
Research Article
J Stem Cell Res Transplant. 2015;2(1): 1016.
Function of Rat Diabetic Islets Improved By Coculturing with Pancreatic Mesenchymal Stromal Cells
Maryam Mohammadi Khajehdehi1, Durdi Qujeq2*, Habibollah Peirovi3, Narges Karbalaie4, Seyed Javad Mowla5
1Department of Genetics, Science & Research Branch, Islamic Azad University, Tehran, Iran
2Department of Biochemistry and Biophysics, Faculty of Medicine, Babol University of Medical Sciences, Babol, Iran
3Nanomedicine & Tissue Engineering Research Center, Taleghani Hospital, Tehran, Iran
4Department of Physiology, Faculty of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran
5Molecular Genetics Department, School of Biological Sciences, Tarbiat Modares University, Tehran, Iran
*Corresponding author: Dr Durdi Qujeq, Department of Biochemistry and Biophysics, Faculty of Medicine, Babol University of Medical Sciences, Babol, Iran
Received: November 26, 2014; Accepted: February 19, 2015; Published: February 24, 2015
Abstract
Developing strategies to prevent beta cells mass reduction and/or increasing their in vivo and in vitro extension are promising choices for cell therapy of type 1 and type 2 diabetes. MSCs (Mesenchymal stem cells)are multipotent stromal cells with the ability to self-renew and differentiate into various cell types of its host tissues. Recently, the effects of transplanted rBMSCs(rat Bone Marrow Mesenchymal Stem Cells) on survival and function of isolated islets have been reported. In this research, rP-MSCs (rat pancreatic Mesenchymal Stem Cells) were isolated and characterized before being co-cultured with injured islets of diabetic rat models in vitro. The effects of rP-MSCs on insulin secretion of islets were examined after 5 days. Furthermore, the extracted cells were labeled by DiI dye and their migration and incorporation within the injured pancreatic islets were visualized after 24 hours. Altogether, our data revealed that after co-culturing of the injured islets with rP-MSCs, the insulin secretion level of the islets were significantly elevated (p value<0.001). This finding implies that rPMSCs have a potential ability in repair of diabetic islets by either releasing some growth and immuno-modulatory factors, or by direct incorporation within the damaged islets.
Keywords: Type 1 diabetes; Pancreatic Stromal Cells; Injured islets; Coculture
Introduction
Diabetes Mellitus is the most common endocrine disorder, with more than 200 million people suffering from the disease worldwide. More importantly, it is estimated that the number of cases will reach to 300 million by 2025 [1-3]. The disease is a chronic metabolic syndrome, and is diagnosed by an elevated sugar level in blood which is referred to hyperglycemia. Type 1 diabetes is usually caused by an autoimmune disturb of pancreatic islet's beta cells which is consequently followed by a shortage or lack of insulin in blood. For this reason, the person will need a permanent supply of external insulin to survive [4]. While the external insulin sources can improve the blood sugar level, managing its routine application, and hence permanent blood's sugar control, is very complicated. Therefore, transplantation of Pancreas tissue or insulin producing cells has been an attractive potential therapy to cure the disease permanently [5].
The main aim of the cell-based therapy that has emerged as a strategic treatment for many human diseases is to replace, repair and/ or enhance the biological function of damaged tissues in an organ. The main biological materials for this purpose are "stem cells" which is obtained either from embryonic or adult tissue-specific stem cells [6]. According to some recent studies, the insulin secreting cells can be generated in vitro from mouse bone marrow stromal cells [7] and mature pancreatic cells including mouse and human pancreatic duct cells [8,9]. In this regard, the epithelial cells of pancreatic duct contain progenitor cells which are involved in pancreas growth and renewal [10]. Furthermore, there are other cell types within the pancreas that are able to proliferate and differentiate to beta cells [11,12]. These cells are recognized by the presence of some cell surface markers that are related to a specific linage of stem cells.
The application of pancreas transplantation, as a therapeutic strategy for diabetic patients, is limited because of a shortage in organ donors [13,14]. In case of islet transplantation to diabetic patients, a good supply of insulin producer cells is required [15]. At the same time, maintaining the survival of islets can be another challenge [16], as the injuries made during islet isolation may lead to a functional drop of islets and transplantation failure [17]. Therefore, improvement of the methods to preserve the function and survival of islets are very important in transplantation success.
