
Mini Review
J Stem Cells Res, Rev & Rep. 2016; 3(1): 1022.
Counting on Mesenchymal Stem Cells: A Hope for Treating Parkinson’s Disease
Equbal Z* and Mukhopadhyay A*
Stem Cell Biology Laboratory, National Institute of Immunology, India
*Corresponding author: Asok Mukhopadhyay, Stem Cell Biology Laboratory, National Institute of Immunology, Aruna Asaf Ali Marg, New Delhi-110067, India
Zaffar Equbal, Stem Cell Biology Laboratory, National Institute of Immunology, Aruna Asaf Ali Marg, New Delhi-110067, India
Received: June 29, 2016; Accepted: September 12, 2016; Published: September 14, 2016
Abstract
Mesenchymal Stem Cells (MSCs) are reported to take part in tissue regeneration both at cellular and molecular levels. Here, we have reviewed the potential role of MSCs for the treatment of Parkinson’s Disease (PD). MSCs, as such, their secretory neurotrophic factors and/or exosomes are found to be involved in partial reversal of the disease symptoms in case of animal model of PD. Since there is no proven long-term effective means for treatment of PD patients, it is extremely desirable that MSC-based cellular therapy is given due importance and more prospective pre-clinical and clinical trials are undertaken.
Keywords: Dopaminergic neurons; Cell therapy; Exosomes; Mesenchymal stem cells; Parkinson’s disease
Introduction
MSCs, commonly known as mesenchymal stem or stromal cells and less popularly known as medicinal signaling cells, are group of cells having fibroblastic morphology with stem cell like properties [1]. MSCs were first isolated from the bone marrow as a Colony Forming Unit Fibroblasts (CFU-Fs) by Friedenstein et al. [2]. Afterwards, these cells were identified in different tissues like adipose, liver, skeletal muscle, pancreas, kidney, placenta, Wharton’s jelly of umbilical cord, etc. [3]. The isolation of these cells were not only restricted to human, mouse and rat; but also from other animals such as buffalo, horse, sheep, dog, goat, etc. [4]. MSCs of different tissues have distinct origins, for example, embryonic MSCs are derived from neuroepithelium [5], whereas MSCs of skeletal muscle, pancreas, adipose tissue and placenta are perivascular in origin [6]. Similarly, dental pulp MSCs are known to be originated from glial cells [7]. Because of their diverse tissue origins, International Society of Cellular Therapy (ISCT) has recommended three minimal criteria for defining MSCs: a) plastic adherence, b) positive expression for CD73, CD90 and CD105 and negative expression for CD45, CD34, CD14 or CD11b, CD79a or CD19 and HLA class II, and c) ability to differentiate into adipocyte, osteocyte and chondrocyte lineages [8]. It has been shown that besides mesenchymal lineage, MSCs can differentiate into other lineages, such as neurons, islet-like cells, lung epithelial cells, etc. [9]. MSCs are also shown to express a few neuron-specific genes and proteins, like MAP2, TUJ1, nestin [10,11]. Apart from their potential of neuronal differentiation, MSCs also secrete many trophic factors that are known to have anti-apototic, neuroprotective and immunomodulatory effects on the target cells [12]. Being immunomodulatory and hypoimmunogenic nature, MSCs have been considered as an ideal candidate for cell-based therapy [13].
Parkinson’s Disease
It is a neurodegenerative disease with the hallmark of tremor, rigidity, bradykinesia and postural instability [14]. In 1817, Dr. James Parkinson in his famous book ÔÇťAn Essay on shaking palsyÔÇŁ described the disease as ‘Shaking Palsy’. Later, it was renamed to ‘Parkinson’s Disease (PD)’ by Dr. Jean Martin Charcot. In PD, neurodegeneration occurs in nigro-striatal neurons that are projected from substantia nigra to caudate putamen. It was reported that the onset of disease was observed only after the degeneration of 43.2% tyrosine hydroxylase-positive cells of substantia nigra and 80.3% of dopaminergic transporter positive cells of striatum, thus considered a slow progressing disease [15].
