Research Article
J Stem Cells Res, Rev & Rep. 2014;1(1): 1004.
Immunohistochemical Localization of Stem Cell-Related Markers (CD133, Nestin and Bmi-1) and mTOR in the Pancreas of Rat Models of Type 1 and Type 2 Diabetes Mellitus
Murata E1, Matsumoto S2, Shuto M1 and Akita M2*
1Department of Health and Medical Care, Saitama Medical University, Japan
2Division of Morphological Science, Biomedical Research Center, Saitama Medical University, Japan
*Corresponding author: Masumi Akita, Division of Morphological Science, Biomedical Research Center, Saitama Medical University, 38 Moroyama, Iruma-gun, Saitama 350-0495, Japan
Received: Aug 07, 2014; Accepted: Aug 20, 2014; Published: Aug 21, 2014
Abstract
Stem cell-related markers (CD133, nestin and Bmi-1) and mammalian target of rapamycin (mTOR) were detected in the pancreas of rat models of type 1 and type 2 Diabetes Mellitus (DM). As a model of type 1 DM, Komeda Diabetes-Prone (KDP) rat and control rat (KND rat) were used. As a model of type 2 DM, spontaneously diabeticTorii (SDT) rat and control rat (SD rat) were used. In each control rat, pancreatic islets were strongly positive for CD133, nestin, Bmi-1and mTOR. In the KDP rat, CD133, nestin, Bmi-1and mTOR positive cells did not show cellular mass like pancreatic islets. These positive cells were associated with invasion by mononuclear cells. Partial acinar cells showed increased immunoreactivity for CD133, Bmi-1 and mTOR, although nestin immunoreactivity was almost negative. In the SDT rat, CD133, nestin, Bmi-1and mTOR positive cells also did not show cellular mass like pancreatic islets. These positive cells were associated with increased fibrillar element. Strong immunoreactivity for CD133, Bmi-1 and mTOR was observed in the pancreatic ductal epithelial cells and partial acinar cells compared with control rat. Nestin immunoreactivity was almost negative. Although β-cell denaturation in the pancreatic islet is present in the rat models of DM, pancreatic acinar and ductal epithelial cells express immunoreactivity for CD133, Bmi-1 and mTOR. Present study suggests that pancreatic acinar and ductal epithelial cells expressing CD133, Bmi-1 and mTOR are considerable as an alternative source of pancreatic stem/progenitors, even individuals who showed a diabetic condition.
Keywords: CD133; Nestin; Bmi-1; mTOR; Type 1 diabetes; Type 2 diabetes; KDP rat; SDT rat; Pancreas
Introduction
Type 1 and type 2 Diabetes Mellitus (DM) are the two main forms of DM. Both types are characterized by progressive β-cell failure in the pancreatic islet [1]. In type 1 DM, this is typically caused by an autoimmune assault against the β-cells, inducing progressive β-cell death [1]. The pathogenesis of type 2 DM is more variable, comprising different degrees of β-cell failure relative to varying degrees of insulin resistance [1]. Pancreatic islet transplantation is a promising method to restore functional islet β-cell mass for patients with DM [2]. Pancreatic islet transplants hold significant potential advantages over whole-gland transplants [3]. However, pancreatic islet transplantation is associated with several clinically relevant risks [4-6]. Because of the limited supply of human donor islets, it is critical that new strategies are explored as alternative renewable sources of transplantation [7]. Stem cells are characterized by extensive proliferation and multilineage differentiation capacity [8]. Mouse embryonic stem cells differentiate into cells of all three primary germ layers including endodermal cells that produce insulin in vitro [9]. Transplanted embryonicstem cells [9-11] and fetal pancreatic progenitor cells [12, 13] have been successfully used to treat DM in murine models. Multipotent adult stem cells are harvested from bone marrow [14,15], adipose tissue [16] and umbilical cord and placenta [17]. Stem cells, as alternative islet cell sources, may be a valuable source for cell replacement therapy. In the pancreatic islet, CD133 [18,19], nestin [7,20-22] and B cell-specific Moloney murine leukemia virus integration site 1 (Bmi-1) [23] have been reported as molecular markers of pancreatic stem cells. Recently, Yilmaz et al. [24] show that reduction in caloric intake inhibits the nutrientsensitive kinase mammalian target of rapamycin (mTOR) pathway and amplify intestinal stem cell numbers during caloric restriction.