Some researchers have tried to overcome the aforementioned problem by co-culturing islets with different cell types. The first study in this subject was performed by [18], when they co-cultured pancreatic islets of new born rats with mouse fibroblasts and observed an increase in survival and function of the cultured islets. Similar efforts have been made by using pancreatic duct cells [19] or bone marrow cells [6,20,21] to improve the islets function.
In this study, we have investigated a new strategy to improve the function of isolated injured islets obtained from rat diabetic models, by co-culturing them with pancreatic mesenchymal stromal cells.
Materials and Methods
Isolation and culture of rP-MSCs
Male Wistar rats (Rattus norvegicus; weight 90-110grams; 3-4 weeks old) were obtained from Pastor Institute in Tehran, and were stored under standard conditions of constant humidity (55-65%) and temperature (22-24°C), 12 hours dark/light cycle, with unrestricted access to food and water. Animal housing and surgical procedures were carried out in accordance with the Animal Care and Use Committee regulations of Tarbiat Modares University (TMU) to reduce animal suffering and the number of used animals.
The animals were anaesthetized with 60mg/kg pentobarbital (Sigma, Germany); their pancreases were removed under sterile conditions, minced into small pieces and washed with cold PBS. The enzymatic digestion was performed by collagenase P (Roche, Germany) enzyme at 1mg/mL in cold PBS, with an incubation time of 13 minutes. Digestion was then stopped with addition of cold PBS. After washing and discarding the supernatant, DMEM (Gibco- Invitrogen, USA) media supplemented with 20% fetal bovine serum (Biowest, USA) was added to the pellet, resuspended and cultured in a 6-well plate.
RNA extraction and reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was isolated from passage 1 of rP-MSCs cell cultures, using Trizol reagent (Invitrogen, USA) and as instructed by the manufacturer. The RNA of PSCs (Pancreatic Stem Cells)and islets (as positive controls) were reverse-transcribed using the primescript™ RT reagent kit (TaKaRa, Japan). The obtained cDNA of PSCs and islets was then added to PCR mix consisted of 10X PCR buffer, 4μl dNTPs, 1μl mix primer, 4μl MgCl2 and 0.25μl TaKaRa Ex Taq™.The PCR products were then analyzed by 2% agarose gel electrophoresis. The sequences of the designed primers for selected genes are listed in Table 1.
Induction of diabetes with Alloxan
Diabetes was induced by intravenous injection of a freshly prepared solution of alloxan (Sigma-Aldrich, USA; 40mg/kg body weight) to rats. The blood sugar level was measured in 48 hours, and animals with glucose ranging from 300-380 mg/dl, showing clear signs of polyuria, polydipsia and polyphagia, were considered as diabetic and were analyzed 48 hours after alloxan treatment.
Isolation of islets from normal and diabetic rats
The rats were starved for 12 hours before surgery, and their pancreatic ducts were clamped. The exposed pancreas was subjected to collagenase P solution (0.5 mg/ml in cold PBS) and incubated in 37°C for 17 minutes. Next, the endocrine parts were isolated from the other parts by PBS washing and suction for several times. The islets were then handpicked under a stereo microscope.
Staining of islets with DTZ
The diabetic and normal islets were stained by DTZ (dithizone), (Millipore,USA). For islets staining, we added 10μl of 100X DTZ stains solution to 1ml culture medium. After 3-5 minutes, the cells were examined under an invert microscope.
Co-culturing of rP-MSCs with normal & diabetic islets
For co-culture experiments, pancreatic stromal cells were employed as feeder cells. Isolated islets (diabetic and normal) were categorized in two groups: half of the strainers (BD Falcon, Mexico) containing islets from each group were cultured with rP-MSCs and the rest of the islets were cultured alone. In order to study the effects of rP-MSCs on islets' function, co-cultures of days 1, 3 and 5 were chosen randomly. At the end of each day, strainers containing islets were incubated first in L-DMEM for 1 hour and then in H-DMEM for another 1 hour. Finally the culture media were collected and the amount of released insulin was measured by rat insulin ELISA kit (Mercodia, Denmark), according to the manufacturer instruction.
Labeling of rP-MSCs by DiI vital dye and monitoring their migration into injured islets
rP-MSCs were labeled with 10μg /ml DiI dye (invitrogen, USA), as instructed with the manufacture. Then, they were examined under a florescent microscope, before and 24 hours after being co-cultured with injured islets.
