
Review Article
J Stem Cells Res, Rev & Rep. 2014;1(2): 1007.
Spatial Effects of Stem Cell Engagement in 3D Printing Constructs
Xiaohong Wang1,2*
1Department of Mechanical Engineering, Tsinghua University, P.R. China
2State Key Laboratory of Materials Processing and Die & Mould Technology, Huazhong University of Science and Technology, P.R. China
*Corresponding author: Xiaohong Wang, Department of Mechanical Engineering, Key Laboratory for Advanced Materials Processing Technology, Ministry of Education & Center of Organ Manufacturing, Tsinghua University, Beijing 100084, P.R. China
Received: Aug 20, 2014; Accepted: Aug 23, 2014; Published: Aug 25, 2014
Abstract
Three-Dimensional Printing (3DP) technology is a remarkable new invention developed for complex organ manufacturing. For the multiple nozzle 3DP techniques, it is capable of placing various cells and Extracellular Matrices (ECMs) into predestinated locations which mimic their respective positions in a living organ. The High-Fidelity (Hi-Fi) constructs hold enormous therapeutic potential in organ transplantation and regenerative medicine. For the one-nozzle 3DP techniques, when one stem cell type is assembled into a spatially organized construct, the predesigned architecture facilitates the stem cells to grow, interact, organize, and differentiate successively with a cocktail combination of growth factor engagement based on their positions within the construct. A series of morphological and functional changes, such as vascularization and angiogenesis, are accompanied during the stem cell differentiation, tissue formation and organ maturation stages. This review mainly focuses on the spatial effects of sequencial differentiation procedures of adipose-derived stem cells in a 3DP construct
Keywords: Stem cells; Gelatin/alginate/fibrin matrices; Three-dimensional printing (3DP); Spatial effects; Inducement/engagement
Introduction
Organs in vivo have very heterogeneous but well-organized Three-Dimensional (3D) tissues [1]. For example, large blood vessel, serving as blood passage channels, has three lamellar cellbased layers: adventitia, media and intima. The three layers, with thicknesses of about hundred microns, are diversified in terms of cell type, Extracellular Matrix (ECM) composition, and functional properties. To date one of the major obstacles in complex organ manufacturing is to incorporate multiple cell types and maintain heterotypic cell viabilities during tissue formation and structural organization [2-4]. Over the last decade various adult cells, such as hepatocytes, cardiomyocyte, Endothelial Cells (ECs), Smooth Muscle Cells (SMCs), fibroblasts, have been used in tissue/organ regeneration [5]. Developing a 3D construct that incorporates heterotypic cell - cell interactions (e.g. endothelial cell - smooth muscle cell) in a well-controlled manner has been proved to be critical for complex organ manufacturing. The formation of organized and functional heterotypic tissues in a 3D construct has been proved to be a very difficult task.
Three-dimensional printing (3DP), also called Rapid Prototyping (RP) and Additive Manufacturing (AM), consists of a series of novel platforms that can simultaneously assemble cells and Extracellular Matrices (ECMs) from digital models in a precisely controlled layerby- layer fashion, including material composition, architecture, and internal pore size, interconnectivity, branching, geometry and orientation [6-11]. The thickness of each layer can be controlled by the parameters of the printers, nozzle size, material concentration, and extruding speed. The printed 3D constructs proved to maintain the multilayered configurations for cells from microscale to macroscale [12]. This is essential to create 3D biomimetic organs because the layered structure of most tissues varies in terms of cellular composition and extracellular matrix properties. Recently, 3DP techniques have become more and more popular with their fabrication abilities of custom complex organs. However, if onenozzle 3D printer is used and one stem cell type is printed, a cocktail growth factor engagement is necessary to induce the stem cells to differentiate into different target cell types [13,14].