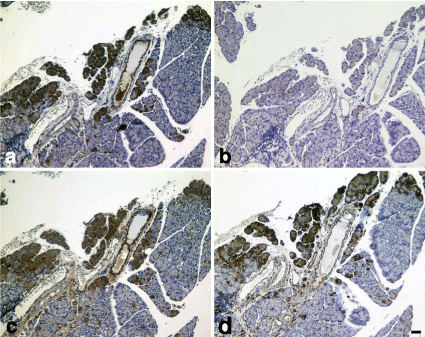
1a) CD133, 2a) nestin, 3a) Bmi-1 and 4a) mTOR immunostaining.CD133, nestin, Bmi-1 and mTOR positive cells (dark brown) were observed with invasion of mononuclear cells (*). Figures 1b-4b: KND rat (control).
1b) CD133, 2b) nestin, 3b) Bmi-1 and 4b) mTOR immunostaining. CD133, nestin, Bmi-1 and mTOR positive cells (dark brown) were observed in the pancreatic islet. There were no invasions of mononuclear cells. Cell nuclei were counterstained with hematoxylin. Scale bar= 20 μm.
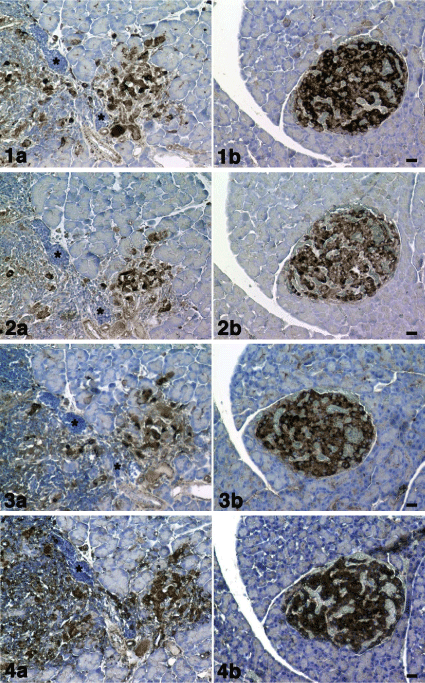
Figure 1: Figure 1a - 4a: KDP rat (model of type 1 DM).
1a) CD133, 2a) nestin, 3a) Bmi-1 and 4a) mTOR immunostaining.CD133,
nestin, Bmi-1 and mTOR positive cells (dark brown) were observed with
invasion of mononuclear cells (*).
Figures 1b-4b: KND rat (control).
1b) CD133, 2b) nestin, 3b) Bmi-1 and 4b) mTOR immunostaining. CD133,
nestin, Bmi-1 and mTOR positive cells (dark brown) were observed in the
pancreatic islet. There were no invasions of mononuclear cells. Cell nuclei
were counterstained with hematoxylin. Scale bar= 20 μm.
Many experimental animal models have contributed to basic and clinical research of DM. Immunohistological analysis using these stem cell-related markers and animal models of type 1 and type 2 DM should provide useful information for understanding the molecular mechanism of DM. However, there is almost no information of these stem cell-related markers in the animal models. In the present study, we detected CD133, nestin, Bmi-1and mTOR in the pancreas of KDP rat, SDT rat and each control rat. Pancreatic islets were strongly positive for CD133, nestin, Bmi-1and mTOR in each control rat. CD133, nestin, Bmi-1 and mTOR positive cells in the pancreas did not show cellular mass like pancreatic islets in both rat models of DM. In the KDP rat, partial acinar cells showed increased Immunoreactivity for CD133, Bmi-1and mTOR. Strong Immunoreactivity for CD133, Bmi-1 and mTOR was shown in the pancreatic ductal epithelial cells and partial acinar cells. Nestin Immunoreactivity in these sites was not observed in both rat models.
Materials and Methods
Animals
Komeda diabetes-prone (KDP) rat: KDP rat developed by Komeda from Long-Evans Tokushima lean (LETL) rat at Tokyo Medical College in 1996. Komeda non-Diabetic (KND) rat was established simultaneously as a control [25]. The KDP rats (male, n=5) and KND rats (male, n=3) were available from Japan SLC (Shizuoka, Japan).
Spontaneously Diabetic Torii (SDT) rat: SDT rats from Japan Tobacco colonies were used in this study. Age-matched Sprague- Dawley (SD) rats were used as control animals. SDT rats (male, n=5) and SD rats (male, n=3) were purchased from CLEA Japan Inc. (Tokyo, Japan).
All animals were given water in drinking water ad libitum for 10 weeks, from 6 to 15 weeks of age. All animals were housed in suspended bracket cages and given a standard laboratory diet (EF, Oriental Yeast Co., Ltd, Tokyo, Japan) in a room with controlled temperature (23 ± 2°C), humidity (55 ± 10%) and lighting (12:12-h light-dark cycle).