Statistical analysis
Each experimental group included 72 islets. Co-culture experiments were performed in 8 different subgroups, and 9 times. Data are reported as mean ± SD by Prism 5 software (GraphPad Prism version 5.0.0.288 for Windows, GraphPad Software, San Diego California USA, www.graphpad.com). Then they were analyzed by two-way ANOVA and paired t-test with the same software. Differences between the experimental and control groups were regarded as statistically significant when p<0.05.
Results
Isolation and Characterization of Rat Pancreatic Mesenchymal Stromal Cells (rP-MSCs)
Isolated cells from enzymatically digested minced pancreases were cultured and their morphology were examined routinely. On first day of culture, fibroblast-like mesenchymal cells adhered to cultured plates (Figure 1A).The cells were then propagated to a confluency of 70% by 72 hours (Figure 1B). The rate of growth and morphology of the cells were not changed during consequent passages. In 8th day of culture, the confluent cells started to generate cell colonies which were morphologically similar to pancreatic islets (Figure 1C). Gradually, more cellular clusters were found in cultures, and step by step maturation took place (Figure 1D).
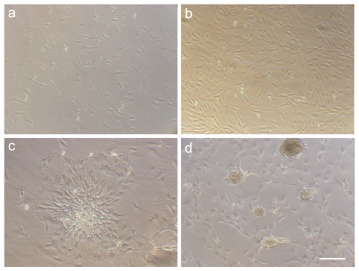
Figure 1: Morphological appearances of rat pancreas stem cells (rP-MSCs) at different time points after culturing: after 24 hours (A) and 72 hours (B) of culturing (C) 8 days after culture (D) 12 days after culture. Note that by continuation of cell culture, clusters of cells formed colonies which resemble the morphology of pancreatic islets (Scale bar = 200μm).
Gene expression profile of rP-MSCs
We examined the expression of some genes which are characteristic factors in MSCsand endocrine progenitor cells, in the aforementioned cell cultures. As a positive control, the extracted RNA from pancreatic islets was used. β-Actin was also employed as an internal control in both RNA samples. As it is evident in Figure 2, Nucleostemin, Vimentin, Cyclin D1, Pdx-1, Isl-1, CD34 and Pax-6 genes were expressed in both pancreatic islets and the isolated cells. However, Ngn3 and CD133 were just expressed in pancreatic islets (Figure 2A).
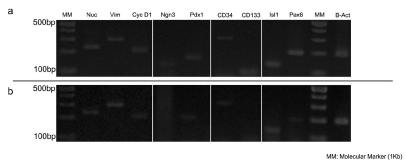
Figure 2: Gel electrophoresis of RT-PCR products. (A) Gene expression in pancreatic islets. (B) Gene expression in rP-MSCs.
Light microscopy of cultured normal & diabetic islets (with and without DTZ)
The number of normal islets which was isolated by aforementioned method was around 300-350 islets per rat. In diabetic rats, this number was much lower, approximately 50-100 islets per animal. To examine the health and morphology of the islets, some of them from each group were stained with DTZ. As it is shown in Figure 3, diabetic islets appeared as hollow and less stained color spheres, due to the loss of their beta cells (Figure 3B, 3D), while normal islets showed a more solid appearance and uniformly stained with the dye (Figure 3A and 3C).
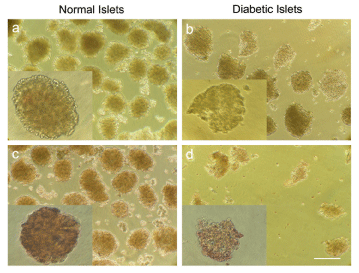
Figure 3: Microscopic images of isolated islets from normal and diabetic rats. (A) Normal islets without DTZ. (B) Diabetic islets without DTZ. (C) Normal islets stained by DTZ. (D) Diabetic islets stained by DTZ (scale bar = 100μm).
Repairing ability of rP-MSCs on diabetic islets
After co-culturing of islets from both groups with rP-MSCs, we employed ELISA test to monitor the level of insulin release within the media. The obtained data demonstrated a significant decrease (p<0.0001) in the level of secreted insulin from normal islets in low glucose medium, from day one toward day five, in both islets cultures with or without rP-MSCs (Figure 4A).