There is increasing experimental evidence that living cells are inherently sensitive to physical, biochemical and chemical stimuli from their surrounding environments [15]. Stem cell differentiation depends on the growth factor inducement and gradients/ concentrations [16]. The local environmental cues or “niches” determine cell-specific recruitment, migration, proliferation, differentiation and the production of the numerous proteins needed for hierarchical tissue organization [17]. Several studies have used stem cell niches to observe the behavior of extending or regenerating axons in response to different in vitro gradients of chemotropic factors [18]. Others built a micro fluidic gradient to study neurite guidance by diffusible factors in a 3D in vitro cell culture model [19]. A growing number of studies report stem cell engagement effects within various natural or synthetic hydrogels [20].
To date, the majority of related studies have used 2D or quasi-3D environments to induce the stem cell differentiation with a single or combination of grow factors [21]. Stem cells therefore were induced into one cell type within these environments. However, in vivo stem cells encounter a heterogeneous mixture of both chemoattractant and chemorepulsive signals [22]. A single administration of these bioactive factors may not be sufficient to yield the numbers of cells and cell types necessary to replace the ones lost due to organ failure, which often involves progressive multiple cell degeneration. Understanding the ectopic stem cell differentiation mechanisms is a key research interest due to its potential clinical applications [23]. A longterm bioactive factor delivery system, with appropriate release kinetics and tuning of relative stem cell differentiation, is essential for correct organ regeneration. Furthermore, among the various stem cell types Adipose-Derived Stem Cells (ADSCs) are easily harvested from patient themselves without immune rejections and have shown multidirectional differentiation potential without ethical controversy [24]. Studies have shown that human ADSCs have the ability to differentiate into osteogenic, adipogenic and neurogenic lineages [25].
Spatial effects of stem cell engagement in 3D printing constructs
In one of our previous studies, ADSCs were used to establish a multicellular system through a one-nozzle cell printing technique. Attempts were made to control the ADSCs differentiation into endothelial cells and adipocytes according to their positions within an orderly 3D construct. After the ADSCs were printed with a gelatin-based hydrogel (gelatin/alginate/fibrin), CaCl2 and thrombin solutions were used to crosslink/polymerize the alginate/fibrin molecules. The crosslinked/polymerized gelatin-based hydrogels have micro-porosity that is critical for fluid penetrating and bioactive factor exchanges during the in vitro engagement stages [26,27]. A Dulbecco’s modified Eagle’s medium (DMEM) culture medium containing 10% Fetal Bovine Serum (FBS), 1 mmol/L insulin, 10 ng/ mL Endothelial Growth Factor (EGF) and 50 U/mL aprotinin was used for 3 d to induce the ADSCs on the channels to differentiate into endothelial cells. Then the culture medium was changed to a DMEM containing 10% FBS, 1 mM insulin, 1 mM dexamethasone (DXM; Sigma), 0.5mmol/L isobutylmethylxanthine (IBMX; Sigma), and 50 u/mL aprotinin for 3 d to induce the ADSCs in the construct to differentiate into adipocytes. With these cocktail growth factor inducements, ADSCs in the construct were differentiated into endothelial cells and adipocytes respectively according to their positions in the construct. After the 3 days engagement with EGF, immunofluorescence staining affirmed that over 90% of the ADSCs on the walls of channels differentiated into mature endothelial cells (CD31 possitive) with typical tubular vessel structures. The mature endothelial cells connected each other to form vessel-like structures with endothelin-1/nitric oxide secretion abilities (Figure 1). After another 3 d engagement with insulin, DXM and IBMX, Oil red O staining confirmed that ADSCs in the construct differentiated into adipocytes with a spherical shape and adipokine (leptin) secretion ability (Figure 2a, 2c, 2e). The differentiations of the ADSCs were based on growth factor inducing (Figure 2c, 2d) and cell position in the 3D construct. Those ADSCs on the surface of walls were easily induced to differentiate into mature endothelial cells and form tubular structures throughout the engineered 3D constructs. While those ADSCs deep in the gelatin-based hydrogels were more sensitive to differentiate into adipocytes during the later inducement stage. In particular, the engineered adipose tissues secreted adipokine, such as leptin, a typical biomark of adipose tissue. The engineered endothelial cells released endothelin-1 (ET-1) and nitric oxide (NO), special biomarks of mature endothelial cells. These functions were coincident with those of in vivo intima and adipose tissues [26,27]. The advantages of this 3DP and engagement model are obvious. It is not only to provide a new approach to engineer orderly vascular adipose-tissues with a predesigned endothelial network, but also to establish a method to evaluate the interaction effects of heterotypic cell - bioactive factor, cell - cell and cell - matrix on different levels.