All animal experiments were approved by the Committee on Animal Experimentation, Saitama Medical University (Permission No. 1214, 1396) and carried out in accordance with the “Guidelines for Animal Experimentation at Saitama Medical University”.
Diabetes was defined as glycosuria positivity and blood glucose levels ≤ 250 mg/dL under ad libitum dietary conditions. Body weight of each rat was measured. Blood glucose levels were measured by a portable glucose meter (Glutest Neo alpha, Sanwa Kagaku Kenkyusho Co, Nagoya, Japan).
Immunohistochemistry
Tissues were fixed in 10% formalin/PBS, and paraffin sections (5μm thick) were prepared. Streptavidin/peroxidase immunohistochemistry for CD133, nestin and Bmi-1 was performed. The specimens were treated with 0.3% H2O2 in methanol to block endogenous peroxidase activity and subsequently incubated with primary antibodies CD133 (1/100 dilution, rabbit polyclonal antibody; Abnova, Taipei, Taiwan), nestin (1/200 dilution, rabbit polyclonal antibody; Santa Cruz Biotechnology, Inc., Santa Cruz, CA), Bmi-1 (1/200 dilution, rabbit polyclonal antibody; Proteintech, Chicago, IL) and mTOR (1/200 dilution, rabbit polyclonal antibody; Abcam, Cambridge, UK) for 18 hours at 4°C. A biotinylated anti-rabbit immunoglobulin was added as a secondary antibody for 1 hour at room temperature. A horseradish peroxidase-labelled streptomycinavidin complex was then used to detect the secondary antibody. Antibody binding was detected by staining with the chromogens 3,3’-diaminobenzidine (DAB). Cell nuclei were counterstained with hematoxylin. Some paraffin sections were stained with hematoxylin and eosin.
Results
Komeda diabetes-prone (KDP) rat (model of type 1 DM)
Pancreatic islets were positive for CD133, nestin, Bmi-1and mTOR in the KND rat (control). In the KDP rat, CD133, nestin and Bmi-1 positive cellular mass observed in the control rat was not observed. CD133, nestin, Bmi-1 and mTOR positive cells were associated with invasion by mononuclear cells (Figures 1-4). Figures 1a-4a are the pancreas of a KDP rat (blood glucose level = 577 mg/dL). Figures 1b-4b are the pancreas of a KND rat (blood glucose level = 95 mg/dL). Partial acinar cells showed the increased Immunoreactivity for CD133, Bmi-1and mTOR, although nestin immunoreactivity was almost negative (Figure 5).

Figure 5: KDP rat (model of type 1 DM).
a) CD133, b)nestin,c) Bmi-1 and d) mTOR immunostaining. Partial acinar
cells (dark brown) showed the increasedimmunoreactivityfor CD133, Bmi-1
and mTOR (Figs. 5a, c, d). Cell nuclei were counterstained with hematoxylin.
Scale bar= 40 μm.
Spontaneously Diabetic Torii (SDT) rat (model of type 2 DM)
Pancreatic islets were positive for CD133, nestin, Bmi-1 and mTOR in the SD rat (control). In the SDT rat, CD133, nestin, Bmi-1 and mTOR positive cellular mass observed in the control rat was not observed. CD133, nestin, Bmi-1 and mTOR positive cells were associated with increasing fibrillar element (Figures 6-9). Decreased numbers of nestin positive cells were observed. These pathological changes in the pancreas of SDT rats differ from KDP rat, in which mononuclear cells infiltration into pancreatic islets is observed. Figures 6a-9a are the pancreas of a SDT rat (blood glucose level = 397 mg/dL). Figures 6b-9b are the pancreas of a SD rat (blood glucose level = 126 mg/dL). The strong Immunoreactivity for CD133, Bmi- 1 and mTOR was observed in the pancreatic ductal epithelial cells (Figures 6a,8a,9a) compared with SD rat (Figures 10a,c,d). Nestin Immunoreactivity was almost negative (Figures 7b,10b).
6a) CD133, 7a) nestin, 8a) Bmi-1 and 9a) mTOR immunostaining. CD133, nestin, Bmi-1 and mTOR positive cells (dark brown) were observed in the increasing fibular element. Decreased numbers of nestin positive cells were observed. Pancreatic ducts (*). Immunoreactivity for CD133 was moderately positive in the epithelial cells of pancreatic ducts. Immunoreactivity for nestin was almost negative. Immunoreactivity for Bmi-1 was strongly positive in the epithelial cells of pancreatic ducts. Immunoreactivity for mTOR was moderately positive in the epithelial cells of pancreatic ducts.
