Abstract
Ischemic heart diseases are largely determined by multifactorial risk factors that are associated with systemic arterial hypertension. Furthermore, high levels of total cholesterol, high body mass index, and high fasting glucose are also major risk factors that can lead to atherosclerosis. Endothelial dysfunction is a characteristic feature of systemic arterial hypertension and an early marker of atherosclerosis. Evidence shows that high blood pressure can result in vascular remodeling and damage to vascular endothelial cells and may partly explain endothelial dysfunction found in hypertensive individuals. There is a consensus that regular exercise can help control blood pressure levels in hypertensive individuals and all leading health organizations consider it the first-line nonpharmacological strategy for blood pressure control. Moreover, regular exercise has shown to improve endothelial function in different populations. However, different modalities of exercises, i.e. aerobic, resistance or combined (aerobic + resistance), could have different effects on blood pressure and endothelial response and they need to be further investigated. Thus, this review aims to better understand the behavior of blood pressure and endothelium-dependent vasodilator capacity in hypertensive individuals engaging in different forms of exercise training.
Keywords: Exercise; Endothelium; Hypertension
Abbreviations
SAH: Systemic Arterial Hypertension; CVD: Cardiovascular Disease; NOS: Nitric Oxide Synthase; NO: Nitric Oxide; eNOS: endothelial Nitric Oxide Synthase; LDL-C: Low-Density Lipoprotein Cholesterol; EPCs: Endothelial Progenitor Cells; BP: Blood Pressure; SBP: Systolic Blood Pressure; DBP: Diastolic Blood Pressure; NOx: Nitrate/Nitrite; 1 RM: One Repetition Maximum
Introduction
Systemic Arterial Hypertension (SAH) is characterized by high Blood Pressure (BP) resulting in a failure of vascular regulation due to malfunction in pressure control mechanisms [1,2]. It is likely that a large number of interrelated factors contribute to increased blood pressure in hypertensive subject, and their relative roles may differ between individuals. Factors associated with SAH are excess sodium intake [3], elevated levels of circulating angiotensin-converting enzyme [4], increased sympathetic nervous system activation [5], genetic factors [6], and endothelial dysfunction [7,8]. It is estimated that, by 2025, approximately 29% of the world population will have hypertension, accounting for about 1.56 billion individuals affected, mostly in developing countries [9]. These data are concerning given the high incidence of coronary artery disease, peripheral artery disease, stroke, and heart failure in this population [10,11]. Of all risk factors for developing coronary artery disease, hypertension is described as the most important, followed by high levels of total cholesterol, high body mass index, and high fasting glucose [12].
Obesity [13,14], physical inactivity [15], and insulin resistance [16] are all risk factors closely associated with SAH. These risk factors may individually contribute to the development of atherosclerosis and consequent mortality from ischemic Cardiovascular Disease (CVD) [17].
The vascular endothelium has an important function in modulating angiogenesis, inflammatory responses, homeostasis as well as and vascular tone and permeability [18]. This important vascular protection function results from a balance of factors released by vascular endothelium that determine vasodilation or vasoconstriction [19]. Vasodilation is primarily mediated by factors such as prostacyclin, Endothelium-Derived Hyperpolarizing Factor (EDHF), and Nitric Oxide (NO), whereas a vasoconstrictor state is mediated by factors such as angiotensin II, Endothelin-1 (ET-1), thromboxane A2, and prostaglandin H2 [20,21]. Among these factors, NO is considered to be the most potent endogenous vasodilator [22,23]. NO is produced from L-arginine and released by the activity of endothelial NO Synthase (eNOS) through the action of chemical agonists that act on specific endothelial chemoreceptors or respond to mechanical forces on mechanoreceptors such as shear stress [24]. A decrease in NO production, low local bioavailability, and inadequate vasomotor response characterize endothelial dysfunction, [19] a hallmark of diabetes mellitus type 2 [25], SAH [26] and dyslipidemia [27] and an early marker of atherosclerosis [28].
Evidence exists that clearly shows that physical exercise can improve the endothelium-dependent vasodilatory capacity in different populations [29]. Increasing physical activity levels and/ or physical exercise is a recommended treatment for SAH in major guidelines [30]. Accumulated evidence supports exercise as a nonpharmacological strategy for the prevention of CVD including ischemic heart disease as endothelial dysfunction precedes the development of these conditions and has a major role in the pathogenesis of atherosclerosis [28].