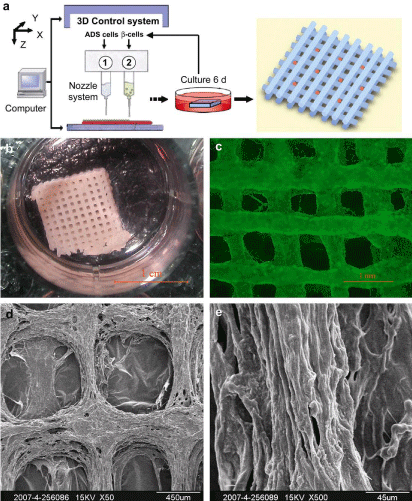
Figure 1: Construction and analyses of the 3DP system. (a) A 3DP system
extruding ADSCs loaded gelatin/alginate/fibrin hydrogel in grid structure. (b)
The 3D structure cultured in a plate. (c) Immunofluorescence staining of the
3D construct with mAb against CD34+ (in green). (d)-(e) Scanning electron
micrographs (SEM) showed the development of extensive ECM and cell
networks in the construct after 6 days of engagement [26,27].
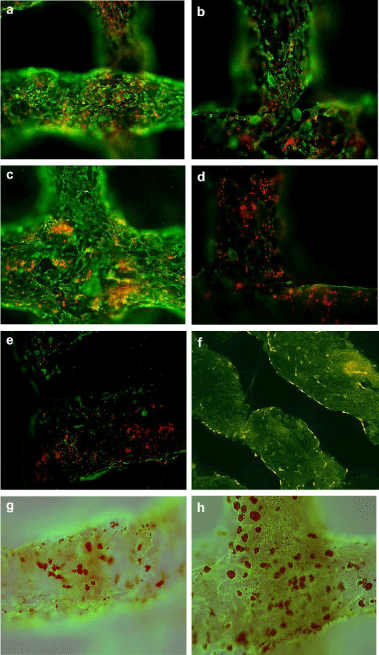
Figure 2: Laser scanning confocal microscopy (LACM) photos showing
ADSCs in the 3D construct controlled to differentiate into endothelial cells
and adipocytes. (a,b) Immunofluorescence staining of the 3D construct using
mAbs for CD34+ cells (ADSCs and endothelial cells) in green and PI for
nuclear in red. (c–f) Immunofluorescence staining of the 3D constructs using
mAbs for CD31+ cells (mature endothelial cells) in green and Propidium
Iodide (PI) for nuclei in red. ADSCs in the 3D construct were cultured with
EGF (a,c,e,f) or without EGF (b,d) (negative control). (e) Construct with
gelatin/alginate hydrogel, without fibrin. (g, h) Immunofluorescences staining
of the 3D construct using mAbs for CD31+ cells in green and Oil red O
staining of the 3D construct for adipocytes in red. ADSCs in 3D construct
were culture with EGF (g) or without EGF (h) for three days, and then treated
with insulin, IBMX and dexamethasone [26,27].