Figures 6b-9b: SD rat (control).
6b)CD133, 7b) nestin, 8b) Bmi-1 and 9b) mTOR immunostaining. CD133, nestin, Bmi-1 and mTOR positive cells (dark brown) were observed in the pancreatic islet. There was no increasing fibular element. Cell nuclei were counterstained with hematoxylin. Scale bar= 20 μm.
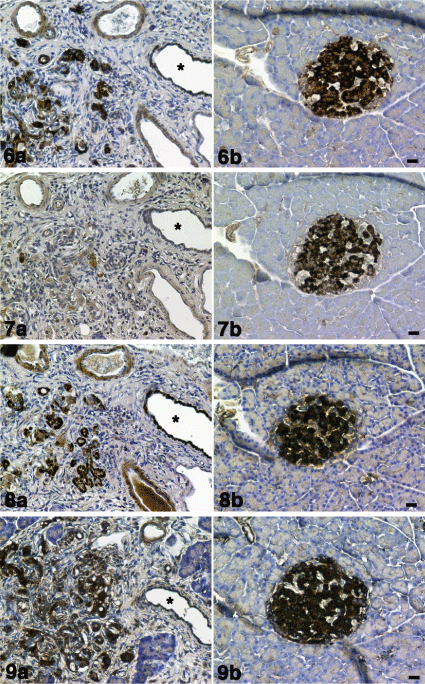
Figure 6-9: SDT rat (model of type 2 DM).
6a) CD133, 7a) nestin, 8a) Bmi-1 and 9a) mTOR immunostaining.
CD133, nestin, Bmi-1 and mTOR positive cells (dark brown) were observed
in the increasing fibular element. Decreased numbers of nestin positive
cells were observed. Pancreatic ducts (*). Immunoreactivity for CD133 was
moderately positive in the epithelial cells of pancreatic ducts. Immunoreactivity
for nestin was almost negative. Immunoreactivity for Bmi-1 was strongly
positive in the epithelial cells of pancreatic ducts. Immunoreactivity for mTOR
was moderately positive in the epithelial cells of pancreatic ducts.
Figures 6b-9b: SD rat (control).
6b)CD133, 7b) nestin, 8b) Bmi-1 and 9b) mTOR immunostaining. CD133,
nestin, Bmi-1 and mTOR positive cells (dark brown) were observed in the
pancreatic islet. There was no increasing fibular element. Cell nuclei were
counterstained with hematoxylin. Scale bar= 20 μm.
a)CD133,b) nestin, c)Bmi-1 and d) mTOR immunostaining. Pancreatic ducts (*). Immunoreactivity for CD133 was negative or slightly positive in some epithelial cells of pancreatic ducts. Immunoreactivity for nestin was almost negative. Immunoreactivity for Bmi-1 was weekly positive in the epithelial cells of pancreatic ducts. Immunoreactivity for mTOR was slightly positive in the epithelial cells of pancreatic ducts. Pancreatic islets (white *).Cell nuclei were counterstained with hematoxylin. Scale bar= 20 μm.
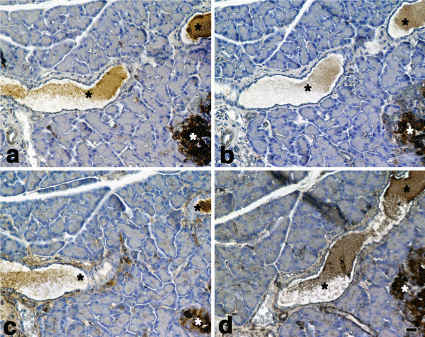
Figure 10: SD rat (control).
a)CD133,b) nestin, c)Bmi-1 and d) mTOR immunostaining.
Pancreatic ducts (*). Immunoreactivity for CD133 was negative or slightly
positive in some epithelial cells of pancreatic ducts. Immunoreactivity for
nestin was almost negative. Immunoreactivity for Bmi-1 was weekly positive
in the epithelial cells of pancreatic ducts. Immunoreactivity for mTOR was
slightly positive in the epithelial cells of pancreatic ducts. Pancreatic islets
(white *).Cell nuclei were counterstained with hematoxylin. Scale bar= 20 μm.
