Abstract
Physical exercise is widely known to be beneficial to health. Physical response of intensive training is associated with various metabolic modifications, related to exercise intensity and/or muscle workload.
Purpose: The purpose of this study was to explore changes in coagulation assessment, as well as inflammatory cytokines, in young male elite cyclists during competitive season training.
Methods: We conducted a prospective study involving 15 elite cyclists of the same team, mean age 19.7 yrs, participating in national and international competitions of the Under 23 category calendar and referring to Sport Medicine Agency of AOU-Careggi, Florence.
Thromboelastometry analysis and biohumoral parameters were obtained at T0 before the training session, T1 one week after the end of the heavy-exercise training season, T2 one week after the end of the cycling race season.
Results: We evidenced a hypercoagulability status related to increased G and MCF values, and a significant increase of ΔMCF (MCF extem-MCF fibtem), expression of platelet contribution to the clot formation (p=0.01). A significant decrease in fibrinolysis activity expressed by ML percentage was observed at T1, which returned to basal levels at T2 (p=0.003). The exercise increased adiponectin concentration from T0 to T2 (p=0.05), as well as TNF- a (P=0.001).
Discussion: Our findings provided evidence for a transient hypercoagulability status at the beginning of the training season in young elite cyclists, a condition requiring attention; this study confirms the long-term beneficial effects of training on metabolism, as well as on the inflammatory function and cardiovascular health.
Keywords: Cycling; Haemostasis; Cytokines; Adiponectin; Exercise; Thromboelastometry
Abbreviations
CT: Clotting Time; MCF: Maximum Clot Firmness; G: Module of Coagulum Elastic Deformation; ML: Maximum Lysis; ΔMCF: Delta Maximum Clot Firmness
Introduction
Physical exercise is widely known to be beneficial to health [1]. Physical response of intensive training is associated with various metabolic modifications, affecting the rate of production or synthesis and the kinetics of the metabolites that are related to exercise intensity and/or muscle damage [2]. Screening for biochemical parameters and their repeated assessment, actually remains a widespread procedure in elite sports athletes. Clinical laboratory analyses useful to understand sport-induced modifications, may allow for providing interventions aimed at optimizing athletes’ performance. During prolonged physical training, the balance between hormones and haemostasis is regulated by an inter-tissue crosstalk. Data from a clinical study, investigating exercise-induced adiponectin response in male rowers during rest and after a period of prolonged exercise training, provided evidence that adiponectin levels changed according to athletes’ performance [3]. The relationship between adiponectin and exercise is object of interest, and findings from literature have shown that the exercise increased serum adiponectin concentration [4].
Beyond hormone response, data from literature suggest that prolonged exercises in cyclists induces increase in pro-inflammatory cytokines, such as TNFa and IL-6, as consequence of an immune response to local damage in the working muscle [5]. Although regular exercise is beneficial for health, physical activity may result from effects on haemostasis, and exercise-related burden of atherothrombotic events may occasionally occur. This is described as “apparent paradox” [6] possibly due to confounding factors such as age, gender, training status, intensity and duration of the exercise. It is noteworthy that the biological relationship between physical exercise and haemostasis, still remain intriguing, and the biological changes induced by physical activity might represent the result of the association between strenuous exercise and a transient hypercoagulability status. Exercise is a potential trigger for clot formation, and different types of exercise may differentially affect haemostatic profile.
Haemostatic variations affect coagulation pathways, and are mainly influenced by exercise intensity, duration and training condition; data from clinical studies suggested that high intensity exercise results in a higher degree of activation of coagulation factors, thus possibly inducing a hypercoagulable status [6].
Cycling is one of the hardest sport disciplines, owing to high demanding training and competition, long-lasting season, high level of completion and high selection within athletes [7].
The aim of this study was to explore changes in coagulation assessment, as well as inflammatory cytokines, in young male elite cyclists during competitive season training.
Methods
Study population
We conducted a prospective study involving 15 elite cyclists of the same team, mean age 19.7 yrs (SD±1), participating in national and international competitions of the Under 23 category calendar and referring to Sport Medicine Agency of AOU-Careggi, Florence, Italy.
They were informed about the experimental procedures and they gave their written informed consent to participate. They belonged to the same sports company to ensure similar training with the same workload and frequency, and they had the same lifestyle. None of them were receiving any medication supplements that might influence the biomedical results. The study was conducted according to policy statement set forth in the Declaration of Helsinki.