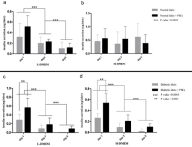
Figure 4: Histogram representation of the data obtained by two-way Annova test. The comparison of the level of insulin released within the media of normal islets alone or co-cultured with rP-MSCSs on days 1, 3, 5 in low glucose medium (L-DMEM, A) and in high glucose medium (H-DMEM, B). Similar comparison is demonstrated for diabetic islets cultured with or without rP-MSCs in L-DMEM on days 1, 3, 5 (C) and in H-DMEM in day 1, 3, 5 (D). Note that insulin secretion was significantly declined from day 1 to day 5 in L-DMEM medium, in both simple and co-culture and in both normal & diabetic islets groups. In H-DMEM medium of normal islets, none of the differences were statistically significant; however, in diabetic islets and in H-DMEM similar results were obtained as for L-DMEM medium.
As it is shown in Figure 4B, there existed some slight changes in the insulin secretion in both groups (normal islets cultured alone or with rP-MSCs) in high glucose medium, however, the observed changes were not statistically significant (p>0.05). For diabetic islets cultured in low and high glucose medium, there was a significant decline in the levels of secreted insulin from day 1 toward day 5 in both cultures, with or without rP-MSCs (P<0.0001; (Figure 4C, 4D)).
Moreover, comparison of the levels of insulin release between simple and co-culture groups of diabetic islets revealed a significant difference after 24 hours of culturing, for both low-glucose and highglucose media P<0.001; (Figure 4C, 4D). However, this difference was not statistically significant for later time points, as determined by ANOVA test (Figure 4C,4D). Interestingly, when the same raw data were re-examined with another statistical test, student T-test, it was determined that the released insulin from diabetic islets co-cultured with rP-MSCs is significantly higher in simple culture of diabetic islets in day 3 (p<0.05) and day 5 (p<0.01) of cultures in high-glucose media, as well as in day 5 (p<0.001), but not in day 3, of cultures in low-glucose media (Figure 5A-5D).
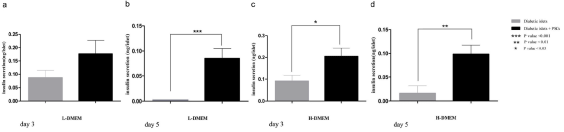
Figure 5: Histogram representation of the data obtained by paired T-test. (A) The comparison of insulin secretion level in diabetic islets of simple and co-cultured with rP-MSC groups in L-DMEM, on day 5. (B) Level of insulin release from diabetic islets in simple and co- cultured groups in H-DMEM on day 3 and (C) on day 5. The obtained P values for each case are provided in the figure.
Light microscopy of DiI labeled cells & migration assay
To monitor the fate of rP-MSCs in our co-culture system, the cells were stained with DiI dye and were observed routinely under a florescent microscope (Figure 6A-6D). Af?ter 24 hours of co-culturing the injured islets with labeled cells, some patches of labeled cells were found in injured islets, confirming the immigration of labeled rPMSCs toward injured islets (Figure 6B,C,E,F).
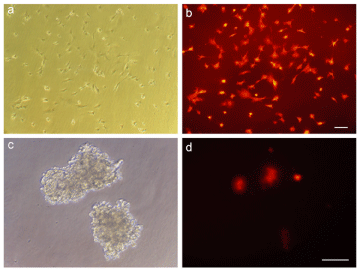
Figure 6: stained rP-MSCs with vital dye DiI and their migration assay within the injured islets. (A, B, C) The images were taken by a phase-contrast microscope. (D, E, F) The images were taken with a florescent microscope to localized the DiI-labeled cells. (scale bar = 100μm).