The reasons for the sequential stem cell differentiation could be explained as: (i) the dissolved growth factors diffuse actively in the culture medium, and can penetrate the micropores of the gelatinbased hydrogels to reach the encapsulated cells; (ii) ADSCs stretch/ migrate more actively on the walls of the 3DP channels than those deep inside the hydrogel; (iii) the growth factor concentrations under the walls of the channels were lower than those on the surface of the channels (due to the molecular diffusion gradient) at the beginning of the inducement [28]; (iv) ADSCs on the walls have more chances to contact the bioactive factors dissolved in the culture medium; (v) the morphological and mechanical properties on the surface of channels were more suitable for the ADSCs to differentiate into endothelial cells [29]; (vi) once differentiated into mature endothelial cells, ADSCs on the walls lose other differentiation potentials; (vii) ADSCs deep in the gelatin-based hydrogel had a delayed opportunity to contact the growth factors. (viii) the spatial effects of the stem cell inducement mainly depended on the growth factor gradients and 3D topological structures. This technique provides a new approach to engineer orderly endothelial vessel networks in vitro and holds the promise to be widely used in the further organ manufacturing, highthroughput drug screening, and anti-tumour mechanism research areas.
In our another previous study, a poly(D,L-lactic-co-glycolic acid) (PLGA) scaffold (10 × 10 × 10 mm3 in size) with interconnected channels in three directions was fabricated using a low-temperature 3DP system (Figure 3). A fibrin/collagen hydrogel was used to load ADSCs into the internal channels of the PLGA scaffold grid (Figure 3c) [30,31]. A similar cocktail procedure was designed to engage the ADSCs in the PLGA channels towards both endothelial and smooth muscle cell lineages. During the engagement stages, the PLGA scaffold had sufficient mechanical properties to support the cell/fibrin/collagen hydrogel inside the predefined channels. The combined 3DP constructs were strong enough for handling and placement under pulsatile culture or anti-stress environments [32- 37]. Similar spatial effects were found during the cocktail growth factor engagement stages. ADSCs on the surface of the fibrin/collagen hydrogel were easily differentiated to endothelial cells during the former inducement stage. While ADSCs encapsulated in the fibrin/ collagen hydrogel were induced to differentiate into smooth muscle cells during the later engagement stage. Vascular-like structures formed corresponding to stem cell locations in the construct (Figure 3-5).

Figure 3: Formation of the cell/fibrin/collagen-poly(D,L-lactic-co-glycolic acid)
(PLGA) combined system: (a) a Computer-Aided Design (CAD) model of the
grid PLGA scaffold; (b) a top view of the grid PLGA scaffold; (c) a top view of
the combined cell/hydrogel-PLGA system [31].
Figure 4 shows the H&E staining results of the combined cell/ hydrogel-PLGA system after 7 days of engagement with VEGF and b-FGF. The pictures look like honeycombs with numerous blood capillary-like structures; the ADSCs encapsulated in the fibrin/ collagen hydrogel form the main content of the honeycomb walls. The capillary-like structures resulted mainly from the high water content fibrin/collagen hydrogel. The spatial effect of the engagement is determined by the hydrogel concentration and stem cell density [31].
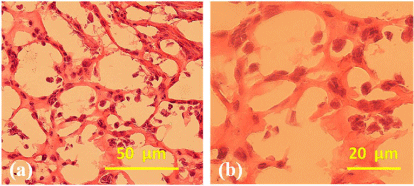
Figure 4: Haematoxylin and Eosin (HE) staining of the cell/hydrogelpoly( D,L-lactic-co-glycolic acid) (PLGA) composite construct after 7 days of engagement with Vascular Endothelial Growth Factor (VEGF) and basic fibroblast growth factor (b-FGF) [31].