Venous blood samples were obtained from all participants before each stage in basal condition for three times during the season, from November 2016 to May 2017. The following sampling times were scheduled: T0: before the training session (November) during which the cyclists were in complete rest; T1: one week after the end of the heavy-exercise training season (December February), cycling training consisted of 20 hours per week during this time (February); T2: one week after the end of the cycling race season during which after the races the number of training hours was the same (May).
Anthropometrics and body composition parameters
For the anthropometric parameter, body height (cm) and mass (kg) were measured using a medical scale (SECA®) with a rod. The Body Mass Index (BMI) was calculated using the body formula mass/ height2 (kg/m2). All the anthropometric measures were performed by the same operator. Whole-body impedance was generated in soft tissues when a current flow passed through the body and was measured using skin electrodes that were placed on the hands and feet (BIA 101, Akern, Florence, Italy). Resistance (RZ, O) was the opposition to the flow of an alternating current, at any current frequency, through intra and extracellular ionic solutions and reflected the amount of body water, while Reactance (XC, O) was the dielectric or capacitive component of cell membranes and organelles, and tissue interfaces. Starting from these variables, the estimate of the body compartments was derived. The participants were subjected to body composition assessments in the same morning with no breakfast, no physical activity in the previous 12 hours and no long trips the previous day [8].
Biochemical analysis
Venous blood samples were obtained from all participants before each stage in basal condition for three times during the season, from November 2016 to May 2017. The following sampling times were scheduled: T0: before the training session (November); T1: one week after the end of the heavy-exercise training season (February); T2: one week after the end of the cycling race season (May).
Samples were analyzed for routine parameters (complete blood count, Prothrombin Time (PT), activated Partial Thromboplastin Time (aPTT), fibrinogen, total colesterol, Low-Density Lipoprotein (LDL), High-Density Lipoprotein (HDL), triglycerides, lipoprotein(a), glucose, alanine aminotransferase, aspartate aminotrasferase, γ-glutamyltrasferase) and several circulating biomarkers will be evaluated:
• Serum levels of total testosterone and cortisol, measured by immunoassay (Immulite 2000, M-Medical System, Italy);
• Adiponectin, interleukin 6 and tumor necrosis factor a levels, measured with commercially available ELISA kits (Mercodia, Uppsala, Sweden and Cloude-Clone Corp., Houston, TX, USA).
Thromboelastometric analysis
Thromboelastometric analysis, which defines whole blood viscoelastic changes during clotting, was achieved by using the ROTEM® system (Werfen Group, Barcelona, Spain). It was performed according to the manufacturer’s protocol within two hours after blood collection and, once initiated, blood coagulation was allowed to run until 60 min. In particular, extrinsic and intrinsic coagulation cascade were studied with EXTEM and INTEM tests; the influence of fibrinogen on clot firmness was estimated with the plateletinactivating FIBTEM test and the effect of fibrinolytic activity were assessed by fibrinolysis inactivating test APTEM. For each test the following ROTEM parameters were analyzed:
• Clotting Time (CT, sec), corresponding to the time from the beginning of the coagulation analysis until an increase in amplitude of 2mm; it reflects the initiation phase of the clotting process;
• Maximum Clot Firmness (MCF, mm), the maximum amplitude in millimeters reached in thromboelastogram, that quantifies the maximum clot firmness of the established whole blood coagulum; it correlates with the platelet count and function as well as with the concentration of fibrinogen;
• G parameter, the shear elastic modulus strength in dyne/ cmq;
• Maximum lysis (ML,%), that quantifies the percentage in reduction of MCF;
• ΔMCF, a calculation of the platelet component to maximum clot firmness (platelet MCF), was derived from ROTEM tests performed with and without platelet inhibition as described by Monaca et al. [9]; it was calculated using the following formula: clot elasticity attributable to platelets was calculated as platelet MCF=MCF EXTEM-MCF FIBTEM.
Statistical analysis
Statistical analysis was performed by using the SPSS (Statistical Package for Social Sciences, Chicago, USA) software for Windows (Version 26.0). The continuos variables were expressed as median ± SD. We used general linear model analysis (ANOVA) in order to assess differences in mean value using the repeated measured variables test. A p-value <0.05 was considered to indicate statistical significance.
Results
Physical characteristics of cyclists are reported in Table 1.
n=15
Age, yrs
19.7±1
Height, cm
178±5
Weight, kg
70.3±6.1
BMI
22.1±1.4
Body Fat, %
7.8±1.2
Fat weight, Kg
7.4±1.5
Fat-free mass, Kg
62.9±5.1
Resistance (Rz)
494±41.2
Reactance (Xc)
62±6
Values are expressed as mean ± SD
Table 1: Physical characteristics of cyclists.