Discussion
There are many rat models of type 1 DM, see review [26] and type 2 DM, see review [27]. In the present study, we used KDP rat as a model of type 1 DM and SDT rat as a model of type 2 DM. The phenotypic features of the KDP rat are resembles human type 1 DM because of autoimmune destruction of the pancreatic β-cells, rapid onset of overt DM with no sex difference, and lack of significant T-cell lymphopenia [25,28]. In the human type 2 DM, inflammation and fibrosis in the pancreatic islets have been described [29]. The phenotypic features of the SDT rat are resembles human type 2 DM because of inflammation and fibrosis in the pancreatic islets [28,30,31].
Present study showed that pancreatic islets were CD133, nestin and Bmi-1 positive in both control rat. In the KDP rat, mononuclear cells infiltration into pancreatic islets is observed. Pancreatic β-cells are the target of an autoimmune assault in type 1 DM, with invasion of the islets by mononuclear cells in an inflammatory reaction termed “insulitis,” leading to loss of most β-cells after prolonged periods of disease [32]. On the other hand, SDT rat characterized by inflammation and fibrosis in and around the islets [28,30,31].
Present study also showed that increased immunoreactivity for CD133 and Bmi-1 was observed in the pancreatic ductal epithelial cells and acinar cells. Bmi-1 is essential for maintenance of adult stem cell self-renewal primarily due to its ability to repress the Ink4a/Arf locus [33-36]. Dhawan et al. [23] reported that Bmi-1-dependent regulation of the Ink4a/Arf locus plays a critical role in regulating pancreatic β-cell proliferation during aging and regeneration. On the other hand, the biological function of CD133 is not well understood. Recently, we reported the co-localization of CD133 with F-actin. It is strongly suggested that CD133 is involved in cell migration [37]. We also reported that CD133 was mainly localized to plasma membrane in the late stages of cultivation. After treatment with CD133 antibody, cell-cell contact was decreased, and apoptosis was increased. It is suggested that CD133-mediated regulation may be required for cell survival [38]. Increased immunoreactivity for CD133 and Bmi-1 may be attributed by the denaturation of β-cells. Insulin and Insulin-Like Growth Factors (IGFs) are prominent inducers of mTOR activity [39,40]. In the diabetic rats used in this study, reduced insulin secretion by the denaturation of β-cells can cause the decreased activity of mTORC1. In SDT rats, blood insulin concentration tended to be lower than in normal SD rats even before the onset of diabetes, and marked hypoinsulinemia developed after the onset of hyperglycemia [31,41,42]. Matsubara et al. [43] noted that the percentage of CD133+ cells inxenograftsfrom pancreatic cells showed the tendency to increase after rapamycin (mTOR inhibitor) treatment, which was not shown in vitro. They noted some differences between in vitro and in vivo. In the present study, increased immunoreactivity of mTOR was observed in the partial acinar and pancreatic ductal epithelial cells. Increased immunoreactivity of mTOR is not attributed insulin. Influence of hyperglycemia must also be considered. mTOR pathway is involved in sensing of nutrient availability and modulation of insulin action in vivo [44]. Um et al. [45] and Khamzina et al. [46] indicate that the activity of the mTOR pathway is increased in rodent models of obesity and that mice deficient in S6 kinase (S6K), a downstream effector of mTOR, are protected against dietinduced insulin resistance. Further study is required to elucidate the issue. Nestin expression was significantly decreased in the heart of diabetic rats [47]. Liu et al. [48] reported that nestin expression in the glomeruli was gradually decreased with the development of diabetic nephropathy. On the other hand, the expression of cyclin-dependent kinase 5 (Cdk5) was increased. They suggest that Cdk5 may relate degradation of nestin.
Koblas [19] used human pancreatic acinar tissue that remains after islet isolation and is discarded. They found that population of human CD133-positive pancreatic cells contains endocrine progenitors expressing neurogenin-3 and cells expressing human telomerase, ABCG2, Oct-3/4, Nanog, and Rex-1, markers of pluripotent stem cells. These cells were able to differentiate into insulin-producing cells in vitro and secreted C-peptide in a glucose-dependent manner [19]. Ohshima et al. [49] isolated mouse pancreatic ductal progenitor cells expressing CD133 by flow cytometric cell sorting. Bonner-Weir et al. [50] showed that human pancreatic ductal epithelial cells could be expanded and differentiated into glucose responsive islet tissue in vitro given insulin. Ramiya et al. [51] isolated murine pancreatic ductal epithelial cells into culture, and induced them into functional islets containing alpha, beta and delta cells. These resulting islets showed temporal changes in mRNA transcripts for islet cell associated differentiation markers, responded to glucose challenge in vitro and reversed insulin-dependent diabetes after being implanted into nonobese diabetic mice. Multipotent stem/progenitors are present in peribiliary glands of extrahepatic biliary trees from humans of all ages and in high numbers in hepato-pancreatic common duct, cystic duct, and hilum [52]. It was shown that extrahepatic bile ducts in mice have β-cells, with secretory granules that are immunoreactive for insulin and that exhibit glucose-stimulated insulin secretion [53]. Their histological studies indicate that the β-cells form directly from the bile duct epithelium in late embryogenesis.