Regular exercise is a non-pharmacological intervention recommended by all leading health organizations because it provides the most comprehensive benefits among modifiable risk factors [31]. Thus, considering that regular exercise is part of antihypertensive therapy and has shown to improve endothelial function in different populations, this review seeks to provide an overview of the effects of different types of physical exercise on BP levels and endothelial function in hypertensive patients.
Endothelium
The endothelium is a single layer of cells lining the tunica intima of blood vessels and it plays a major role in the modulation of vascular angiogenesis, inflammatory responses, and vascular tone and permeability [18,32]. Endothelial protection particularly involves the activity of eNOS, an enzyme involved in the production of NO.
Endothelium-dependent NO release can be stimulated by different physiological factors including physical stress (shear stress), circulating hormones (catecholamines, vasopressin, and aldosterone), platelet agonists (serotonin and adenosine diphosphate), autacoids (histamine and bradykinin), and prostaglandins [33]. The NO dilates all types of blood vessels by stimulating soluble guanylyl cyclase and increasing cyclic GMP in smooth muscle cells [34]. A decreased production of NO, low local bioavailability, and/or insufficient vasomotor response characterize endothelial dysfunction evidenced in patients with SAH, [19] which has an important role in the pathogenesis of atherosclerosis (Figure 1) [28,35].
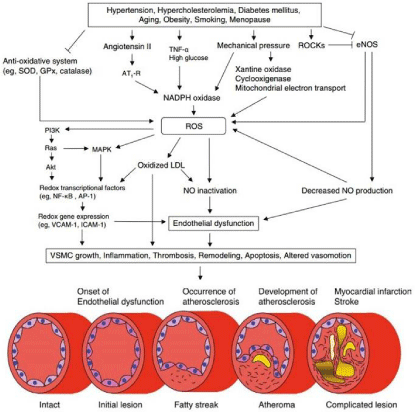
Figure 1: Mechanisms involved in endothelial dysfunction and consequent atherosclerotic disease progression [29].
NO: Nitric Oxide; ROCK: Rho-Associated Kinase; SOD: Superoxide Dismutase; NADH/NADPH: Nicotinamide Adenine Dinucleotide Phosphate; ROS: Reactive
Oxygen Species; HSP: Heat Shock Protein; HIF-1: Hypoxia-Induced Factor-1; VEGF: Vascular Endothelial Growth Factor; eNOS: endothelial NO Synthase; PI3K:
Phosphatidyl-Inositol-3-Kinase; MAPK: Mitogen-Activated Protein Kinase; NF-κB: Nuclear Factor κB; AP-1: Plasminogen Activator Inhibitor-1; VCAM-1: Vascular
Cell Adhesion Molecule-1; ICAM-1: Intracellular Adhesion Molecule-1; VSMC: Vascular Smooth Muscle Cell. Adapted from Higashi Y et al. [35].
In the pathogenesis of atherosclerosis, there is first a qualitative change of the monolayer of endothelial cells lining the inner surface of the arteries when exposed to irritating stimuli such as high BP, dyslipidemia or pro-inflammatory mediators. This change induces endothelial cells to express adhesion molecules that recruit leukocytes to the surface. At the same time there are changes in the permeability and extracellular matrix composition of the endothelium promoting the entry and retention of Low-Density Lipoprotein Cholesterol (LDL-C) [36]. Chemically modified particles in these components may induce leukocyte adhesion, and modified intact particles are endocytosed by macrophages leading to intracellular accumulation of cholesterol. During the progression of an atherosclerotic plaque, leukocytes directly migrate into the intima the innermost layer of an artery, where monocytes differentiate into macrophages that engulf lipoprotein particles and become foam cells [37]. The recruitment of smooth muscle cells of the tunica media to the intima is also involved in the formation of atherosclerotic plaque (atheroma) [37].
After migrating from the middle layer to the intima, smooth muscle cells proliferate in response to mediators such as plateletderived growth factors, and produce extracellular matrix molecules, including interstitial collagen and elastin, to form a fibrous cap covering the plaque [37]. The atherosclerotic plaque is generally rich in foam cells, dead smooth muscle cells, and released lipids that accumulate at its center. Ineffective release of dead cells can promote the accumulation of cellular debris and extracellular lipids creating a lipid-rich necrotic core (Figure 1) [38].
Atherosclerotic plaques can produce the clinical manifestation of angina pectoris with a positive exercise stress test and perfusion defects at imaging studies [39]. Blood flow restriction and stenosis lead to tissue ischemia or thrombus formation which may disrupt blood flow or break loose and become lodged in distal arteries causing acute thrombotic vascular disease including myocardial infarction, cerebral vascular accident, and sudden death [36].