Discussion
In this research, we have investigated the potential application of rP-MSCs to improve the recovery of diabetic islets in rats. Recent studies on rodents have demonstrated that the mature pancreas contains several types of endocrine progenitor cells, with the ability to differentiate to beta cells [22]. These cells could be identified via the presence of several ES-specific cell surface markers. We chose some of these markers to characterize the isolated MSCs from rat islets, based on previous reports of ours and other groups. These markers include: Nucleostemin that is expressed in high level in pluripotent as well as highly dividing cells [23,24]. Cyclin D1 is primarily expressed in highly dividing cells including stem and cancerous cells, and it has a key role for progression from G1 to S phases [25]. Vimentin is a specific protein for mesenchymal cells and it is also a marker for proliferation rate and undifferentiated state of the cells [26]. The expression of these markers in our isolated cells from rat pancreas, along with their morphological features, identified them as putative pancreatic mesenchymal stem cells.As described previously mesenchymal stem cell -derived factors showed positive effects on islets. MSCs-derived trophic molecules have the potential to inhibit islet cell death [27]. Previous study conducted by other investigators has shown that MSCs undergo to differentiation into an insulin-releasing phenotype after co-culture with pancreatic islets [28].
CD34 is one of the pancreatic stem cell/progenitor markers[29], whereas CD133 is recognized as a pancreatic duct progenitor marker. According to [30]. findings, rat pancreatic duct progenitors which were isolated by FACS were CD133+/CD34- (2007). The isolated cells in our work were CD133-/CD34+; and it seems that those cells did not have a ductal origin. Indeed, the extracted cells seem to be originated from the endocrine part of the pancreas, and since they are CD34+, they are probably arisen from the pancreatic stem cells. Moreover, the cultured cells were Pdx-1+, Isl-1+ and Pax 6+. Since Pdx-1 is a transcription factor responsible for pancreas morphology and functioning of the beta cells [31,32] and that Isl-1 and Pax 6 are specific markers for endocrine pancreas [33], we can conclude that the isolated cells are probably originated from the endocrine part of the pancreas.
Based on our data, a significant decrease in the level of insulin release was observed in low-glucose medium of normal islets cultured with or without rP-MSCs, from day 1 to day 5. In contrast, we failed to show a similar observation in high-glucose medium. Moreover, in the co-culture system of day 1, 3, and 5, rP-MSCs did not have any significant influence on insulin secretion of normal islets. Examining the effects of human bone marrow mesenchymal stem cells cocultured with pancreatic islets [34], reported a significant drop in the beta cell population in cultured islets alone from day 3 toward day 45, which in turn leads to a significant drop in the level of insulin release. Consistent with our findings in H-DMEM in day 5, they observed that the level of insulin released from islets co-cultured with BM-MSCs was lower than islets cultured alone, in days 3 and 14 of cultivation.
In a research by [21], the insulin level in treated islets with STZ after 14 days had a significant decrease, as compared with normal islets. Moreover, the intracellular insulin level of the islets co-cultured with stem cells showed a significant elevation, compared to the islets cultured alone. These data are in agreement with our findings, and confirmed our conclusion. In another research by [20], co-culturing of islets with BM cells elevated the survival and functioning of the islets; which is consistent with our results.
Several reports demonstrated the migration ability of MSCs to damaged areas [35-37]. Much evidence has accumulated in recent years, indicating that bone marrow mesenchymal stem cells had the ability to migrate to pancreaticislets and provide an apparent overall preservation for islet function. Furthermore pancreatic islets showed a better survival and function by co-culturing with bone marrow mesenchymal stem cells [38]. The ability of BMSCs to migrate into pancreatic islets was first reported by [39]; using bone marrow cells from GFP+ transgenic mice and transplanting the GFP+ cells to recipient mice confirmed the presence of GFP+ bone marrow cells around and into pancreatic islets. Their results were similar to ours in examination of cell migration to islets, however, for considering cellular migration, we were labeled cells by DiI dye and the experiments were done in vitro. The later finding was consistent with our data on migration of Di1 labeled cells to the cultured islets; however, it is possible that some released markers of rPSCs be effective on increasing of insulin secretion level of injured islets.
Acknowledgment
This study was supported by a grant (number 418/A/87/157, 87/2/1) from the Hematology-Oncology and Stem Cell Transplantation Research Center, Shariati Hospital, Tehran University of Medical Sciences, Tehran, Iran.
References
- 1. Amos A, McCarty D, Zimmet P. The rising global burden of diabetes and its complications, estimates and projections to the year 2010. Diabetic Med 1997; 14: S1-S85.
- 2. King H, Aubert R, Herman W. Global burden of diabetes, 1995-2025. Prevalence, numerical estimates and projections. Diabetes Care 1998;21: 1414-1431.