Pancreatic ductal epithelial cells and acinar cells expressing CD133, Bmi-1 and mTORare considerable as an alternative source of stem/progenitors for targeted therapies and regenerative medicine for diabetes.
Conclusion
Stem cell-related markers (CD133, nestin and Bmi-1) and mTOR were detected in the pancreas of rat models of type 1 and type 2 DM. Although β-cell denaturation in the pancreatic islet was present in both rat models of DM, pancreatic acinar and ductal epithelial cells express CD133, Bmi-1 and mTOR. Nestin immunoreactivity in these sites was not observed in both rat models. Pancreatic acinar and ductal epithelial cells expressing CD133, Bmi-1 and mTOR are considerable as an alternative source of stem/progenitors, even individuals who showed a diabetic condition.
Acknowledgment
This work was partly supported by the programs of the Grant-in- Aid SMU-FHMC Grant 14-013.
References
- Cnop M, Welsh N, Jonas JC, Jörns A, Lenzen S, Eizirik DL. Mechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes: many differences, few similarities. Diabetes. 2005; 54: S97-107.
- Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Wamock GL, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000; 343: 230-238.
- Robertson RP, Davis C, Larsen J, Stratta R, Sutherland DE. American Diabetes Association. Pancreas and islet transplantation in type 1 diabetes. Diabetes Care. 2006; 29: 935.
- Ryan EA, Paty BW, Senior PA, Shapiro AM. Risks and side effects of islet transplantation. Curr Diab Rep. 2004; 4: 304-309.
- Nikolic B, Faintuch S, Goldberg SN, Kuo MD, Cardella JF. Stem cell therapy: a primer for interventionalists and imagers. J Vasc Interv Radiol. 2009; 20: 999-1012.
- Gaba RC, Garcia-Roca R, Oberholzer J. Pancreatic islet cell transplantation: an update for interventional radiologists. J Vasc Interv Radiol. 2012; 23: 583-594.
- Wei R, Yang J, Hou W, Liu G, Gao M, Zhang L, et al. Insulin-Producing cells derived from human embryonic stem cells: Comparison of definitive endoderm- and nestin-positive progenitor-based differentiation strategies. PLoS ONE. 2013; 8: e72513.
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998; 282: 1145-1147.
- Blyszczuk P, Czyz J, Kania G, Wagner M, Roll U, St-Onge L, et al. Expression of Pax4 in embryonic stem cells promotes differentiation of nestin-positive progenitor and insulin-producing cells. Proc Natl Acad Sci U S A. 2003; 100: 998-1003.
- Hori Y, Rulifson IC, Tsai BC, Heit JJ, Cahoy JD, Kim SK. Growth inhibitors promote differentiation of insulin-producing tissue from embryonic stem cells. Proc Natl Acad Sci U S A. 2002; 99: 16105-16110.
- Kroon E, Martinson LA, Kadoya K, Bang AG, Kelly OG, Eliazer S, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008; 26: 443-452.
- Zhang Y, Ren Z, Zou C, Wang S, Luo B, Li F, et al. Insulin-producing cells from human pancreatic islet-derived progenitor cells following transplantation in mice. Cell Biol Int. 2011; 35: 483-490.
- Zhang WJ, Xu SQ, Cai HQ, Men XL, Wang Z, Lin H, et al. Evaluation of islets derived from human fetal pancreatic progenitor cells in diabetes treatment. Stem Cell Res Ther. 2013; 4: 141.
- Ianus A, Holz GG, Theise ND, Hussain MA. In vivo derivation of glucose-competent pancreatic endocrine cells from bone marrow without evidence of cell fusion. J Clin Invest. 2003; 111: 843-850.
- Hess D, Li L, Martin M, Sakano S, Hill D, Strutt B, et al. Bone marrow-derived stem cells initiate pancreatic regeneration. Nat Biotechnol. 2003; 21: 763-770.