The balance between endothelial injury and recovery is critical to continuously maintaining endothelial function [40]. The bone marrow releases a progenitor cell subtype called Endothelial Progenitor Cells (EPCs) that migrate to the peripheral circulation and differentiate into mature endothelial cells to maintain vascular integrity [27]. EPC levels have been associated with cardiovascular risk factors [41], endothelium-dependent vasodilation [42], and clinical outcomes including hospitalization and cardiovascular death rates [43]. In patients with cardiovascular risk factors such as SAH [44], smoking [45], metabolic syndrome and diabetes mellitus [46,47] circulating levels of EPCs can be reduced compared with healthy individuals of matching age and sex. Endothelial dysfunction has been associated with decreased number and impaired function of EPCs in patients with SAH, diabetes mellitus and metabolic syndrome, and thus increased risk of atherosclerosis would be expected [48].
There is good evidence in the literature of the role of regular exercise in healthy individuals. Regular aerobic exercise is able to improve endothelial function [29], reduce arterial stiffness [49], increase the number of EPCs [50], reduce blood pressure levels [51], and improve cardiopulmonary fitness [52] in health individuals. Resistance training for healthy individuals can improve endothelial function [29] and reduce blood pressure levels [51]; however, it does not appear to have positive effects on arterial stiffness [53]. Compared to aerobic exercise, resistance training stands out by increasing muscle mass [54], bone density [55], and muscle strength [56].
Considering that regular exercise is part of antihypertensive therapy and that it improves endothelial function in healthy subjects, an overview of the effect of exercise on endothelial function in patients with SAH is required.
Exercise in Hypertensive Individuals
Factors associated with potential antihypertensive effects of long-term exercise are not yet fully understood; although it has been proposed exercise can reduce the sympathetic activity [57] and promote a balance between vasodilation and vasoconstriction [58]. Regular exercise, also known as exercise training, produces effects on the cardiovascular system and has a proven action restoring endothelial function even in the presence of cardiovascular disease [59]. It promotes changes in cholesterol and its subfractions; accelerates the removal of chylomicrons and LDL-C from circulation; increases the release of NO that seems to generate increased expression of NOS; increases the number of circulating EPCs in healthy individuals as well as in individuals with established cardiovascular disease [50,60], and improves plasma glucose [61], which has a direct link to impaired endothelial function [62].
This assumption is consistent with findings in healthy individuals [63], individuals with diabetes mellitus type 2 [64] and patients with CVD showing that exercise training improved vascular function and overall health [65]. Particularly aerobic exercise can contribute to reducing mortality due to CVD by about 30%. There is evidence that regular aerobic exercise can prevent loss in endothelium-dependent vasodilation and restore previous levels in middle-aged men, the elderly, and physically inactive individuals [66,67].
The relationship between aerobic exercise and SAH has been widely investigated. Studies have showed a reduction in BP with exercise in the short and long term [31,68]. In a meta-analysis of studies conducted between 1976 and 2012 Cornelissen et al. (2013) provided a recent comprehensive view of the effects of aerobic training as an intervention on BP reduction [51]. They found a mean reduction of 4.7 mmHg in the Systolic Blood Pressure (SBP) and 2.8 mmHg in the Diastolic Blood Pressure (DBP) among males and a lower reduction among females of 0.8 mmHg in SBP and 0.5 mmHg in DBP. Regarding exercise frequency per week, no differences in BP reduction were seen when exercise frequency was less than three times a week (3.9 mmHg in SBP; 2.8 mmHg in DBP) compared to 3 to 4 times (3.5 mmHg in SBP; 2.4 mmHg in DBP) or more than 4 times (3.2 mmHg in SBP; 2.4 mmHg in DBP). These authors also assessed the relationship between BP reduction and exercise duration and found a difference in DBP reduction only for 30 to 45 minute (3.8 mmHg in SBP; 3.3 mmHg in DBP) and more than 45-minute sessions (2.8 mmHg in SBP; 1.9 mmHg in DBP). The magnitude of BP reduction in response to exercise intensity was similar in moderate (4.8 mmHg in SBP; 2.3 mmHg in DBP) and vigorous exercise (3.6 mmHg in SBP; 3.1 mmHg in DBP) [51].