In addition, to study the effects of different engagement times, three groups of independent experiments were set. After the combined cell/gelatin/fibrin-PLGA constructs were cultured in a 10% FBS containing DMEM for 7 days, ADSCs in each group were engaged towards ECs for 3, 5, or 7 days with 50 ng/mL VEGF and 10 ng/ml Basic Fibroblast Growth Factor (b-FGF), followed by 6 days’ inducement to SMCs with 0.5 ng/mL HGF, 50 ng/mL human Platelet-Derived Growth Factor (PDGF-BB), 10 ng/mL transforming growth factor β1 (TGF-β1) and 2.5 ng/mL b-FGF. Immunofluorescence staining pictures revealed the engagement results of the three combined groups with different engagement times (Figure 5). In the first group, the combined construct was first engaged to an EC lineage with VEGF and b-FGF for 3 days (Figure 5a) and then to a SMC lineage with HGF, PDGF-BB, TGF-β1and b-FGF for 6 days (Figure 5b). In Figure 5a, only a few separated ADSCs express the EC marker FacVIII after 3 days engagement to EC, and almost all the other ADSCs express the SMC marker α-SMA during the following 6 days engagement (Figure 5b). In the second group, the combined construct was first engaged to the EC lineage with VEGF and b-FGF for 5 days (Figure5c) and then to the SMC lineage with HGF, PDGFBB, TGF-β1and b-FGF for 6 days (Figure 5d). In Figure 5c, ADSCs in the centre of the channel express FacVIII and form tube-like structures. These cells are surrounded by α-SMA marked SMCs, as shown in Figure 5d. In the last group, almost all the ADSCs express the EC marker FacVIII during the first 7 days engagement to EC with VEGF and b-FGF, with only a few cells expressing the SMC marker α-SMA during the following 6 days engagement of the SMC lineage with HGF, PDGFBB, TGF-β 1and b-FGF. The fibrin/collagen-loaded ADSCs formed honeycomb-like structures by expressing CD31, CD34, VIII factors and α-SMA in different layers. The second group holds the possibility of developing a real vascular system with both intima and media cell layers [31]. These results have further confirmed the hypotheses that spatial effects of the stem cell engagement mainly depend on the growth factor gradients and 3D topological structures. This technique allows in vitro vascularization of the predefined PLGA channels and therefore holds the potential to be widely used in the future complex organ manufacturing, high-throughput drug screening, and antitumour mechanism research areas.
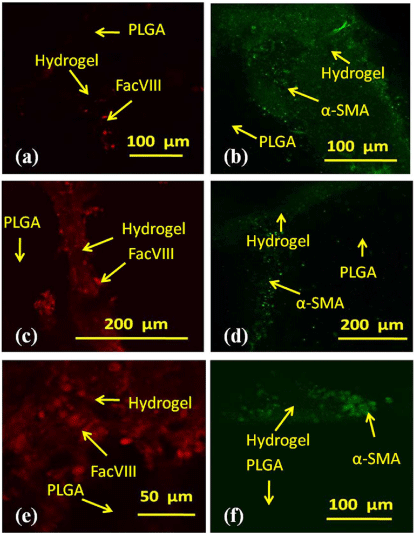
Figure 5: Immunofluorescence staining of the cell/hydrogel-poly (D, L-lacticco-
glycolic acid) (PLGA) combined system after different engagement times.
(a) The construct was first engaged towards Endothelial Cell (EC) lineage
using vascular endothelial cells growth factor (VEGF) and basic fibroblast
growth factor (b-FGF) for 3 days with a few cells in red color expressed EC
marker FacVIII. (b) After engagement towards the EC lineage for 3 days,
the construct was engaged towards Smooth Muscle Cell (SMC) lineage with
Hepatocyte Growth Factor (HGF), human Platelet-Derived Growth Factor
(PDGF-BB), transforming growth factor β1 (TGF-β1) and basic fibroblast
growth factor (b-FGF) for 6 days. Almost all the cells in coloured green
expressed SMC markera-Smooth Muscle Actin (SMA). (c) The construct was
first engaged towards the EC lineage using VEGF and b-FGF for 5 days.
