Res Ther. 2014; 10: 84-88.
- Randelli P, Randelli F, Ragone V, Menon A, D'Ambrosi R, Cucchi D, et al. Regenerative medicine in rotator cuff injuries. Biomed Res Int. 2014; 2014: 129515.
- Petrou IG, Grognuz A, Hirt-Burri N, Raffoul W, Applegate LA. Cell therapies for tendons: old cell choice for modern innovation. Swiss Med Wkly. 2014; 144: w13989.
- Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002; 418: 41-49.
- Grange S. Current issues and regulations in tendon regeneration and musculoskeletal repair with mesenchymal stem cells. Curr Stem Cell Res Ther. 2012; 7: 110-114.
- Rubio D, Garcia-Castro J, Martin MC, De La Fuente R, Cigudosa JC, Lloyd AC, et al. Spontaneous human adult stem cell transformation. Cancer Res. 2005; 65: 3035-3039.
- Dhinsa BS, Mahapatra AN, Khan WS. Sources of adult mesenchymal stem cells for ligament and tendon tissue engineering. Curr Stem Cell Res Ther. 2014; 10: 26-30.
- Cipolleschi MG, Dello Sbarba P, Olivotto M. The role of hypoxia in the maintenance of hematopoietic stem cells. Blood. 1993; 82: 2031-2037.
- Hubbi ME, Semenza GL. Regulation of cell proliferation by hypoxia-inducible factors. Am J Physiol Cell Physiol. 2015; 309: 775-782.