Current PD treatment, involves medication of levodopa along with carbidopa and Deep Brain Stimulation (DBS). However long-term remission have not been observed as these procedures neither counteract the progression of neuron degeneration nor show effectiveness at the advance stages of the disease [16]. Above limitations has been the impetus on a cell-based therapy for PD patients [17].
Several attempts have been made by transplanting human mesencephalic tissue in caudate putamen of PD patients [18-20]. Study of long-term efficacy of these cells showed that patients were free from the pathological signs even after 14 years of transplantation [21] and the normal functional improvement continued till 18 years post therapy [22]. However, due to limited availability and ethical issue of using fetal mesencephalic tissues, alternate cell sources have been explored for the treatment of PD. In this regard Embryonic Stem (ES) cells, induced Pluripotent Stem (iPS) cells, neural stem cells and MSCs are most familiar. The use of ES cells is linked with the risk of tumour formation; moreover their clinical applications have been ethically restricted in many countries [23]. The iPS cells, on the other hand, besides inducing teratoma can also transmit concomitant illness to the patient [24]. Whereas, the neural stem cells are limited due to the shortage of donors [25]. Out of all the possible stem cells types that can be used for therapy, MSCs are considered highly promising owing to simple isolation and culture procedure, hypoimmunogenic (do not express HLA-II and but express HLA-G), immunomodulatory (secrete prostaglandin E2, TGF-β1, HGF, SDF-1╬▒, indoleamine-2,3-dioxygenase, IL-4, IL-6 and IL-10), antiapoptotic (secrete VEGF, HGF, IGF-1) properties, and ability to differentiate into neuronal lineage [26].
Cell Therapy using Differentiated MSCs
Owing to transdifferentiation potential towards neuronal lineage, MSCs have been used to generate midbrain specific dopaminergic neurons. Different protocols were developed for the formation of dopaminergic neurons. Culture of MSCs in the presence of cocktail of growth factors (FGF2, SHH, FGF8) or with small molecules like forskolin or the combine use of DMSO and Butylated Hydroxyl Anisole (BHA) resulted change in cell fate [27-31]. Gene transfection of Notch Intracellular Domain (NICD) followed by treatment with FGF-2, forskolin and Ciliary Neurotrphic Factor (CNTF) resulted in the generation of dopaminergic neurons without the formation of glial cells in case of both rat and human MSCs [32]. The microRNAs (miR-29a, miR-9, miR-124) were also found to promote differentiation of MSCs into neuronal lineage [33-35]. The differentiation of MSCs into neuronal lineage has also been achieved by treating these cells with PC-12 cell-secreted exosomes containing miR-125b [36]. Recently neurons were successfully generated from human DPSCs (Dental Pulp Stem Cells) via intermediate neurosphere stage [37]. This intermediary neurosphere stage promotes cell-to-cell contact thereby playing a crucial role in neural commitment [38,39]. These differentiated cells when transplanted in PD model (6-OHDA lessoned mice, rat and monkey) not only effectively integrated in the tissue but also secreted dopamine in the striatum or caudate putamen of the recipients [28,40,41]. Although there have been many reports of successful differentiation of MSCs into dopaminergic neurons, detail characterisation of the cells at epigenetic level remains obscure. Moreover, it is of utmost importance to decipher the mechanism that directs cellular fate change. The stability of differentiated cells remains debatable as the various studies suggests that the use of small molecules like forskolin, induces transient differentiation [30,42,43]. This proposes a real challenge for using transdifferentiated cells in cell based therapy. Considering the efficiency of transdifferentiation, there is always a risk of having undifferentiated cells in the pool of differentiated cells, which when transplanted may cause undesirable side effects to the recipients [44-48]. This limitation was partially addressed by sorting dopaminergic primed transgenic stem cells expressing GFP under the control of either Hes5 or Nurr1 or Pitx3. Transplantation with Nurr1 based sorted cells showed the greatest number of DA neuron survivability [49,50].