Metabolic and hormonal parameters
Exercise performed in the different stages (from T0 to T2) induced a significant decrease in glucose levels (p=0.05), as well as LDL-c concentrations (p=0.04), whereas a significant increase in HDL-c was found (p=0.01). We observed significant differences in haematological parameters at the considered time points (from T0 to T2) (Table 2).
T0 (n=15)
T1 (n=15)
T2 (n=15)
p
Glucose (mg/dL)
83±8.1
83±5.5
75±8.2
0.05
Triglycerides (mg/dL)
48±12
53±10.6
87 ±41.6
ns
Total Cholesterol (mg/dL)
170±40.4
177±35.8
169±31.1
ns
HDL (mg/dL)
68±16.1
82±16.1
74±10.4
0.01
LDL (mg/dL)
93±30.7
91±27.6
83±40.16
0.04
Lipoprotein (a) (mg/dL)
389±281
345±231.5
345±319.7
ns
Leukocytes (x109/L)
5.24±1.4
4.54±1
5.40±1.01
0.001
Red blood cells (x1012/L)
5.14±0.3
4.88±0.3
4.77 ±0.3
<0.0001
Hemoglobin (g/dL)
15.4±1
14.7±0.9
14.2±0.9
0.003
Hematocrit (%)
45±2.1
44±2.6
43±2.3
0.02
Platelets (x109/L)
200±42.5
191±50.8
200±31.6
ns
Values are expressed as mean +SD
Table 2: Biohumoral, hormonal and haematological parameters of cyclists.
As concerns markers of liver function (AST, ALT, GGT), no significant changes during training timing were observed. Testosterone showed fluctuations during exercise, with a decrease at the first determination (T1), and a restoration of baseline values at T2 (p=0.006). As concerns cortisol levels, an increase from T0 to T2, even though not significant, was observed.
Coagulative parameters
Interestingly, as concerns coagulative assessment, a significant increase of Prothrombin Time (PT) and fibrinogen according to time points was observed (p=0.004 and p=0.01, respectively), and aPTT values decreased, even if not significantly, from T0 to T2 (Table 3).
T0 (n=15)
T1 (n=15)
T2 (n=15)
p
PT, %
86±10.3
93±8.5
96±10.1
0.004
aPTT, sec
32±3.5
29±4.5
30±3.1
ns
Fibrinogen, (mg/dL)
333.4±97.3
358.3±110.5
357.7±69.5
0.01
CT, sec
62±7.3
59±9.4
61±5.9
ns
MCF, sec
58.5±4.1
60.6±4.3
59.8±4.6
0.06
Δ MCF, sec
46±2.9
48.7±2.7
46.6±3.7
0.01
ML (%)
8±3.6
4±4.1
8±3.4
0.003
G
7250±1291
7639±1548
7668±1615
Ns
Values are expressed as mean +SD. CT: Clotting Time; MCF: Maximum Clot Firmness; ML: Maximum Lysis; G: module of coagulum elastic deformation.
Table 3: Coagulative and thromboelastometry parameters of cyclists.
We performed a further analysis by evaluating thromboelastometry parameters, a global test able to provide information on both coagulative and fibrinolytic pathway. Our findings provided evidence for a hypercoagulability status related to increased G and MCF values; we found a significant increase of ΔMCF (MCF extem–MCF fibtem), expression of platelet contribution to the clot formation (p=0.01). Moreover, a significant decrease in fibrinolysis activity expressed by ML percentage was observed at T1, which returned to basal levels at the end of cycling race season (T2) (p=0.003) (Table 3).
Adiponectin and pro-inflammatory cytokines (IL-6 and TNF-a) are reported in Figure 1. The exercise increased adiponectin concentration from T0 to T2 (p=0.05), as well as TNF- a (P=0.001). IL-6 levels increased from T0 to T2, even if not significantly.
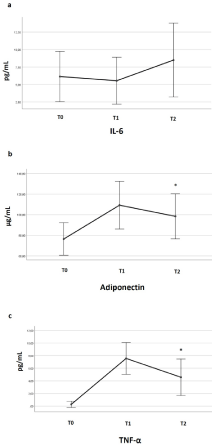
Figure 1: The impact of cycling training and race season on cytokines.