Nearly all the cells on the surface of the fibrin/collagen hydrogel expressed
FacVIII marker (red). (d) After engagement towards the EC lineage for 5 days,
the construct was engaged towards the SMC lineage with HGF, PDGF-BB,
TGF-β1 and b-FGF for 6 days. Most of the cells deep in the fibrin/collagen
hydrogel expressed the a-SMA marker (bright green); (e) The construct was
first engaged towards the EC lineage using VEGF and b-FGF for 7 days.
Much more cells expressed FacVIII marker (red); (f) After engagement
towards the EC lineage for 7 days, the construct was engaged towards the
SMC lineage with HGF, PDGF-BB, TGF-β1and b-FGF for 6 days. A thin layer
of cells expressed a-SMA marker (bright green) [31].
Generally, low temperature 3D printing techniques were used to fabricate synthetic polymer scaffolds for hard tissue substitutes with strong mechanical strength [32-37]. These polymer scaffolds, such as grid polyurethane (PU) and PLGA scaffolds with interconnected channels, could withstand more stress compared to natural hydrogels, which is insufficient to maintain the stability of the whole construct under in vitro or in vivo conditions [38,39]. The natural hydrogels with excellent biocompatibilities could offer a transient, spatial advantageous environment to stimulate stem cells differentiated into different target cell lineages in the 3D constructs. This raises the possibility that the combinatorial synthetic and natural polymer systems could be also used to control the stem cell fates. Similar spatial inducement effects were found compared with the pure natural gelatin-based hydrogel systems. This experiment further verifies that a well-controlled environment is able to activate a series of specific responses of stem cells to differentiate into different target cell types. The reproduction of similarly sophisticated behavior in a combined synthetic and natural polymer system would be of great scientific and technological value in the future complex organ manufacturing.
Conclusion and Prospects
In summary, 3DP techniques can provide more information on how microenvironments, especially local spatial structures, influence cell behaviors. By culturing cells under different locations in a 3DP construct the relationship between cell function and microenvironment can be studied in more details. Subsequent control of the large number of parameters that mutually influence cells, tissues and organs in increasingly complex 3D constructs is possible. It is important to utilize a cocktail combination of bioactive factors to stimulate stem cells to differentiate into different target cell types in the 3DP constructs. Future work to encompass additional growth factor incorporation, as well characterize the fate and function of renewal cell types, will take steps closer to potential clinical applications. Long-term heterotypic cell growth abilities in the 3DP constructs might be more challenging based on the spatial effects of the cocktail growth factor engagements.
Acknowledgment
The Project was supported by the State Key Laboratory of Materials Processing and Die & Mould Technology, Huazhong University of Science and Technology (No. 2012-P03), the Cross- Strait Tsinghua Cooperation Basic Research (No.2012THZ02-3), the National Natural Science Foundation of China (No. 81271665 & 30970748), the National Natural Science Foundation of China (NSFC) and Japanese Society for the Promotion of Science (JSPS) Bilateral Cooperation Program and the National High Tech 863 Grant (No. 2009AA043801).
References
- Wang XH, Tuomi J, Mäkitie AA, Poloheimo KS, Partanen J, Yliperttula M. The integrations of biomaterials and rapid prototyping techniques for intelligent manufacturing of complex organs. In: Advances in Biomaterials Science and Applications in Biomedicine, Lazinica R editor. In Tech: Rijeka, Croatia. 2013; 437-463.
- Wang X, Yan Y, Zhang R. Rapid prototyping as a tool for manufacturing bioartificial livers. Trends Biotechnol. 2007; 25: 505-513.
- Wang X, Yan Y, Zhang R. Recent trends and challenges in complex organ manufacturing. Tissue Eng Part B Rev. 2010; 16: 189-197.
- Wang X. Intelligent freeform manufacturing of complex organs. Artif Organs. 2012; 36: 951-961.