Cell Therapy using Undifferentiated MSCs
Due to several properties attributed to MSCs, as mentioned above, direct use of MSCs has also become popular in cell therapy [51-53]. Human trials of MSCs in PD patients thus far have shown encouraging results [26,54]. The basis of the study was due to secretion of many trophic (e.g. SCF, LIF, FGF-2, VEGF, IL-6) and neuroprotective factors (e.g. NGF, GDNF and BDNF) by MSCs [55,56]. Furthermore, due to secretion of anti-apoptotic factors, these cells have been tested for their ability to restrict the progression of neurodegeneration. Bone marrow MSCs, when transplanted into a 6-OHDA rat model of PD, were not only found to secrete trophic factors like EGF, VEGF, Neurotrophin-3 (NT3), and BDNF without acquiring neuronal phenotype but also were effective in endogenous repair of the damaged neurons [57]. In another study, MSCs were able to exert neuroprotection in 6-OHDA rat model of PD via secretion of neuroprotective factor SDF-1╬▒ [58]. When transplanted into ventricles of E15.5 days mice, these cells generated both migratory neurons as well as radial glial cells [59]. A mean improvement of 17.92% during ‘ON’ and 31.21% during ‘OFF’ period on the basis of UPDRS (unified Parkinson’s disease rating scale) was observed during allogenic transplantation of adult human bone marrow MSCs in PD patient [60].
Though MSCs transplantation has been designated safe by USFDA [61], the biggest challenge associated with transplantation has been considered due to the risk associated with their maldifferentiation [46]. There is also a risk of propagation of Lewy-bodies from host to the grafted tissue in autologous therapy [62,63]. Moreover, MSCs can differentiate into Tumour Associated Fibroblasts (TAFs), thus may promote the formation of metastatic tumours [64]. To circumvent these problems, the current approach relies on the use of secretome or exosomes of MSCs.
Therapeutic Potential of MSC-Secreted Exosomes
MSCs secrete various paracrine factors either directly into the media or through exosomes when cultured on adherent plate [65]. Exosomes are a subclass of nano vesicles of sizes ranging from 50 to 100 nm with an average density of 1.15 g/cm3 [66]. Exosomes have been isolated from the culture media by ultracentrifugation and characterised by assessing their size as well as the expression of CD9, CD63 and CD81 [67]. In addition to CD9, CD63 and CD81, MSCs derived exosomes also express CD29, CD44 and CD73 [65]. The generation of exosomes from MSCs and their uptake by the target cells are depicted in the Figure 1. The role of exosomes derived from MSCs was first reported in 2009 where it was shown to mediate cardioprotective effect in mice model of myocardial ischemia [68]. Exosomes serve as repertoire of protein, lipids, mRNAs as well as miRNA. Recently, miRNA profiling of human MSCs has revealed that the parent cells retain some miRNAs completely while gets devoid of others after secreting them via exosomes [69]. Exosomes pre-treated with RNase were found to be completely ineffective in casting protective effect in a Kidney injury animal model [70]. Human adipose derived MSCs have been shown to secrete neprilysinbound exosomes that helps in the degradation of both Aβ40 and Aβ42 in experimental model of Alzheimer disease [71]. In cerebral artery occlusion rat model, miR-133b present in MSCs derived exosomes promoted neurite outgrowth and functional recovery after stroke [72,73]. Genetically engineering MSCs to over-express GDNF along with modifying its 3’ UTR to contain 25 nt binding site for miR1289 can prove effective in treatment of Parkinson disease [74- 76]. MSCs when engineered to secrete exogenous miR-124 and miR- 145 through exosomes, promoted neuronal differentiation of neural progenitor cells [77]. Low immunogenicity and high permeability towards blood brain barrier makes exosomes- mediated therapy less cumbersome. The major concern with exosomes-mediated therapy is to harness these vesicles in large quantities and their continuous delivery. Exosomes release from infected cells can carry infectious protein like abnormally folded Prion Protein (PrP), Scrapie (PrPsc) on their surface, which needs to be taken care prior to their therapeutic applications [78].
