
Mini Review
J Stem Cells Res, Rev & Rep. 2014;1(3): 1012.
Perspectives of Mesenchymal Stem Cell-Based Neuroregeneration
Katarzyna Roszek* and Joanna Czarnecka
Department of Biochemistry, Faculty of Biology and Environment Protection, Nicolaus Copernicus University in Torun, Poland
*Corresponding author:Roszek K, Department of Biochemistry, Faculty of Biology and Environment Protection, Nicolaus Copernicus University, Gagarin St. 7, 87-100 Torun, Poland
Received: September 23, 2014; Accepted: October 09, 2014; Published: October 09, 2014
Abstract
Stem cell-based therapy seems to be a promising strategy to treat neurodegenerative diseases, in particular those that are fatal and difficult to treat. However, the sources of stem cells are required to comply with the requirement of availability and expanding in culture, to overcome ethical objections and concerns of graft rejection. Mesenchymal stem cells (MSCs) are population of adult stem cells that fulfill all these criteria. These cells are able to differentiate into osteoblasts, chondrocytes and adipocytes as well as cardiomyocytes, hepatocytes, endothelial and pancreatic cells. The most recent studies have been focused on the neurogenic potential of mesenchymal stem cells expressed by their ability to differentiate into neural and glial cell types. The article focuses on current approaches to the mesenchymal stem cell-based neuroregeneration and their perspectives. The anti-inflammatory action of MSCs and their potential effects on neuroprotection and neuroregeneration has been described in respect to the central nervous system (CNS) disorders. Some representative clinical and experimental trials using MSCs in CNS therapies have been specified.
Keywords: Mesenchymal stem cells; Neural lineage differentiation; Genetically modified stem cells; Neuroregeneration; Central nervous system disorders
Introduction
The Mesenchymal Stem Cells (MSCs) have received considerable attention as a promising population of adult stem cells. They can be derived from various adult tissues, such as bone marrow, umbilical blood and cord or adipose tissue and they easily expand in culture. Human mesenchymal stem cells are attractive tool for regeneration because of their plasticity and potential to differentiate into multiple cell lineages. Their ability to differentiate in vitro into osteoblasts, chondrocytes and adipocytes is one of the minimal criteria for defining human MSCs [1]. These cells were also found to differentiate into cardiomyocytes, hepatocytes, endothelial and pancreatic cells. However, MSCs derived from different sources express distinct biological potential - some of them are easy to proliferate whereas the others have a greater potential to differentiate [2,3]. The most recent studies have been focused on the neurogenic potential of mesenchymal stem cells represented by their ability to differentiate into neural and glial cell types (as summarized in [3]). Additionally, MSCs have shown the extraordinary immunomodulatory properties by suppressing pro-inflammatory cytokines production, and therefore enable autologous and, what is more beneficial for patients, heterologous transplantation without the need of immunosuppression [4-6]. Thus, MSCs exhibit a promising therapeutic potential in numerous disease models, including the Central Nervous System (CNS) disorders. In this review we describe the current approaches to the mesenchymal stem cell-based neuroregeneration and their therapeutic prospects.
The MSC-based experimental therapies of CNS disorders can be classified into a three major groups, based on the transplantation of naive undifferentiated MSCs, MSC-derived neural cells and genetically modified MSCs – Figure 1.
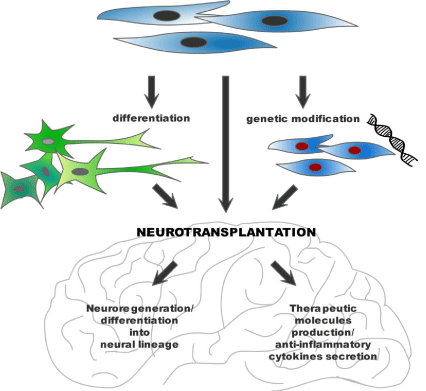
Figure 1: Current approaches to the mesenchymal stem cell-based neuroregeneration (see text for details).