- Bartolo LD, Leindlein A, Hofmann D, Bader A, de Grey A, Curcio E, et al. Bio-hybrid organs and tissues for patient therapy: A future vision for 2030. Chemical Engineering and Processing 2012; 51: 79-87.
- Yan YN, Wang XH, Pan YQ, Liu H, Cheng J, Xiong Z, et al. Fabrication of viable tissue-engineered constructs with 3D cell-assembly technique. Biomaterials. 2005; 26: 5864-5871.
- Yan YN, Wang XH, Xiong Z, Liu HX, Liu F, Lin F, Wu RD, Zhang RJ, Lu QP. Direct construction of a three-dimensional structure with cells and hydrogel. J Bioact Compa Polym 2005; 20: 259-269.
- Wang XH, Yan YN, Pan YQ, Xiong Z, Liu HX, Cheng J, et al. Generation of three-dimensional hepatocyte/gelatin structures with rapid prototyping system. Tissue Engineering. 2006; 12: 83-90.
- Li SJ, Xiong Z, Wang XH, Yan YN, Liu HX, Zhang RJ. Direct fabrication of a hybrid cell/hydrogel construct via a double-nozzle assembling technology. J Bioact Compa Polym. 2009; 24: 249-264.
- Li SJ, Yan YN, Xiong Z, Weng CY, Zhang RJ, Wang XH. Gradient hydrogel construct based on an improved cell assembling system. J Bioact Compa Polym. 2009; 24: 84-99.
- Xu W, Wang XH, Yan YN, Zheng W, Xiong Z, Lin F, et al. Rapid prototyping of three-dimensional cell/gelatin/fibrinogen constructs for medical regeneration. J Bioact Compa Polym. 2007; 22: 363-377.
- Wang XH, Yan YN, Zhang RJ. Gelatin-based hydrogels for controlled cell assembly. Ottenbrite RM editor. In: Biomedical Applications of Hydrogels Handbook. New York: Springer. 2010; 269-84.
- Yao R, Zhang RJ, Yan YN, Wang XH. In vitro angiogenesis of 3D tissue engineered adipose tissue. J Bioact Compa Polym. 2009; 24: 5-24.
- Yao R, Zhang RJ, Wang XH. Design and evaluation of a cell microencapsulating device for cell assembly technology. J Bioact Compa Polym. 2009; 24: 48-62.
- Hutmacher DW, Singh H. Computational fluid dynamics for improved bioreactor design and 3D culture. Trends Biotechnol. 2008; 26: 166-172.
- Kuhl PR, Griffith-Cima LG. Tethered epidermal growth factor as a paradigm for growth factor-induced stimulation from the solid phase. Nat Med. 1996; 2: 1022-1027.
- Cao X, Shoichet MS. Defining the concentration gradient of nerve growth factor for guided neurite outgrowth. Neuroscience. 2001; 103: 831-840.
- Conway A, Schaffer DV. Biomaterial microenvironments to support the generation of new neurons in the adult brain. Stem Cells. 2014; 32: 1220-1229.
- Kothapalli CR, van Veen E, de Valence S, Chung S, Zervantonakis IK, Gertler FB, et al. A high-throughput microfluidic assay to study neurite response to growth factor gradients. Lab Chip. 2011; 11: 497-507.
- Leslie-Barbick Julia E, Shen Colette, Chen Christopher, West JL. Micron-scale spatially patterned, covalently immobilized vascular endothelial growth factor on hydrogels accelerates endothelial tubulogenesis and increases cellular angiogenic responses. Tissue Eng A. 2011; 17: 221-229.
- Lugert S, Basak O, Knuckles P, Haussler U, Fabel K, Götz M, et al. Quiescent and active hippocampal neural stem cells with distinct morphologies respond selectively to physiological and pathological stimuli and aging. Cell Stem Cell. 2010; 6: 445-456.
- Keung AJ, Kumar S, Schaffer DV. Presentation counts: microenvironmental regulation of stem cells by biophysical and material cues. Annu Rev Cell Dev Biol. 2010; 26: 533-556.