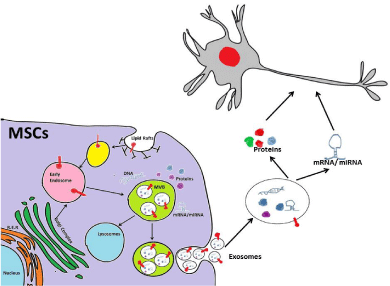
Figure 1: Exosomes are generated by a subgroup of late endosomes,
called Micro Vesicle Bodies (MVBs) that by inward budding engulf proteins,
lipids, mRNA and miRNA. Upon fusion with plasma membrane of the cells,
these MVBs releases exosomes into the extracellular compartment that are
internalised by target cells thereby mediate cell-to-cell communication.
Conclusion
Due to limitation in current therapy for the treatment of PD, the new focus has been emerged in cell-based therapy. Dopaminergic neurons derived from different stem cell types (e.g. ESCs, iPSCs, NSCs); have shown remarkable therapeutic benefits in ameliorating parkinsonian phenotype in animal models and limited extent in human. Owing to the ethical and scientific issues associated with these cells, MSCs has become a popular choice for cell based therapy. Abundance and less invasive isolation procedure associated with MSCs, makes them ideal for autologous transplantation. Allogenic transplantation of MSCs and their derived cells are also possible due to their hypoimmunogenic and immunomodulatory properties. Transplantation of undifferentiated MSCs or dopaminergic neurons derived from MSCs remains a favourite option with a very high success rate. Ability to secrete many trophic factors and exosomes have made the use of MSCs more attractive in treatment of PD. Having small size and/or soluble nature, these secreted products can cross blood brain barrier, involve less complication in delivery, thus considered promising in the future.
References
- Caplan AI. What's in a name? Tissue Eng Part A. 2010; 16: 2415-2417.
- Friedenstein AJ, Chailakhyan RK, Gerasimov UV. Bone marrow osteogenic stem cells: in vitro cultivation and transplantation in diffusion chambers. Cell Tissue Kinet. 1987; 20: 263-272.
- Nombela-Arrieta C, Ritz J, Silberstein LE. The elusive nature and function of mesenchymal stem cells. Nat Rev Mol Cell Biol. 2011; 12: 126-131.
- Calloni R, Viegas GS, Turck P, Bonatto D, Pegas Henriques JA. Mesenchymal stromal cells from unconventional model organisms. Cytotherapy. 2014; 16: 3-16.
- Takashima Y, Era T, Nakao K, Kondo S, Kasuga M, Smith AG, et al. Neuroepithelial cells supply an initial transient wave of MSC differentiation, Cell. 2007; 129: 1377-1388.
- Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008; 3: 301-313.
- Kaukua N, Shahidi MK, Konstantinidou C, Dyachuk V, Kaucka M, Furlan A, et al. Glial origin of mesenchymal stem cells in a tooth model system. Nature. 2014; 513: 551-554.
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006; 8: 315-317.
- Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008; 8: 726-736.
- Blondheim NR, Levy YS, Ben-Zur T, Burshtein A, Cherlow T, Kan I, et al. Human mesenchymal stem cells express neural genes, suggesting a neural predisposition. Stem Cells Dev. 2006; 15: 141-164.
- Foudah D, Monfrini M, Donzelli E, Niada S, Brini AT, Orciani M, et al. Expression of neural markers by undifferentiated mesenchymal-like stem cells from different sources. J Immunol Res. 2014: 987678.