Changes in cytokines: IL-6 (a), adiponectin (b), and TNF-a (c) during the
season at T0 (before the training session), T1 (one week after the end of the
heavy-exercise training season, T2 (one week after the end of the cycling
race season).
Values are means ± SD. *p<0.05.
Discussion
In this study, we evidenced that elite cyclists respond to a strenuous and long-duration by increasing inflammatory cytokines and influencing haemostasis, with transient increase in blood hypercoagulability.
We observed a significant increase of TNF-a levels from resting to the end of the cycling race season, with the greatest peak occurred one week after the end of the heavy-exercise training session (T1). This inflammatory response may be possibly due to skeletal muscle workload that could be related to the synthesis of stress factors by the muscle [10].
On the other side, IL-6 showed an inverse trend in comparison to that observed in TNF-a levels, possibly hypothesizing an antiinflammatory effect able to counterbalance TNF-a inflammatory response. It is known that during and following exercise, the skeletal muscle markedly increases both cellular and circulating levels of IL-6 and that this transient rise in circulating concentrations of IL-6 is responsible for a subsequent increase levels of the anti-inflammatory cytokines [11]. Moreover, IL-6 could also exerts anti-inflammatory effect simultaneously stimulating the release of cortisol from the adrenal glands, stress hormone secreted one week after the end of the cycling race season (T2) [12], which may suppress inflammation by increasing the synthesis of several anti-inflammatory proteins [13]. It has been described reduced serum concentration of total testosterone after a successful period of heavy-exercise training season. Our data are in keeping with this observation, thus permitting to support the hypothesis that an upregulation of testosterone was associated with increased androgen receptor expression [14].
It is noteworthy that pro-inflammatory cytokines are capable of activating the coagulation system.
In our study, we observed an increase of PT percentage and an aPTT reduction, as well as fibrinogen increase after heavy-exercise training season and after cycling race season, which could suggest a pro-coagulant milieu.
Among laboratory tests, recently the viscoelastic properties of blood clot have been studied in athletes by using thromboelastometry, a whole blood point of care coagulation testing, widely used to diagnose coagulopathy [15].
Coagulation activation in response to exercise may be supported by pro-coagulant changes documented by ROTEM analysis, which assess the haemostatic profile of whole blood. Our findings showed an increased MCF, expression of a stronger clot, as well as G (shear elastic modulus strength) progressively increase during training and cycling race season (from T0 to T2).
At variance with previous data reporting increased platelet count in long-term strenuous exercise [6], we did not demonstrate changes in platelet count during training and cycling race season. Nevertheless, we evidenced a significant increase of platelets contribution to clot strength, by calculating ΔMCF parameter, representing difference in amplitude between the EXTEM and the FIBTEM MCF [9]. Therefore, we could hypothesized platelet activation mediated by an inflammatory status heavy exercise-related, in particular by increased TNF-a levels.
Unexpectedly, data from ROTEM evidenced a reduced fibrinolytic activity, expressed by Maximum Lysis (ML) one week after the end of the heavy-exercise training season, thus restoring basal values one week after the end of the cycling race season.
This finding is at variance with data from literature, nevertheless, confounding variables, such as behavioral conditions, type, intensity and duration of exercise, laboratory variability undermined available studies. In our study, we could hypothesize that hydration, not optimal at the first evaluation, or the adrenergic stimulation, which occurs by transiting from a period of rest to one of intense training, may explain our results.
Notably, adiponectin is involved in the pathogenesis of atherosclerosis and cardiovascular diseases through several mechanisms related to glucose and lipid metabolism. Moreover, adiponectin could exert beneficial effects by reducing inflammatory reactions [16] and in increasing endothelial function [17]. Our findings showed that heavy-exercise was associated to increased adiponectin concentrations and with an improvement in metabolic profile, related to decreased glucose and LDL-c concentrations, and increased HDL-c levels.
Few data described chronic effects of exercise on adipokines, but the role of adiponectin in response to prolonged heavy training stress in highly trained rowers was clearly demonstrated, thus suggesting that there should be a wide reserve to increase stress hormone responses to exercise [3].
The present study has a limitation that needs to be addressed. Among specific adipocytokines related to prolonged exercise training, we did not evaluate leptin, since we are aware that adiponectin and leptin may give the advantage of knowing the amount of stress affecting the organism.
Conclusion
Our findings provided evidence for a transient hypercoagulability status at the beginning of the training season in young elite cyclists, a condition requiring attention; on the other hand, this study confirms the long-term beneficial effects of training on metabolism, as well as on the inflammatory function and cardiovascular health.