- Orive G, Hernández RM, Rodríguez Gascón A, Calafiore R, Chang TM, de Vos P, et al. History, challenges and perspectives of cell microencapsulation. Trends Biotechnol. 2004; 22: 87-92.
- Poulsom R, Alison MR, Forbes SJ, Wright NA. Adult stem cell plasticity. J Pathol. 2002; 197: 441-456.
- Banas A, Teratani T, Yamamoto Y, Tokuhara M, Takeshita F, Quinn G, et al. Adipose tissue-derived mesenchymal stem cells as a source of human hepatocytes. Hepatology. 2007; 46: 219-228.
- Xu MN, Yan YN, Liu HX, Yao Y, Wang XH. Control adipose-derived stromal cells differentiation into adipose and endothelial cells in a 3-D structure established by cell-assembly technique. J Bioact Compat Polym. 2009; 24: 31-47.
- Xu ME, Wang XH, Yan YN, Yao R, Ge YK. A cell-assembly derived physiological 3D model of the metabolic syndrome, based on adipose-derived stromal cells and a gelatin/alginate/fibrinogen matrix. Biomaterials. 2010; 31: 3868-3877.
- Lander AD. Morpheus unbound: reimagining the morphogen gradient. Cell. 2007; 128: 245-256.
- Dahl SL, Vaughn ME, Hu JJ, Driessen NJ, Baaijens FP, Humphrey JD, et al. A microstructurally motivated model of the mechanical behavior of tissue engineered blood vessels. Ann Biomed Eng. 2008; 36: 1782-1792.
- Zhao XR, Wang XH. Preparation of an adipose-derived stem cell/fibrin-poly (D, L-lactic-co-glycolic acid) construct based on a rapid prototyping technique. J Bioact Compat Polym. 2013; 28: 191-203.
- Zhao X, Liu L, Wang J, Xu Y, Zhang W, Khang G, et al. In vitro vascularization of a combined system based on a 3D printing technique. J Tissue Eng Regen Med. 2014.
- Cui T, Yan Y, Zhang R, Liu L, Xu W, Wang X. Rapid prototyping of a double-layer polyurethane-collagen conduit for peripheral nerve regeneration. Tissue Eng Part C Methods. 2009; 15: 1-9.
- Cui TK, Wang XH, Yan YN, Zhang RJ. Rapid prototyping a new polyurethane-collagen conduit and its Schwann cell compatibility. J Bioact Compat Polym. 2009; 24: 5-17.
- Wang XH, Cui TK, Yan YN, Zhang RJ. Peroneal nerve regeneration along a new polyurethane-collagen guide conduit. J Bioact Compat Polym. 2009; 24: 109-127.
- He K, Wang XH. Rapid prototyping of tubular polyurethane and cell/hydrogel construct. J Bioact Compat Polym. 2011; 26: 363-374.
- Huang Y, He K, Wang X. Rapid prototyping of a hybrid hierarchical polyurethane-cell/hydrogel construct for regenerative medicine. Mater Sci Eng C Mater Biol Appl. 2013; 33: 3220-3229.
- Wang X, Xu H. Incorporation of DMSO and dextran-40 into a gelatin/alginate hydrogel for controlled assembled cell cryopreservation. Cryobiology. 2010; 61: 345-351.
- Wang X, Sui S. Pulsatile culture of a poly(DL-lactic-co-glycolic acid) sandwiched cell/hydrogel construct fabricated using a step-by-step mold/extraction method. Artif Organs. 2011; 35: 645-655.
- Wang XH, Mäkitie AA, Paloheimo K-S, Tuomi J, Paloheimo M, Sui SC, et al. Characterization of a PLGA sandwiched cell/fibrin tubular construct and induction of the adipose derived stem cells into smooth muscle cells. Mater Sci Eng C. 2011; 31: 801-808.