- Murphy MB, Moncivais K, Caplan AI. Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Experimental & Molecular Medicine. 2013; 45.
- Ninagawa N, Murakami R, Isobe E, Tanaka Y, Nakagawa H, Torihashi S. Mesenchymal stem cells originating from ES cells show high telomerase activity and therapeutic benefits. Differentiation. 2011; 82: 153-164.
- Lees AJ, Hardy J, Revesz T. Parkinson's disease. Lancet. 2009; 373: 2055-2066.
- Bezard E, Dovero S, Prunier C, Ravenscroft P, Chalon S, Guilloteau D, et al. Relationship between the appearance of symptoms and the level of nigrostriatal degeneration in a progressive 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned macaque model of Parkinson's disease. J Neurosci 2001; 21: 6853-6861.
- Jankovic J, Aguilar LG. Current approaches to the treatment of Parkinson's disease. Neuropsychiatr Dis Treat. 2008; 4: 743-757.
- Lindvall O, Kokaia Z. Stem cells in human neurodegenerative disorders--time for clinical translation? J Clin Invest. 2010; 120: 29-40.
- Spencer DD, Robbins RJ, Naftolin F, Marek KL, Vollmer T, Leranth C, et al. Unilateral transplantation of human fetal mesencephalic tissue into the caudate nucleus of patients with Parkinson's disease. N Engl J Med. 1992; 327: 1541-1548.
- Piccini P, Brooks DJ, Bjorklund A, Gunn RN, Grasby PM, Rimoldi O, et al. Dopamine release from nigral transplants visualized in vivo in a Parkinson's patient. Nat Neurosci. 1999; 2: 1137-1140.
- Freed CR, Greene PE, Breeze RE, Tsai WY, DuMouchel W, Kao R, et al. Transplantation of embryonic dopamine neurons for severe Parkinson's disease. N Engl J Med. 2001; 344: 710-719.
- Mendez I, Vinuela A, Astradsson A, Mukhida K, Hallett P, Robertson H, et al. Dopamine neurons implanted into people with Parkinson's disease survive without pathology for 14 years. Nat Med. 2008; 14: 507-509.
- Hallett PJ, Cooper O, Sadi D, Robertson H, Mendez I, Isacson O. Long-term health of dopaminergic neuron transplants in Parkinson's disease patients. Cell Rep. 2014; 7: 1755-1761.
- Arnhold S, Klein H, Semkova I, Addicks K, Schraermeyer U. Neurally selected embryonic stem cells induce tumor formation after long-term survival following engraftment into the subretinal space. Invest Ophthalmol Vis Sci. 2004; 45: 4251-4255.
- Herberts CA, Kwa MS, Hermsen HP. Risk factors in the development of stem cell therapy. J Transl Med. 2011; 9: 29.
- Galvin KA, Jones DG. Adult human neural stem cells for cell-replacement therapies in the central nervous system. Med J Aust. 2002; 177: 316-318.
- Wei X, Yang X, Han ZP, Qu FF, Shao L, Shi YF. Mesenchymal stem cells: a new trend for cell therapy. Acta Pharmacol Sin. 2013; 34: 747-754.
- Trzaska KA, Kuzhikandathil EV, Rameshwar P. Specification of a dopaminergic phenotype from adult human mesenchymal stem cells. Stem Cells. 2007; 25: 2797-2808.
- Khoo ML, Tao H, Meedeniya AC, Mackay-Sim A, Ma DD. Transplantation of neuronal-primed human bone marrow mesenchymal stem cells in hemiparkinsonian rodents. PLoS One. 2011; 6: e19025.
- Jang S, Cho HH, Cho YB, Park JS, Jeong HS. Functional neural differentiation of human adipose tissue-derived stem cells using bFGF and forskolin. BMC Cell Biol. 2010; 11: 25.