Cross-sectional studies have shown that individuals that exercise regularly have lower arterial stiffness when compared to sedentary ones [62]. A prospective study of 18 healthy young volunteers who followed an aerobic training program on a treadmill for eight weeks found reduced arterial stiffness, which returned to baseline values after a four-week period of detraining [69]. Higashi et al. (1999) assessed forearm blood flow in 17 patients with mild SAH in response to an aerobic training program [66]. After a 12-week training, forearm blood flow values were greater post-training compared to pre-training. This beneficial effect on endothelium-dependent vasodilation is mediated by an increase in endothelial NO. In another study 13 middle-aged sedentary men underwent a 12-week running program (45-min sessions, 5-6 days a week, with gradual intensity increase up to 75% of maximum heart rate) [69]. There was an increase in forearm endothelial function by approximately 30% [70].
Aerobic exercise performed for one hour on an exercise bike, three times a week, for six months at an intensity of 50% of heart rate reserve was shown to increase plasma concentrations of nitrate/ nitrite (NOx), which are metabolites used to indirectly measure NO levels in postmenopausal women with hypertension [71].
Interestingly, increased nitric oxide negatively correlated with BP changes [71]. It is thus evident aerobic exercise training plays an important role in reducing BP and this benefit is associated with improved endothelium-dependent dilation. However, low-intensity aerobic exercise performed on an upper body ergometer, three times a week for 12 weeks, was shown to reduce BP levels without changes in endothelium-dependent dilation in hypertensive subjects [72]. This same response has been described in other studies after moderateintensity aerobic training on a treadmill [58] which confirms that multiple factors are involved in reducing BP in individuals with SAH.
Unlike aerobic training, few studies have investigated the effects of resistance training on SBP and DBP with inconsistent results. Bermudes et al. (2004) did not find any differences in SBP and DBP 24 hours after circuit sessions of resistance training compared to the group who did not exercise [67]. Nascimento et al. (2014) evaluated hypertensive elderly women after a resistance training program [73]. The training sessions were held twice a week for 14 weeks and consisted of six types of exercises, three sets of 8-12 repetitions with a rest period of two minutes at moderate intensity based on Borg’s rating scale of perceived exertion [6-20 point scale]. The authors found reduced SBP and DBP compared to pre-exercise values, which remained after up to 14 weeks of detraining [73].
To assess whether resistance training can be used for SAH prevention and treatment, a meta-analysis reviewed 29 studies and compared normotensive, pre-hypertensive, and hypertensive patient groups [51]. Overall, the analysis showed that resistance training could lead to a mean reduction of 1.8 mmHg in SBP and 3.2 mmHg in DBP. However, no reduction in BP was found when they analyzed in hypertensive patients [51].
Stensvold et al. (2010) have shown that a three-month program of resistance exercises, three times a week, with three sets of 8-12 repetitions at vigorous intensity (80% of One Repetition Maximum - 1 RM) improved endothelial function in patients with metabolic syndrome [74]. This response was independent of changes in total body weight, fasting glucose, lipid profile and BP levels. Interestingly, this result was similar to those found when the patients exposed to aerobic training or combined aerobic and resistance in the same week [74].
The positive effects of resistance training on BP may also be associated with improved endothelium-dependent vasodilation in healthy subjects [75]. However, the effect of resistance training on endothelial function, as well as its relationship to BP in patients with SAH, still needs further investigation. The European Society of Hypertension and the American College of Sports Medicine guidelines for lifestyle changes recommend regular moderateintensity dynamic aerobic exercise for a minimum of 30 minutes 5-7 days a week for the treatment of hypertension [30,76]. Strength exercise training is also recommended as part of a weekly program for the prevention and treatment of hypertension. However, since the amount of evidence on this form of training is more scant, [51] aerobic exercise is often a more recommended first choice. There are few reports of the effects of concurrent or combined training that involves a combination of resistance and aerobic exercise in one training session. Keese et al. (2011) investigated the effects of resistance training followed by aerobic training and found a reduction in SBP that remained for 2 hours after exercise when compared to the control group [77]. A reduction in DBP occurred at 50 minutes post-exercise. Another study assessed the effect of a training program consisting of three concurrent training sessions at different aerobic intensities (50%, 65% and 80% of VO2 max) [78]. The authors found that post-exercise hypotension was longer after the most intense session. However, after the session at an intensity of 65% VO2 max, the post-exercise hypotensive effect on SBP lasted for up to 2 hours and on DBP for 40 minutes [78]. Despite its promising findings, a meta-analysis by Cornelissen and Smart did not show any beneficial effects of concurrent training on BP in hypertensive patients [51]. A study by Kawano et al. (2006) reported increased arterial compliance in young healthy men after combined training (resistance training at 80% of maximum load and 30 minutes of aerobic exercise) [79]. In a physical training protocol proposed by Walsh et al. (2003), they noted an increase in endothelium-dependent and endotheliumindependent vasodilatation in patients with heart failure after an eight-week circuit-training program [80]. The program consisted of a combination of resistance (55-65% of 1-RM) and aerobic training (70-85% HR peak), lasting 40 minutes per session [80].