This represents a “pilot” study, which through a more accurate and in-depth global haemostatic test, might contribute to clarify the role of prolonged heavy exercise inducing a transient hypercoagulable state in elite cyclists. Moreover, our findings might be useful to improve training planning, performance and the recovery strategies of cyclists. Larger and further studies are warranted to support our results.
References
- Pedersen BK. Anti-inflammatory effects of exercise: role in diabetes and cardiovascular disease. Eur J Clin Invest. 2017; 47: 600-611.
- Jürimäe J, Mäestu J, Jürimäe T, Mangus B, von Duvillard SP. Peripheral signals of energy homeostasis as possible markers of training stress in athletes: a review. Metabolism. 2011; 60: 335-350.
- Jürimäe J, Purge P, Jürimäe T. Adiponectin and stress hormone responses to maximal sculling after volume-extended training season in elite rowers. Metabolism. 2006; 55: 13-19.
- Sirico F, Bianco A, D’Alicandro G, Castaldo C, Montagnani S, Spera R, et al. Effects of Physical Exercise on Adiponectin, Leptin, and Inflammatory Markers in Childhood Obesity: Systematic Review and Meta-Analysis. Child Obes. 2018; 14: 207-217.
- Cordova A, Monserrat J, Villa G, Reyes E, Alvarez-Mon Soto M. Effects of AM3 (Inmunoferon) on Increased Serum Concentrations of interleukin-6 and Tumour Necrosis Factor Receptors I and II in Cyclists. J Sports Sci. 2006; 24: 565-573.
- Posthuma JJ, van der Meijden PEJ, Ten Cate H, Spronk HMH. Short- And Long-term Exercise Induced Alterations in Haemostasis: A Review of the Literature. Blood Rev. 2015; 29: 171-178.
- Lombardi G, Lanteri P, Fiorella PL, Simonetto L, Impellizzeri FM, Bonifazi M, et al. Comparison of the Hematological Profile of Elite Road Cyclists During the 2010 and 2012 GiroBio Ten-Day Stage Races and Relationships With Final Ranking. PLoS One. 2013; 30; 8: e63092.
- Mascherini G, Petri C, Calà P, Bini V, Galanti G. Lifestyle and resulting body composition in young athletes. Minerva Pediatr. 2016.
- Monaca E, Strelow H, Jüttner T, Hoffmann T, Potempa T, Windolf J, et al. Assessment of hemostaseologic alterations induced by hyperbaric oxygen therapy using point-of-care analyzers. Undersea Hyperb Med. 2014; 41: 17- 26.
- Córdova Martínez A, Martorell Pons M, Gomila SA, Tur Marí JA, Pons Biescas A. Changes in Circulating Cytokines and Markers of Muscle Damage in Elite Cyclists During a Multi-Stage Competition. Clin Physiol Funct Imaging. 2015; 35: 351-358.
- Gleeson M, Bishop NC, Stensel DJ, Lindley MR, Mastana SS, Nimmo MA. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol. 2011; 11: 607-615.
- Cosio-Lima LM, Desai BV, Schuler PB, Keck L, Scheeler L. A comparison of cytokine responses during prolonged cycling in normal and hot environmental conditions. J Sports Med. 2011; 2: 7-11.
- Barnes PJ. Anti-inflammatory actions of glucocorticoids: molecular mechanisms. Clin Sci (Lond). 1998; 94: 557-572.
- Sylta Ø, Tønnessen E, Sandbakk Ø, Hammarström D, Danielsen J, Skovereng K, et al. Effects of High-Intensity Training on Physiological and Hormonal Adaptions in Well-Trained Cyclists. Med Sci Sports Exerc. 2017; 49: 1137-1146.
- Serraino GF, Murphy GJ. Effects of cerebral near-infrared spectroscopy on the outcome of patients undergoing cardiac surgery: a systematic review of randomised trials. BMJ Open. 2017; 7: e016613.
- Yanai H, Yoshida H. Beneficial Effects of Adiponectin on Glucose and Lipid Metabolism and Atherosclerotic Progression: Mechanisms and Perspectives. Int J Mol Sci. 2019; 20: 1190.
- Torigoe M, Matsui H, Ogawa Y, Murakami H, Murakami R, Wu Cheng X, et al. Impact of the High-Molecular-Weight Form of Adiponectin on Endothelial Function in Healthy Young Men. Clin Endocrinol (Oxf). 2007; 67: 276-281.