- Rooney GE, Howard L, O'Brien T, Windebank AJ, Barry FP. Elevation of cAMP in mesenchymal stem cells transiently up regulates neural markers rather than inducing neural differentiation. Stem Cells Dev. 2009; 18: 387-398.
- Woodbury D, Schwarz EJ, Prockop DJ, Black IB. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000; 61: 364-370.
- Dezawa M, Kanno H, Hoshino M, Cho H, Matsumoto N, Itokazu Y, et al. Specific induction of neuronal cells from bone marrow stromal cells and application for autologous transplantation. J Clin Invest. 2004; 113: 1701-1710.
- Duan P, Sun S, Li B, Huang C, Xu Y, Han X, et al. miR-29a modulates neuronal differentiation through targeting REST in mesenchymal stem cells. PLoS One. 2014; 9: e97684.
- Han R, Kan Q, Sun Y, Wang S, Zhang G, Peng T, et al. MiR-9 promotes the neural differentiation of mouse bone marrow mesenchymal stem cells via targeting zinc finger protein 521. Neurosci Lett. 2012; 515: 147-152.
- Zhao Y, Jiang H, Liu XW, Xiang LB, Zhou DP, Chen JT. MiR-124 promotes bone marrow mesenchymal stem cells differentiation into neurogenic cells for accelerating recovery in the spinal cord injury. Tissue Cell. 2015; 47: 140-146.
- Takeda YS, Xu Q. Neuronal Differentiation of Human Mesenchymal Stem Cells Using Exosomes Derived from Differentiating Neuronal Cells. PLoS One. 2015; 10: e0135111.
- Gervois P, Struys T, Hilkens P, Bronckaers A, Ratajczak J, Politis C, et al. Neurogenic maturation of human dental pulp stem cells following neurosphere generation induces morphological and electrophysiological characteristics of functional neurons. Stem Cells Dev. 2015; 24: 296-311.
- Tarnok K, Pataki A, Kovacs J, Schlett K, Madarasz E. Stage-dependent effects of cell-to-cell connections on in vitro induced neurogenesis. Eur J Cell Biol. 2002; 81: 403-412.
- Schlett K, Czirok A, Tarnok K, Vicsek T, Madarasz E. Dynamics of cell aggregation during in vitro neurogenesis by immortalized neuroectodermal progenitors. J Neurosci Res. 2000; 60:184-194.
- Hayashi T, Wakao S, Kitada M, Ose T, Watabe H, Kuroda Y, et al. Autologous mesenchymal stem cell-derived dopaminergic neurons function in parkinsonian macaques. J Clin Invest. 2013; 123: 272-284.
- Kumar A, Dudhal S, Sundari TA, Sunkara M, Usman H, Varshney A, et al. Dopaminergic-primed fetal liver mesenchymal stromal-like cells can reverse parkinsonian symptoms in 6-hydroxydopamine-lesioned mice. Cytotherapy. 2016; 18: 307-319.
- Bertani N, Malatesta P, Volpi G, Sonego P, Perris R. Neurogenic potential of human mesenchymal stem cells revisited: analysis by immunostaining, time-lapse video and microarray. J Cell Sci. 2005; 118: 3925-3936.
- Lu P, Blesch A, Tuszynski MH. Induction of bone marrow stromal cells to neurons: differentiation, transdifferentiation, or artifact? J Neurosci Res. 2004; 77: 174-191.
- Cuiffo BG, Campagne A, Bell GW, Lembo A, Orso F, Lien EC, et al. MSC-regulated microRNAs converge on the transcription factor FOXP2 and promote breast cancer metastasis. Cell Stem Cell. 2014; 15: 762-774.
- Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, et al. Mesenchymal stem cells within tumour stromapromote breast cancer metastasis. Nature. 2007; 449: 557-563.