Conclusion
This brief review showed that overall exercise training could reduce blood pressure, improve the effectiveness of antihypertensive drugs and decrease cardiovascular risk. Various forms of exercise training at different intensities and durations lead to endothelial vascular modulation in hypertensive individuals. Thus, the review addressed new issues such as the effect of resistance exercise training on endothelium modulators in CVD, especially SAH. In brief, moderate-intensity aerobic exercise (60-75% of heart rate reserve), with an average duration of 40 minutes, seems to be the most suitable for improving endothelium-dependent and hypotensive effects. Resistance training at an average intensity of 60-85% of the maximum repetition test (1-RM) also produces significant improvement in endothelial function, although the hypotensive effect is still controversial. Alternatively, an exercise program consisting of a combination of aerobic (60-75% of heart rate reserve) and resistance (50-80% of 1-RM) exercises in a single session (concurrent or combined training) can improve endothelial function and lower blood pressure.
References
- Beevers G, Lip GY, O'Brien E. ABC of hypertension: The pathophysiology of hypertension. BMJ. 2001; 322: 912-916.
- Neal B, MacMahon S, Chapman N; Blood Pressure Lowering Treatment Trialists' Collaboration. Effects of ACE inhibitors, calcium antagonists, and other blood-pressure-lowering drugs: results of prospectively designed overviews of randomised trials. Blood Pressure Lowering Treatment Trialists' Collaboration. Lancet. 2000; 356: 1955-1964.
- Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, Obarzanek E. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med. 2001; 344: 3-10.
- Neal B, MacMahon S, Chapman N; Blood Pressure Lowering Treatment Trialists' Collaboration. Effects of ACE inhibitors, calcium antagonists, and other blood-pressure-lowering drugs: results of prospectively designed overviews of randomised trials. Blood Pressure Lowering Treatment Trialists' Collaboration. Lancet. 2000; 356: 1955-1964.
- Gudmundsdottir H, Strand AH, Høieggen A, Reims HM, Westheim AS, Eide IK, et al. Do screening blood pressure and plasma catecholamines predict development of hypertension? Twenty-year follow-up of middle-aged men. Blood Press. 2008; 17: 94-103.
- Lin Y, Lai X, Chen B, Xu Y, Huang B, Chen Z, et al. Genetic variations in CYP17A1, CACNB2 and PLEKHA7 are associated with blood pressure and/or hypertension in She ethnic minority of China. Atherosclerosis. 2011; 219: 709-714.
- Sander M, Chavoshan B, Victor RG. A large blood pressure-raising effect of nitric oxide synthase inhibition in humans. Hypertension. 1999; 33: 937-942.
- Rossi R, Chiurlia E, Nuzzo A, Cioni E, Origliani G, Modena MG. Flow-mediated vasodilation and the risk of developing hypertension in healthy postmenopausal women. J Am Coll Cardiol. 2004; 44: 1636-1640.
- Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005; 365: 217-223.
- Messerli FH, Williams B, Ritz E. Essential hypertension. Lancet. 2007; 370: 591-603.
- Arima H, Murakami Y, Lam TH, Kim HC, Ueshima H, Woo J, et al. Effects of prehypertension and hypertension subtype on cardiovascular disease in the Asia-Pacific Region. Hypertension. 2012; 59: 1118-1123.
- Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012; 380: 2224-2260.
- Paffenbarger RS Jr, Jung DL, Leung RW, Hyde RT. Physical activity and hypertension: an epidemiological view. Ann Med. 1991; 23: 319-327.
- Fagard RH. Physical activity, physical fitness and the incidence of hypertension. J Hypertens. 2005; 23: 265-267.
- de Simone G, Devereux RB, Chinali M, Roman MJ, Best LG, Welty TK, et al. Risk factors for arterial hypertension in adults with initial optimal blood pressure: the Strong Heart Study. Hypertension. 2006; 47: 162-167.
- Seligman BG, Polanczyk CA, Santos AS, Foppa M, Junges M, Bonzanini L, et al. Intensive practical lifestyle intervention improves endothelial function in metabolic syndrome independent of weight loss: a randomized controlled trial. Metabolism. 2011; 60: 1736-1740.