Undifferentiated mesenchymal stem cells
One of the earliest neurotransplantations of mesenchymal stem cells into experimental animals was performed in 1998 by Azizi and collaborators. Human bone marrow-derived MSCs were directly injected into the striatum of rat brain. Only 20% of the infused cells had engrafted, but there was no evidence of an inflammatory response or rejection. After infusion into the brain, the human MSCs lost their immunoreactivity. The cells migrated from the site of injection to successive areas of the brain [7]. In another experiment, mesenchymal stem cells implanted to embryonic rat brain ventricles differentiated in vivo in a regionally and temporally specific manner. Whereas donor cells localized near to the subventricular zone continued to express nestin, a marker of neural precursors, the cells in the neocortex and midbrain expressed mature neuronal markers like Microtubule-Associated Protein Tau (MAPT) or Microtubule- Associated Protein 2 (MAP-2) [8]. On the other hand, Tondreau and colleagues found that more than 80% of mesenchymal stem cells cultured in vitro constitutively expressed nestin and ß-III tubulin. Moreover, MSCs cultured in non-differentiating medium after fifth passage started to express mature neuronal or glial markers as: Tyrosine Hydroxylase (TH), Microtubule-Associated Protein 2 (MAP-2) and Glial Fibrillary Acidic Protein (GFAP) [9]. It must be remembered that MSCs derived from different sources hold distinct differentiation potential. For example, the in vitro expanded UCB-derived MSCs harbor a small unique population of cells that possesses huge inherent neurogenic potential and differentiate using simple protocol of neuronal induction. These “pluripotent progenitors” generate cells expressing neural progenitor markers and are responsible for the immediate neuronal differentiation in vivo [3,10].
The huge advantage of MSCs is that they can bypass the bloodbrain barrier [11]. When injected systemically they migrate into the various brain areas, home precisely to the site of injury, in particular to hypoxic, apoptotic or inflamed parts and easily integrate into the nervous tissue. MSCs are known to secrete a variety of cytokines and growth factors that have both paracrine and autocrine activities for damaged tissues. To date, the experimental and clinical evidence showed no “danger signal” in tissues after MSCs injection. Thus, MSCs have been widely tested as a promising therapeutic tool in animal models of neurological diseases, such as multiple sclerosis (MS), Huntington’s disease (HD), Amyotrophic Lateral Sclerosis (ALS) as well as in traumatic brain injury [12-15].
Differentiated mesenchymal stem cells
The in vitro pre-differentiation of MSCs into neural lineage can be achieved by culturing them in the induction media. Such media consist of a cocktail of growth factors and small molecules that drive cells to the neural fate. In early experiments, Sanchez-Ramos and collaborators demonstrated that human and mouse bone marrowderived MSCs can be induced to differentiate into neural cells under special culture conditions. MSCs cultured in the presence of epidermal growth factor (EGF) or brain-derived neurotrophic factor (BDNF) expressed nestin, GFAP and neuron-specific nuclear protein (NeuN) [16]. The differentiation to neural lineage is more effective when human and mouse bone marrow-derived MSCs were co-cultured with rat foetal mesencephalic or striatal cells [16]. However, it has not been shown conclusively that mature neurons with signalling capacity can be generated from MSCs [17]. The role of differentiation media composition and cell culture substrates in in vitro pre-differentiation of MSCs into neural lineage was studied by Kim et al. [18]. The tested culture media contained basic fibroblast growth factor (bFGF), Nerve Growth Factor (NGF) and retinal acid (RA). The cell culture substrates coated with laminin, gelatin, collagen and fibronectin were examined. Combination of RA and bFGF together with fibronectin-coated dishes provoke pre- neuronal differentiation in 40% MSCs [18]. According to Kaka and co-authors other molecules important for neural lineage differentiation are dimethyl sulfoxide (DMSO), platelet-derived growth factor (PDGF) and heregulin (HRG), followed by triiodothyronine (T3) [19]. Numerous strategies have been employed to achieve MSC-derived neural phenotypes from different cell sources (extensively reviewed in [3]). It is suggested that origin and biological potential of the human mesenchymal stem cells is essential for the study on inducing them to neuronal differentiation. Nevertheless, the choice of the best source for treating neurodegeneration still remains unclear [3]. The most important advantage of pre-differentiation into neural lineage may be concern for safety, as some studies have noted formation of tumors in undifferentiated murine MSCs cultures, whereas studies on human MSCs transformation are insufficient.