- 3. Zimmet P. Globalization, coca-colonization and the chronic disease epidemic: can the Doomsday scenario be averted? J Med 2000; 247: 301-310.
- 4. Atkinson MA, Eisenbarth GS. Type I diabetes: new perspectives on disease pathogenesis and treatment. Lancet 2001; 358: 221-229.
- 5. Halban PA. Cellular sources of new pancreatic beta cells and therapeutic implications for regenerative medicine. Nat Cell Bio 2004;6: 1021-1025.
- 6. Karaoz E, Ayhan S, Okcu A, Aksoy A, Bayazit G, et al. Bone marrow-derived mesenchymal stem cells co-cultured with pancreatic islets display β cell plasticity. J Tissue EngRegen Med 2010a (2011); 5: 491-500.
- 7. Gao R, Ustinov J, Pulkkinen MA, Lundin K, Korsgren O, et al. Characterization of endocrine progenitor cells and critical factors for their differentiation in human adult pancreatic cell culture. Diabetes 2003; 52: 2007-2015.
- 8. Bonner-Weir S, Taneja M, Weir GC, Tatarkiewicz K, Song KH, et al. In vitro cultivation of human islets from expanded ductal tissue. ProcNatlAcadSci2000;97: 7999-8004.
- 9. Yang L, Li S, Hatch H, Ahrens K, Cornelius JG, et al. In vitro trans-differentiation of adult hepatic stem cells into pancreatic endocrine hormone-producing cells. ProcNatlAcadSci USA 2002; 99: 8078-8083.
- 10. Sharma A, Zangen DH, Reitz P, Taneja M, Lissauer ME, et al. The homeodomain protein IDX-1 increases after an early burst of proliferation during pancreatic regeneration. Diabetes 1999; 48: 507-513.
- 11. Zulewski H, Abraham EJ, Gerlach MJ, Daniel PB, Moritz W, et al. Multipotentialnestin-positive stem cells isolated from adult pancreatic islets differentiate ex vivo into pancreatic endocrine, exocrine, and hepatic phenotypes. Diabetes 2001; 50: 521-533.
- 12. Wu F, Jagir M, Powell JS. Long-term correction of hyperglycemia in diabetic mice after implantation of cultured human cells derived from fetal pancreas. Pancreas 2004; 29: e23-e29.
- 13. Ryan EA, Paty BW, Senior PA. Five-year follow-up after clinical islet transplantation. Diabetes 2005;54: 2060-2069.
- 14. Balamurugan AN, Bottino R, Giannoukakis N, Smetanka C. Prospective and challenges of islet transplantation for the therapy of autoimmune diabetes. Pancreas 2006; 32: 231-243.
- 15. Noguchi H, Iwanaga Y, Okitsu T. Evaluation of islet transplantation from non-heart beating donors. Am J Transplant 2006;6: 2476-2482.
- 16. Shapiro AM, Ricordi C, Hering BJ, Auchincloss H, Lindblad R, et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med 2006; 355: 1318-1330.
- 17. Ichii H, Wang X, Messinger S, Alvarez A, Fraker C, et al. Improved human islet isolation using nicotinamide. Am J Transplant 2006;6: 2060-2068.
- 18. Rabinovitch A, Russell T l, Mintz DH. Factors from fibroblasts promote pancreatic islet B cellsurvival in tissue culture. Diabetes 1979;28: 1108-1113.
- 19. Gatto C, Callegari M, Folin M, Conconi M, Paolin A, et al. Effects of cryopreservation and coculture with pancreatic ductal epithelial cells on insulin secretion from human pancreatic islets. Int J Mol Med 2003; 12: 851-854.
- 20. Luo L, Badiavas E, Luo JZ, Maizel A. Allogeneic bone marrow supports human islet beta cell survival and function over six months. BiochemBiophys Res Commun2007;361: 859-864.
- 21. Karaoz E, Genc ZS, Demircan PC, AksoyA, Duruksu G. Protection of rat pancreatic islet function and viability by coculture with rat bone marrow-derived mesenchymal stem cells. Cell Death and Disease 2010b; 1 e36.
- 22. Soria B, Bedoya FJ, Martin F. Gastrointestinal stem cells: Pancreatic stem cells. Am J PhysiolGastrointest Liver Physiol2005;289: G177-G180.