- Cornelissen VA, Arnout J, Holvoet P, Fagard RH. Influence of exercise at lower and higher intensity on blood pressure and cardiovascular risk factors at older age. J Hypertens. 2009; 27: 753-762.
- Feletou M, Vanhoutte PM. Endothelial dysfunction: a multifaceted disorder (The Wiggers Award Lecture). Am J Physiol Heart Circ Physiol. 2006; 291: 985-1002.
- Soloviev MA, Kulakova NV, Semiglazova TA, Borodulina EV, Udut VV. Correction of endothelial dysfunction in patients with arterial hypertension. Bull Exp Biol Med. 2011; 151: 183-185.
- Woodman CR, Price EM, Laughlin MH. Selected Contribution: Aging impairs nitric oxide and prostacyclin mediation of endothelium-dependent dilation in soleus feed arteries. J Appl Physiol (1985). 2003; 95(5): 2164-2170.
- Cediel E, Vazquez-Cruz B, Navarro-Cid J, de las Heras N, Sanz-Rosa D, Cachofeiro V, et al. Role of endothelin-1 and thromboxane A2 in renal vasoconstriction induced by angiotensin II in diabetes and hypertension. Kidney international Supplement. 2002; 2-7.
- Fitzgerald SM, Bashari H, Cox JA, Parkington HC, Evans RG. Contributions of endothelium-derived relaxing factors to control of hindlimb blood flow in the mouse in vivo. Am J Physiol Heart Circ Physiol. 2007; 293: 1072-1082.
- Zellers TM, Wu YQ, McCormick J, Vanhoutte PM. Prostacyclin-induced relaxations of small porcine pulmonary arteries are enhanced by the basal release of endothelium-derived nitric oxide through an effect on cyclic GMP-inhibited-cyclic AMP phosphodiesterase. Acta pharmacologica Sinica. 2000; 21: 131-138.
- Johnson BD, Mather KJ, Newcomer SC, Mickleborough TD, Wallace JP. Brachial artery flow-mediated dilation following exercise with augmented oscillatory and retrograde shear rate. Cardiovasc Ultrasound. 2012; 10: 34.
- Hodes RJ, Lakatta EG, McNeil CT. Another modifiable risk factor for cardiovascular disease? Some evidence points to arterial stiffness. J Am Geriatr Soc. 1995; 43: 581-582.
- Cheetham C, Green D, Collis J, Dembo L, O'Driscoll G. Effect of aerobic and resistance exercise on central hemodynamic responses in severe chronic heart failure. J Appl Physiol (1985). 2002; 93: 175-180.
- Tousoulis D, Papageorgiou N, Androulakis E, Siasos G, Latsios G, Tentolouris K, et al. Diabetes mellitus-associated vascular impairment: novel circulating biomarkers and therapeutic approaches. J Am Coll Cardiol. 2013; 62: 667-676.
- Anderson TJ, Uehata A, Gerhard MD, Meredith IT, Knab S, Delagrange D, Lieberman EH. Close relation of endothelial function in the human coronary and peripheral circulations. J Am Coll Cardiol. 1995; 26: 1235-1241.
- Ashor AW, Lara J, Siervo M, Celis-Morales C, Oggioni C, Jakovljevic DG, Mathers JC. Exercise modalities and endothelial function: a systematic review and dose-response meta-analysis of randomized controlled trials. Sports Med. 2015; 45: 279-296.
- Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Böhm M, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013; 34: 2159-2219.
- Cornelissen VA, Fagard RH, Coeckelberghs E, Vanhees L. Impact of resistance training on blood pressure and other cardiovascular risk factors: a meta-analysis of randomized, controlled trials. Hypertension. 2011; 58: 950-958.
- Tomiyama H, Yamashina A. Non-invasive vascular function tests: their pathophysiological background and clinical application. Circ J. 2010; 74: 24-33.
- Förstermann U, Münzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation. 2006; 113: 1708-1714.
- Vanhoutte PM. Endothelial dysfunction: the first step toward coronary arteriosclerosis. Circ J. 2009; 73: 595-601.
- Higashi Y, Noma K, Yoshizumi M, Kihara Y. Endothelial function and oxidative stress in cardiovascular diseases. Circ J. 2009; 73: 411-418.
- Tabas I, Williams KJ, Borén J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation. 2007; 116: 1832-1844.
- Glass CK, Witztum JL. Atherosclerosis. the road ahead. Cell. 2001; 104: 503-516.