- Chandra V, Swetha G, Muthyala S, Jaiswal AK, Bellare JR, Nair PD, et al. Islet-like cell aggregates generated from human adipose tissue derived stem cells ameliorate experimental diabetes in mice. PLoS One. 2011; 6: e20615.
- Kadam S, Govindasamy V, Bhonde R. Generation of functional islets from human umbilical cord and placenta derived mesenchymal stem cells. Methods Mol Biol. 2012; 879: 291-313.
- Hori Y, Fukumoto M, Kuroda Y. Enrichment of putative pancreatic progenitor cells from mice by sorting for prominin1 (CD133) and platelet-derived growth factor receptor beta. Stem Cells. 2008; 26: 2912-2920.
- Koblas T, Pektorova L, Zacharovova K, Berkova Z, Girman P, Dovolilova E, et al. Differentiation of CD133-positive pancreatic cells into insulin-producing islet-like cell clusters. Transplant Proc. 2008; 40: 415-418.
- Zulewski H, Abraham EJ, Gerlach MJ, Daniel PB, Moritz W, Müller B, et al. Multipotential nestinK-positive stem cells isolated from adult pancreatic islets differentiate ex vivo into pancreatic endocrine, exocrine, and hepatic phenotypes. Diabetes. 2001; 50: 521-533.
- Lumelsky N, Blondel O, Laeng P, Velasco I, Ravin R, McKay R. Differentiation of embryonic stem cells to insulin-secreting structures similar to pancreatic islets. Science. 2001; 292: 1389-1394.
- Zhang L, Hong TP, Hu J, Liu YN, Wu YH, Li LS. Nestin-positive progenitor cells isolated from human fetal pancreas have phenotypic markers identical to mesenchymal stem cells. World J Gastroenterol. 2005; 11: 2906-2911.
- Dhawan S, Tschen SI, Bhushan A. Bmi-1 regulates the Ink4a/Arf locus to control pancreatic beta-cell proliferation. Genes Dev. 2009; 23: 906-911.
- Yilmaz ÖH, Katajisto P, Lamming DW, Gültekin Y, Bauer-Rowe KE, Sengupta S, et al. mTORC1 in the Paneth cell niche couples intestinal stem-cell function to calorie intake. Nature. 2012; 486: 490-495.
- Komeda K, Noda M, Terao K, Kuzuya N, Kanazawa M, Kanazawa Y. Establishment of two substrains, diabetes-prone and non-diabetic, from Long-Evans Tokushima Lean (LETL) rats. Endocr J. 1998; 45: 737-744.
- Mordes JP, Bortell R, Blankenhorn EP, Rossini AA, Greiner DL. Rat models of type 1 diabetes: genetics, environment, and autoimmunity. ILAR J. 2004; 45: 278-291.
- Nugent DA, Smith DM, Jones HB. A review of islet of Langerhans degeneration in rodent models of type 2 diabetes. Toxicol Pathol. 2008; 36: 529-551.
- Yokoi N. Identification of a major gene responsible for type 1 diabetes in the Komeda diabetes-prone rat. Exp Anim. 2005; 54: 111-115.
- Clark A, Nilsson MR. Islet amyloid: a complication of islet dysfunction or an aetiological factor in Type 2 diabetes? Diabetologia. 2004; 47: 157-169.
- Fuse M, Yokoi N, Shinohara M, Masuyama T, Kitazawa R, Kitazawa S, et al. Identification of a major locus for islet inflammation and fibrosis in the spontaneously diabetic Torii rat. Physiol Genomics. 2008; 35: 96-105.
- Masuyama T, Komeda K, Hara A, Noda M, Shinohara M, Oikawa T, et al. Chronological characterization of diabetes development in male Spontaneously Diabetic Torii rats. Biochem Biophys Res Commun. 2004; 314: 870-877.
- Klöppel G, Löhr M, Habich K, Oberholzer M, Heitz PU. Islet pathology and the pathogenesis of type 1 and type 2 diabetes mellitus revisited. Surv Synth Pathol Res. 1985; 4: 110-125.
- Jacobs JJ, Kieboom K, Marino S, DePinho RA, van Lohuizen M. The oncogene and Polycomb-group gene bmi-1 regulates cell proliferation and senescence through the ink4a locus. Nature. 1999; 397: 164-168.
- Lessard J, Sauvageau G. Bmi-1 determines the proliferative capacity of normal and leukaemic stem cells. Nature. 2003; 423: 255-260.
- Molofsky AV, Pardal R, Iwashita T, Park IK, Clarke MF, Morrison SJ. Bmi-1 dependence distinguishes neural stem cell self-renewal from progenitor proliferation. Nature. 2003; 425: 962-967.