- Kunter U, Rong S, Boor P, Eitner F, Muller-Newen G, Djuric Z, et al. Mesenchymal stem cells prevent progressive experimental renal failure but maldifferentiate into glomerular adipocytes. J Am SocNephrol. 2007; 18: 1754-1764.
- Fiorina P, Jurewicz M, Augello A, Vergani A, Dada S, La Rosa S, et al. Immunomodulatory function of bone marrow-derived mesenchymal stem cells in experimental autoimmune type 1 diabetes. J Immunol. 2009; 183: 993-1004.
- Sundin M, Orvell C, Rasmusson I, Sundberg B, Ringden O, Le Blanc K. Mesenchymal stem cells are susceptible to human herpes viruses, but viral DNA cannot be detected in the healthy seropositive individual. Bone Marrow Transplant. 2006; 37: 1051-1059.
- Ganat YM, Calder EL, Kriks S, Nelander J, Tu EY, Jia F, et al. Identification of embryonic stem cell-derived midbrain dopaminergic neurons for engraftment. J Clin Invest. 2012; 122: 2928-2939.
- Thompson LH, Bjorklund A. Transgenic reporter mice as tools for studies of transplantability and connectivity of dopamine neuron precursors in fetal tissue grafts. Prog Brain Res. 2009; 175: 53-79.
- Yang ZX, Han ZB, Ji YR, Wang YW, Liang L, Chi Y, et al. CD106 identifies a subpopulation of mesenchymal stem cells with unique immunomodulatory properties. PLoS One. 2013; 8: e59354.
- Saeidi M, Masoud A, Shakiba Y, Hadjati J, MohyeddinBonab M, Nicknam MH, et al. Immunomodulatory effects of human umbilical cord Wharton's jelly-derived mesenchymal stem cells on differentiation, maturation and endocytosis of monocyte-derived dendritic cells. Iran J Allergy Asthma Immunol. 2013; 12: 37-49.
- English K, French A, Wood KJ. Mesenchymal stromal cells: facilitators of successful transplantation? Cell Stem Cell. 2010; 7: 431-442.
- Wang S, Qu X, Zhao RC. Clinical applications of mesenchymal stem cells. J Hematol Oncol. 2014; 5: 19.
- Gu W, Zhang F, Xue Q, Ma Z, Lu P, Yu B. Transplantation of bone marrow mesenchymal stem cells reduces lesion volume and induces axonal regrowth of injured spinal cord. Neuropathology. 2010; 30: 205-217.
- Wilkins A, Kemp K, Ginty M, Hares K, Mallam E, Scolding N. Human bone marrow-derived mesenchymal stem cells secrete brain-derived neurotrophic factor which promotes neuronal survival in vitro. Stem Cell Res. 2009; 3: 63-70.
- Cova L, Armentero MT, Zennaro E, Calzarossa C, Bossolasco P, Busca G, et al. Multiple neurogenic and neurorescue effects of human mesenchymal stem cell after transplantation in an experimental model of Parkinson's disease. Brain Res. 2010; 1311: 12-27.
- Wang F, Yasuhara T, Shingo T, Kameda M, Tajiri N, Yuan WJ, et al. Intravenous administration of mesenchymal stem cells exerts therapeutic effects on parkinsonian model of rats: focusing on neuroprotective effects of stromal cell-derived factor-1alpha. BMC Neurosci. 2010; 11: 52.
- Munoz-Elias G, Marcus AJ, Coyne TM, Woodbury D, Black IB. Adult bone marrow stromal cells in the embryonic brain: engraftment, migration, differentiation, and long-term survival. J Neurosci. 2004; 24: 4585-4595.
- Venkataramana NK, Pal R, Rao SAV, Naik AL, Jan M, Nair R, et al. Bilateral Transplantation of Allogenic Adult Human Bone Marrow-Derived Mesenchymal Stem Cells into the Subventricular Zone of Parkinson’s Disease: A Pilot Clinical Study. Stem Cells International. 2012; 2012: 12.