- Rudijanto A. The role of vascular smooth muscle cells on the pathogenesis of atherosclerosis. Acta Med Indones. 2007; 39: 86-93.
- Libby P, DiCarli M, Weissleder R. The vascular biology of atherosclerosis and imaging targets. J Nucl Med. 2010; 51: 33-37.
- Mattei P, Virdis A, Ghiadoni L, Taddei S, Salvetti A. Endothelial function in hypertension. J Nephrol. 1997; 10: 192-197.
- Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, et al. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001; 89: 1-7.
- Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003; 348: 593-600.
- Werner N, Kosiol S, Schiegl T, Ahlers P, Walenta K, Link A, et al. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med. 2005; 353: 999-1007.
- Liu X, Zhang GX, Zhang XY, Xia WH, Yang Z, Su C, et al. Lacidipine improves endothelial repair capacity of endothelial progenitor cells from patients with essential hypertension. Int J Cardiol. 2013; 168: 3317-3326.
- Puls M, Schroeter MR, Steier J, Stijohann L, Hasenfuss G, Konstantinides S, et al. Effect of smoking cessation on the number and adhesive properties of early outgrowth endothelial progenitor cells. Int J Cardiol. 2011; 152: 61-69.
- Devaraj S, Jialal I. Dysfunctional endothelial progenitor cells in metabolic syndrome. Exp Diabetes Res. 2012; 2012: 585018.
- António N, Fernandes R, Soares A, Soares F, Lopes A, Carvalheiro T, et al. Reduced levels of circulating endothelial progenitor cells in acute myocardial infarction patients with diabetes or pre-diabetes: accompanying the glycemic continuum. Cardiovasc Diabetol. 2014; 13: 101.
- Zerbini G, Lorenzi M, Palini A. Tumor angiogenesis. N Engl J Med. 2008; 359: 763.
- Ashor AW, Lara J, Siervo M, Celis-Morales C, Mathers JC. Effects of exercise modalities on arterial stiffness and wave reflection: a systematic review and meta-analysis of randomized controlled trials. PLoS One. 2014; 9: 110034.
- Silva JF, Rocha NG, Nobrega AC. Mobilization of endothelial progenitor cells with exercise in healthy individuals: a systematic review. Arquivos brasileiros de cardiologia. 2012; 98: 182-191.
- Cornelissen VA, Smart NA. Exercise training for blood pressure: a systematic review and meta-analysis. J Am Heart Assoc. 2013; 2: 004473.
- Montero D, Díaz-Caestro C. Endurance training and maximal oxygen consumption with ageing: Role of maximal cardiac output and oxygen extraction. Eur J Prev Cardiol. 2016; 23: 733-743.
- Miyachi M. Effects of resistance training on arterial stiffness: a meta-analysis. Br J Sports Med. 2013; 47: 393-396.
- Peterson MD, Sen A, Gordon PM. Influence of resistance exercise on lean body mass in aging adults: a meta-analysis. Med Sci Sports Exerc. 2011; 43: 249-258.
- Zhao R, Zhao M, Xu Z. The effects of differing resistance training modes on the preservation of bone mineral density in postmenopausal women: a meta-analysis. Osteoporosis International: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2015; 26: 1605-1618.
- Borde R, Hortobágyi T, Granacher U. Dose-Response Relationships of Resistance Training in Healthy Old Adults: A Systematic Review and Meta-Analysis. Sports Med. 2015; 45: 1693-1720.
- Martinez DG, Nicolau JC, Lage RL, Toschi-Dias E, de Matos LD, Alves MJ, et al. Effects of long-term exercise training on autonomic control in myocardial infarction patients. Hypertension. 2011; 58: 1049-1056.
- Hansen AH, Nyberg M, Bangsbo J, Saltin B, Hellsten Y. Exercise training alters the balance between vasoactive compounds in skeletal muscle of individuals with essential hypertension. Hypertension. 2011; 58: 943-949.
- James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, Lackland DT. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014; 311: 507-520.
- Goto C, Higashi Y, Kimura M, Noma K, Hara K, Nakagawa K, et al. Effect of different intensities of exercise on endothelium-dependent vasodilation in humans: role of endothelium-dependent nitric oxide and oxidative stress. Circulation. 2003; 108: 530-535.
- Moraes MR, Bacurau RF, Simes HG, Campbell CS, Pudo MA, Wasinski F, et al. Effect of 12 weeks of resistance exercise on post-exercise hypotension in stage 1 hypertensive individuals. J Hum Hypertens. 2012; 26: 533-539.