Genetically modified mesenchymal stem cells
Combining the benefits of undifferentiated MSCs transplantation with blood-brain barrier bypassing and drug or therapeutic gene delivery may be realized with genetically engineered MSCs. Ryu and collaborators in their study [20] evaluated the therapeutic effects for CNS disorders using human bone marrow-derived mesenchymal stem cells, engineered to secrete interferon-ß, as delivery vehicles. MSCs injected intravenously preferentially migrate to the sites of inflammation and may therefore be used for tissue directed immunosuppression, or delivery of therapeutic molecules to the injuried site. Another potent strategy for suppression of the inflammatory processes throughout the CNS may be mesenchymal stem cells genetically modified with NTPDase (nucleoside triphosphate diphosphohydrolase, CD39) gene. Elevated concentrations of extracellular ATP in the local microenvironment of the injury mark the damaged site and contribute to the promotion of primary immune response [21]. NTPDases capable of ATP cleavage contribute to the decrease in ATP concentration, thus terminating the prolonged inflammatory response. MSCs engineered to express therapeutic enzyme could effectively combine the high enzymatic activity with its local delivery to maintain the low concentration of ATP (see also [22]).
Some other experiments used mesenchymal stem cells genetically engineered to over-express brain- derived neurotrophic factor or nerve growth factor. The modified cells were injected into the striatum of YAC 128 mouse model of Huntington’s disease. It effected in supporting the injured neurons and suppression of the neurodegenerative processes [15,23]. Sadan and colleagues have modified bone marrow-derived mesenchymal stem cells into neurotrophic factor-secreting cells, thus combining stem cell-based regenerative therapy with the NTF-based neuroprotection. The engineered MSCs injected to the rat model of Parkinson’s disease induced regeneration in the damaged striatal dopaminergic nerves. In these experiments, the neurotrophic factor-secreting cells were more effective than unmodified MSCs [24].
Mesenchymal stem cells have the potential as drugs or other molecules carriers to treat patients with neural diseases and neuropathologies for which limited treatment options exist. It seems that genetically manipulated MSCs provide attractive platforms with lesion-targeting capability for the sustained production of therapeutic proteins in vivo. When considering the limitations of current methods of drug delivery to the brain, MSCs have the potential to become a safe cellular delivery vehicle. Progress along these cell lines has been made in rodent models of neurodegenerative disorders and ischemic stroke (described in [4]).
Clinical and experimental trials in CNS disorders
Mesenchymal stem cells have numerous advantages of potential clinical importance, that make them ideal candidates for stem cellbased therapy. There have been several clinical studies using MSCs during recent years, some of them focused on the central nervous system disorders [25]. There are also several ongoing clinical trials with genetically modified MSCs as delivery vehicles for the treatment of number of CNS pathologies including brain ischemia, amyotrophic lateral sclerosis and multiple sclerosis [11]. The representative clinical and experimental trials using MSCs were collected in Table 1.
Disease
Cells
Route of delivery
References
Traumatic Brain Injury
MSCs
intravenous
NCT01649700
Parkinson Disease
MSCs
intravenous
NCT01446614
Multiple Sclerosis
MSCs
intravenous
NCT01377870
MSC-derived neural progenitors
intravenous
NCT01933802
Ischemic stroke
MSCs
intravenous
NCT01468064
Amyotrophic Lateral Sclerosis
MSCs
intrathecal
NCT02116634
Neurotrophic factors- secreting mesenchymal stromal cells
intramuscular, intrathecal
NCT02017912
Table 1: The representative clinical trials using MSCs [compiled from: 3,11,25,26].
There are still some unanswered questions that need to be addressed before such experimental therapies become widespread. One important problem is the time of injection, as it has been suggested that the best results can be obtained when therapy is initiated immediately after the injury. Currently unanswered question is also the dosage and the mode of MSCs administration, among others [3]. The ongoing clinical trials will shed some light onto these problems in next few years.
Conclusion
Although studies demonstrating the presence of neural stem cells in the brain of many rodents, as well as finding of new neurons in the adult human neurogenic areas are presently indisputable, a significant part of the central nervous system of adult mammals is nonneurogenic in physiological conditions [14,27,28]. There is currently a great deal of interest in the use of MSCs to treat neurodegenerative diseases, in particular those that are fatal and difficult to treat. No effective treatments are currently available for brain neurological disorders such as stroke/cerebral ischemia, traumatic brain injury and neurodegenerative disorders. Cell-based therapy is a promising strategy, although cells are required to overcome ethical, tissue availability and graft rejection concerns. Mesenchymal stem cells fulfill all these criteria. In vitro expanded undifferentiated MSCs can be used across allogeneic barrier, they are able to cross the blood brain barrier and home to regions of brain tissue pathology. These advantages underscore why MSCs are gaining growing attention for brain disorders therapy.