- Parekkadan B, Milwid JM. Mesenchymal stem cells as therapeutics. Annu Rev Biomed Eng. 2010; 12: 87-117.
- Li JY, Englund E, Holton JL, Soulet D, Hagell P, Lees AJ, et al. Lewy bodies in grafted neurons in subjects with Parkinson's disease suggest host-to-graft disease propagation. Nat Med. 2008; 14: 501-503.
- Kordower JH, Chu Y, Hauser RA, Freeman TB, Olanow CW. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson's disease. Nat Med. 2008; 14: 504-506.
- Spaeth EL, Dembinski JL, Sasser AK, Watson K, Klopp A, Hall B, et al. Mesenchymal stem cell transition to tumor-associated fibroblasts contributes to fibrovascular network expansion and tumor progression. PLoS One. 2009; 4: e4992.
- Yu B, Zhang X, Li X. Exosomes derived from mesenchymal stem cells. Int J Mol Sci. 15: 4142-4157.
- Livshits MA, Khomyakova E, Evtushenko EG, Lazarev VN, Kulemin NA, Semina SE, et al. Isolation of exosomes by differential centrifugation: Theoretical analysis of a commonly used protocol. Sci Rep. 2015; 5: 17319.
- Thery C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. CurrProtoc Cell Biol. 2006; 3: 3.22.
- Lai RC, Arslan F, Lee MM, Sze NS, Choo A, Chen TS, et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010; 4: 214-222.
- Collino F, Deregibus MC, Bruno S, Sterpone L, Aghemo G, Viltono L, et al. Microvesicles derived from adult human bone marrow and tissue specific mesenchymal stem cells shuttle selected pattern of miRNAs. PLoS One. 2010; 5: e11803.
- Reis LA, Borges FT, Simoes MJ, Borges AA, Sinigaglia-Coimbra R, Schor N. Bone marrow-derived mesenchymal stem cells repaired but did not prevent gentamicin-induced acute kidney injury through paracrine effects in rats. PLoS One. 2012; 7: e44092.
- Katsuda T, Tsuchiya R, Kosaka N, Yoshioka Y, Takagaki K, Oki K, et al. Human adipose tissue-derived mesenchymal stem cells secrete functional neprilysin-bound exosomes. Sci Rep. 2013; 3: 1197.
- Xin H, Li Y, Buller B, Katakowski M, Zhang Y, Wang X, et al. Exosome-mediated transfer of miR-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. Stem Cells; 2012; 30: 1556-1564.
- Xin H, Li Y, Liu Z, Wang X, Shang X, Cui Y, et al. MiR-133b promotes neural plasticity and functional recovery after treatment of stroke with multipotent mesenchymal stromal cells in rats via transfer of exosome-enriched extracellular particles. Stem Cells. 2013; 31: 2737-2746.
- Bolukbasi MF, Mizrak A, Ozdener GB, Madlener S, Strobel T, Erkan EP, et al. miR-1289 and "Zipcode"-like Sequence Enrich mRNAs in Microvesicles. MolTher Nucleic Acids. 2012; 1: e10.
- Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F. GDNF: A glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993; 260:1130-1132.
- Gill SS, Patel NK, Hotton GR, O'Sullivan K, McCarter R, Bunnage M, et al. Direct brain infusion of glial cell line-derived neurotrophic factor in Parkinson disease. Nat Med. 2003; 9: 589-595.
- Lee HK, Finniss S, Cazacu S, Xiang C, Brodie C. Mesenchymal stem cells deliver exogenous miRNAs to neural cells and induce their differentiation and glutamate transporter expression. Stem Cells Dev. 2014; 23: 2851-2861.
- Fevrier B, Vilette D, Archer F, Loew D, Faigle W, Vidal M, et al. Cells release prions in association with exosomes. ProcNatlAcadSci USA. 2004; 101: 9683-9688.