- Madhura M, Sandhya TA. Effect of short duration aerobic exercise training on reflection index, stiffness index and pulse wave velocity. Indian J Physiol Pharmacol. 2012; 56: 15-20.
- Shi L, Morrison JA, Wiecha J, Horton M, Hayman LL. Healthy lifestyle factors associated with reduced cardiometabolic risk. Br J Nutr. 2011; 105: 747-754.
- Montero D, Walther G, Benamo E, Perez-Martin A, Vinet A. Effects of exercise training on arterial function in type 2 diabetes mellitus: a systematic review and meta-analysis. Sports Med. 2013; 43: 1191-1199.
- Vuckovic KM, Piano MR, Phillips SA. Effects of exercise interventions on peripheral vascular endothelial vasoreactivity in patients with heart failure with reduced ejection fraction. Heart Lung Circ. 2013; 22: 328-340.
- Phillips SA, Das E, Wang J, Pritchard K, Gutterman DD. Resistance and aerobic exercise protects against acute endothelial impairment induced by a single exposure to hypertension during exertion. J Appl Physiol (1985). 2011; 110: 1013-1020.
- Taddei S, Galetta F, Virdis A, Ghiadoni L, Salvetti G, Franzoni F, et al. Physical activity prevents age-related impairment in nitric oxide availability in elderly athletes. Circulation. 2000; 101: 2896-2901.
- Higashi Y, Sasaki S, Kurisu S, Yoshimizu A, Sasaki N, Matsuura H, et al. Regular aerobic exercise augments endothelium-dependent vascular relaxation in normotensive as well as hypertensive subjects: role of endothelium-derived nitric oxide. Circulation. 1999; 100: 1194-1202.
- DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, et al. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation. 2000; 102: 1351-1357.
- Zaros PR, Pires CE, Bacci M, Moraes C, Zanesco A. Effect of 6-months of physical exercise on the nitrate/nitrite levels in hypertensive postmenopausal women. BMC Womens Health. 2009; 9: 17.
- Westhoff TH, Schmidt S, Gross V, Joppke M, Zidek W, van der Giet M, et al. The cardiovascular effects of upper-limb aerobic exercise in hypertensive patients. J Hypertens. 2008; 26: 1336-1342.
- Bermudes AM, Vassallo DV, Vasquez EC, Lima EG. Ambulatory blood pressure monitoring in normotensive individuals undergoing two single exercise sessions: resistive exercise training and aerobic exercise training. Arq Bras Cardiol. 2004; 82: 65-71, 57-64.
- Nascimento DaC, Tibana RA, Benik FM, Fontana KE, Ribeiro Neto F, Santana FS, et al. Sustained effect of resistance training on blood pressure and hand grip strength following a detraining period in elderly hypertensive women: a pilot study. Clin Interv Aging. 2014; 9: 219-225.
- Stensvold D, Tjønna AE, Skaug EA, Aspenes S, Stølen T, Wisløff U, Slørdahl SA. Strength training versus aerobic interval training to modify risk factors of metabolic syndrome. J Appl Physiol (1985). 2010; 108: 804-810.
- Gonzales JU, Thompson BC, Thistlethwaite JR, Scheuermann BW. Association between exercise hemodynamics and changes in local vascular function following acute exercise. Appl Physiol Nutr Metab. 2011; 36: 137-144.
- Pescatello LS, Franklin BA, Fagard R, Farquhar WB, Kelley GA, Ray CA; American College of Sports Medicine. American College of Sports Medicine position stand. Exercise and hypertension. Med Sci Sports Exerc. 2004; 36: 533-553.
- Keese F, Farinatti P, Pescatello L, Monteiro W. A comparison of the immediate effects of resistance, aerobic, and concurrent exercise on postexercise hypotension. J Strength Cond Res. 2011; 25: 1429-1436.
- Keese F, Farinatti P, Pescatello L, Cunha FA, Monteiro WD. Aerobic exercise intensity influences hypotension following concurrent exercise sessions. Int J Sports Med. 2012; 33: 148-153.
- Kawano H, Tanaka H, Miyachi M. Resistance training and arterial compliance: keeping the benefits while minimizing the stiffening. J Hypertens. 2006; 24: 1753-1759.
- Walsh JH, Bilsborough W, Maiorana A, Best M, O'Driscoll GJ, Taylor RR, Green DJ. Exercise training improves conduit vessel function in patients with coronary artery disease. J Appl Physiol (1985). 2003; 95: 20-25.