Effects of MSCs transplantation on neuroregenerative processes include: in vivo and in vitro differentiation into neurons and glial cells, promoting endogenous neuronal growth, reducing demyelination and encouraging synaptic connection from damaged neurons. The set of growth factors and other small molecules secreted by MSCs significantly contributes to tissue repair, through stimulating angiogenesis, reducing oxidative stress and decreasing apoptosis. Due to anti- inflammatory cytokines production, MSCs modulate microglial activation and suppress pathological T, B and NK cell responses.
Although most of the described approaches still remain in the experimental stage, continuing effort in developing new therapeutic strategies of CNS disorders will enable faster and widespread adoption of these techniques in clinical applications. MSCs have advantages over other stem cells with regards to their use in cell therapy. However, the application of engineered mesenchymal stem cells combines the benefits of neuroregenerative properties of MSCs with active molecules delivery. It becomes a promising strategy for efficient drug/enzyme/growth factor delivery to CNS tissues. In our opinion, the genetically modified MSCs and their therapeutic capability for neuroregeneration will focus the growing research interest and efforts in the nearest future.
References
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini FC, Krause DS, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006; 8: 315-317.
- Roszek K, Bomastek K, Drozdzal M, Komoszynski M. Dramatic differences in activity of purines metabolizing ecto-enzymes between mesenchymal stem cells isolated from human umbilical cord blood and umbilical cord tissue. See comment in PubMed Commons below Biochem Cell Biol. 2013; 91: 519-525.
- Taran R, Mamidi MK, Singh G, Dutta S, Parhar IS, John JP, et al. In vitro and in vivo neurogenic potential of mesenchymal stem cells isolated from different sources. See comment in PubMed Commons below J Biosci. 2014; 39: 157-169.
- Azari MF, Mathias L, Ozturk E, Cram DS, Boyd RL, Petratos S. Mesenchymal stem cells for treatment of CNS injury. See comment in PubMed Commons below Curr Neuropharmacol. 2010; 8: 316-323.
- De Miguel MP, Fuentes-Julián S, Blázquez-Martínez A, Pascual CY, Aller MA, Arias J, et al. Immunosuppressive properties of mesenchymal stem cells: advances and applications. See comment in PubMed Commons below Curr Mol Med. 2012; 12: 574-591.
- Lotfinegad P, Shamsasenjan K, Movassaghpour A, Majidi J, Baradaran B. Immunomodulatory nature and site specific affinity of mesenchymal stem cells: a hope in cell therapy. See comment in PubMed Commons below Adv Pharm Bull. 2014; 4: 5-13.
- Azizi SA, Stokes D, Augelli BJ, DiGirolamo C, Prockop DJ. Engraftment and migration of human bone marrow stromal cells implanted in the brains of albino rats--similarities to astrocyte grafts. See comment in PubMed Commons below Proc Natl Acad Sci U S A. 1998; 95: 3908-3913.
- Muñoz-Elias G, Marcus AJ, Coyne TM, Woodbury D, Black IB. Adult bone marrow stromal cells in the embryonic brain: engraftment, migration, differentiation, and long-term survival. See comment in PubMed Commons below J Neurosci. 2004; 24: 4585-4595.
- Tondreau T, Lagneaux L, Dejeneffe M, Massy M, Mortier C, Delforge A. Bone marrow derived mesenchymal stem cells already express specific neural proteins before any differentiation. Differentiation. 2004; 72: 319-326.
- Divya MS, Roshin GE, Divya TS, Rasheed VA, Santhoshkumar TR, Elizabeth KE, et al. Umbilical cord blood-derived mesenchymal stem cells consist of a unique population of progenitors co-expressing mesenchymal stem cell and neuronal markers capable of instantaneous neuronal differentiation. Stem Cell Res Ther. 2012; 3: 57.