- Bruggeman SW, Valk-Lingbeek ME, van der Stoop PP, Jacobs JJ, Kieboom K, Tanger E, et al. Ink4a and Arf differentially affect cell proliferation and neural stem cell self-renewal in Bmi1-deficient mice. Genes Dev. 2005; 19: 1438-1443.
- Akita M, Tanaka K, Murai N, Matsumoto S, Fujita K, Takaki T, et al. Detection of CD133 (prominin-1) in a human hepatoblastoma cell line (HuH-6 clone 5). Microsc Res Tech. 2013; 76: 844-852.
- Akita M, Matsumoto S, Murai N, Komatsu K, Fujita K. Sub cellular localization of CD133 and interleukin-6 receptor (IL-6R) in human hepatoblastoma cell-line (HuH-6 Clone-5). J Stem Cell Res DevTher. 2014; 1 [in press].
- Sengupta S, Peterson TR, Sabatini DM. Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol Cell. 2010; 40: 310-322.
- Sinnett-Smith J, Kisfalvi K, Kui R, Rozengurt E. Metformin inhibition of mTORC1 activation, DNA synthesis and proliferation in pancreatic cancer cells: dependence on glucose concentration and role of AMPK. Biochem Biophys Res Commun. 2013; 430: 352-357.
- Ohta T, Matsui K, Miyajima K, Sasase T, Masuyama T, Shoda T, et al. Effect of insulin therapy on renal changes in spontaneously diabetic Torii rats. Exp Anim. 2007; 56: 355-362.
- Ohta T, Miyajima K, Yamada T. Pathophysiological changes in pre-diabetic stage of spontaneously diabetic Torii (SDT) rats. J Anim Vet Adv. 2011;10: 813-817.
- Matsubara S, Ding Q, Miyazaki Y, Kuwahata T, Tsukasa K, Takao S. mTOR plays critical roles in pancreatic cancer stem cells through specific and stemness-related functions. Sci Rep. 2013; 3: 3230.
- Krebs M, Brunmair B, Brehm A, Artwohl M, Szendroedi J, Nowotny P, et al. The Mammalian target of rapamycin pathway regulates nutrient-sensitive glucose uptake in man. Diabetes. 2007; 56: 1600-1607.
- Um SH, Frigerio F, Watanabe M, Picard F, Joaquin M, Sticker M, et al. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature. 2004; 431: 200-205.
- Khamzina L, Veilleux A, Bergeron S, Marette A. Increased activation of the mammalian target of rapamycin pathway in liver and skeletal muscle of obese rats: possible involvement in obesity-linked insulin resistance. Endocrinology. 2005; 146: 1473-1481.
- El-Helou V, Proulx C, Béguin P, Assimakopoulos J, Gosselin H, Clement R, et al. The cardiac neural stem cell phenotype is compromised in streptozotocin-induced diabetic cardiomyopathy. J Cell Physiol. 2009; 220: 440-449.
- Liu W, Zhang Y, Liu S, Liu Q, Hao J, Shi Y, et al. The expression of intermediate filament protein nestin and its association with cyclin-dependent kinase 5 in the glomeruli of rats with diabetic nephropathy. Am J Med Sci. 2013; 345: 470-477.
- Oshima Y, Suzuki A, Kawashimo K, Ishikawa M, Ohkohchi N, Taniguchi H. Isolation of mouse pancreatic ductal progenitor cells expressing CD133 and c-Met by flow cytometric cell sorting. Gastroenterology. 2007; 132: 720-732.
- Bonner-Weir S, Taneja M, Weir GC, Tatarkiewicz K, Song KH, Sharma A, et al. In vitro cultivation of human islets from expanded ductal tissue. Proc Natl Acad Sci U S A. 2000; 97: 7999-8004.
- Ramiya VK, Maraist M, Arfors KE, Schatz DA, Peck AB, Cornelius JG. Reversal of insulin-dependent diabetes using islets generated in vitro from pancreatic stem cells. Nat Med. 2000; 6: 278-282.
- Cardinale V, Wang Y, Carpino G, Cui CB, Gatto M, Rossi M, et al. Multipotent stem/progenitor cells in human biliary tree give rise to hepatocytes, cholangiocytes, and pancreatic islets. Hepatology. 2011; 54: 2159-2172.
- Dutton JR, Chillingworth NL, Eberhard D, Brannon CR, Hornsey MA, Tosh D, et al. Beta cells occur naturally in extrahepatic bile ducts of mice. J Cell Sci. 2007; 120: 239-245.