- 23. Liu SJ, Cai ZW, Liu YJ, Dong MY, Sun LQ, et al. Role of nucleostemin in growth regulation of gastric cancer, liver cancer and other malignancies. World Journal of Gastroenterology 2004; 10: 1246-1249.
- 24. Kafienah W, Mistry S, Williams C, Hollander AP. Nucleostemin is a marker of proliferating stromal stem cells in adult human bone marrow. Stem Cells 2006;24: 1113-1120.
- 25. Yaghoobi MM, Mowla SJ, Tarihi T.Nucleostemin, a coordinator of self-renewal, is expressed in rat marrow stromal cells and turns off after induction of neural differentiation. Neurosci Lett 2005; 890: 81-86.
- 26. Stenger AM, Garre ML, Andreussi L, Cama A, Callea F. Expression of histone H3 cell cycle-related gene, vimentin and MYC genes in pediatric brain tumors. A preliminary analysis showing the different malignant cell growth potential. Molecular Brain Research 1992; 13: 273-275.
- 27. Park KS, Kim YS, Kim JH, Choi BK, Kim SH, et al. Influence of Human Allogenic bone marrow and cord blood-derived mesenchymal stem cell secreting trophic factors on ATP (adenosine-5=-triphosphate)/ADP (adenosine-5=-diphosphate) ratio and insulin secretory function of isolated human islets from cadaveric donor. Transplantation Proceedings 2009; 41: 3813-3818.
- 28. Scuteri A, Donzelli E, Rodriguez-Menendez V, Ravasi M, Bonandrini B, et al. Differentiation of mesenchymal stem cells towards an insulin-releasing phenotype after co-culture with pancreatic islets. Italian Journal of Anatomy and Embryology 2012; 117: 174.
- 29. Puglisi MA, Giuliani L, Fierabracci A. Identification and characterization of a novel expandable adult stem/progenitor cell population in the human exocrine pancreas. J Endocrinol Invest 2008; 31: 563-572.
- 30. Oshima Y, Suzuki A, Kawashimo K, Ishikawa M, Ohkohchi N, et al. Isolation of mouse pancreatic ductal progenitor cells expressing CD133 and c-Met by flow cytometric cell sorting. Gastroenterology 2007;132: 720-732.
- 31. Ahlgren U, Jonsson J &Jonsson. L Beta-cell-specific inactivation of the mouse Ipf1/Pdx1 gene results in loss of the beta-cell phenotype and maturity onset diabetes. Genes Dev1998; 12: 1763-1768.
- 32. Sander M, Sussel L, Conners J. Homeobox gene Nkx6.1 lies downstream of Nkx2.2 in the major pathway of beta-cell formation in the pancreas. Development 2000;127: 5533-5540.
- 33. St-Onge L, Sosa-Pineda B, Chowdhury K. Pax6 is required for differentiation of glucagon-producing alpha-cells in mouse pancreas. Nature 1997; 387: 406-409.
- 34. Wang Zh, Xiong F, Hassani M, Luo JZQ, Luo LG. Bone marrow increases human islets insulin positive cells in co-culture: quantification with flow cytometry. Journal of Diabetes Mellitus 2011;1: 109-117.
- 35. Barbash IM, Chouraqui P, Baron J. Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium: feasibility, cell migration, and body distribution. Circulation 2003; 108: 863-868.
- 36. Hou LL, Zheng M, Wang DM. Migration and differentiation of human bone marrow mesenchymal stem cells in the rat brain. Sheng Li XueBao2003; 55: 153-159.
- 37. Ji JF, He BP, Dheen ST, Tay SS. Interactions of chemokines and chemokine receptors mediate the migration of mesenchymal stem cells to the impaired site in the brain after hypoglossal nerve injury. Stem Cells 2004;22: 415-427.
- 38. Lin P, Chen L, Li D, Yang N, Sun Y, et al. Dynamic analysis of bone marrow mesenchymal stem cells migrating to pancreatic islets using coculture microfluidic chips: An accelerated migrating rate and better survival of pancreatic islets were revealed. NeuroEndocrinolLett 2009; 30: 204-208.
- 39. Sordi V, Malosio ML, Marchesi F, Mercalli A, Melzi R, et al. Bone marrow mesenchymal stem cells express a restricted set of two pancreatic islets functionally active chemokine receptrs capable of promoting migration. Blood 2005; 106: 419-427.