- Aleynik A, Gernavage KM, Mourad YSh, Sherman LS, Liu K, Gubenko YA, et al. Stem cell delivery of therapies for brain disorders. See comment in PubMed Commons below Clin Transl Med. 2014; 3: 24.
- Kassis I, Grigoriadis N, Gowda-Kurkalli B, Mizrachi-Kol R, Ben-Hur T, Slavin S, et al. Neuroprotection and immunomodulation with mesenchymal stem cells in chronic experimental autoimmune encephalomyelitis. See comment in PubMed Commons below Arch Neurol. 2008; 65: 753-761.
- Sadan O, Shemesh N, Barzilay R, Bahat-Stromza M, Melamed E, Cohen Y, et al. Migration of neurotrophic factors-secreting mesenchymal stem cells toward a quinolinic acid lesion as viewed by magnetic resonance imaging. See comment in PubMed Commons below Stem Cells. 2008; 26: 2542-2551.
- Joyce N, Annett G, Wirthlin L, Olson S, Bauer G, Nolta JA. Mesenchymal stem cells for the treatment of neurodegenerative disease. See comment in PubMed Commons below Regen Med. 2010; 5: 933-946.
- Olson SD, Pollock K, Kambal A, Cary W, Mitchell GM, Tempkin J, et al. Genetically engineered mesenchymal stem cells as a proposed therapeutic for Huntington's disease. See comment in PubMed Commons below Mol Neurobiol. 2012; 45: 87-98.
- Sanchez-Ramos J, Song S, Cardozo-Pelaez F, Hazzi C, Stedeford T, Willing A, et al. Adult bone marrow stromal cells differentiate into neural cells in vitro. See comment in PubMed Commons below Exp Neurol. 2000; 164: 247-256.
- Hardy SA, Maltman DJ, Przyborski SA. Mesenchymal stem cells as mediators of neural differentiation. See comment in PubMed Commons below Curr Stem Cell Res Ther. 2008; 3: 43-52.
- Kim BJ, Seo JH, Bubien JK, Oh YS. Differentiation of adult bone marrow stem cells into neuroprogenitor cells in vitro. See comment in PubMed Commons below Neuroreport. 2002; 13: 1185-1188.
- Kaka GR, Tiraihi T, Delshad A, Arabkheradmand J, Kazemi H. In vitro differentiation of bone marrow stromal cells into oligodendrocyte-like cells using triiodothyronine as inducer. See comment in PubMed Commons below Int J Neurosci. 2012; 122: 237-247.
- Ryu CH, Park KY, Hou Y, Jeong CH, Kim SM, Jeun SS. Gene therapy of multiple sclerosis using interferon ß-secreting human bone marrow mesenchymal stem cells. See comment in PubMed Commons below Biomed Res Int. 2013; 2013: 696738.
- Bours MJ, Swennen EL, Di Virgilio F, Cronstein BN, Dagnelie PC. Adenosine 5'-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. See comment in PubMed Commons below Pharmacol Ther. 2006; 112: 358-404.
- Roszek K, Czarnecka J. Is ecto-nucleotidase based therapy of central nervous system disorders possible? Mini Rev Med Chem. 2014.
- Dey ND, Bombard MC, Roland BP, Davidson S, Lu M, Rossignol J, et al. Genetically engineered mesenchymal stem cells reduce behavioral deficits in the YAC 128 mouse model of Huntington's disease. Behavioural Brain Research 2010; 214: 193-200.
- Sadan O, Bahat-Stromza M, Barhum Y, Levy YS, Pisnevsky A, Peretz H, et al. Protective effects of neurotrophic factor-secreting cells in a 6-OHDA rat model of Parkinson disease. See comment in PubMed Commons below Stem Cells Dev. 2009; 18: 1179-1190.
- Paul G, Anisimov SV. The secretome of mesenchymal stem cells: potential implications for neuroregeneration. See comment in PubMed Commons below Biochimie. 2013; 95: 2246-2256.
- A service of the U.S. National Institutes of Health.
- Roszek K, Czarnecka J, KomoszyA ski M. Ependyma and subependyma of adult mammalian brain as a source of neural stem cells. Adv Cell Biol. 2011; 38: 379-393.
- Jessberger S, Gage FH. Adult neurogenesis: bridging the gap between mice and humans. See comment in PubMed Commons below Trends Cell Biol. 2014; 24: 558-563.