
Review Article
J Stem Cell Res Transplant. 2019; 6(1): 1029.
Pediatric Stroke and Cell-Based Treatment – Pivotal Role of Brain Plasticity
Arne Jensen*
Campus Clinic Gynecology, Ruhr-University Bochum, Germany
*Corresponding author: Arne Jensen, Campus Clinic Gynecology, Ruhr-University Bochum, Universitätsstr. 140, 44799 Bochum, Germany
Received: March 15, 2019; Accepted: April 27, 2019; Published: May 04, 2019
Abstract
Pediatric cerebrovascular disorders like stroke are among the top 10 causes of death in children, with rates highest in the first year of life, while survivors may face lifelong sequelae, disability, and/or cerebral palsy for which there is no cure at present. Individual treatments using human autologous cord blood Mononuclear Cells (hucbMNC) containing stem cells have yielded promising results. However, stroke is an entity with heterogenic etiologies including arterial ischemic stroke, hemorrhagic stroke, hemorrhagic infarction, and sinus venous thrombosis in newborns, infants, children, and adults. Hence, any attempt to examine care, non-cellular or cell-based therapies has to merit the specific characteristics of this heterogeneity as far as age, symptoms, diagnostics, pathophysiology, histopathology, clinical course and prevalence are concerned. This review describes the various etiologies of stroke for the Neonatal/Perinatal and childhood subsets of the pediatric population as a basis for established non-cellular and novel cell-based therapeutic approaches using cord blood mononuclear cells in the preclinical and clinical setting. In addition, due to its fundamental importance for cell-based therapeutic strategies for stroke, an account of brain plasticity along with blood-brain barrier function and the distinctions between the developing brain and the adult brain is provided. It is concluded that on the present balance of evidence the pathophysiological characteristics associated with plasticity of the developing brain are closely linked to the pharmacological action of hucbMNC in such a way that cell-based treatment might be more efficacious in pediatric stroke than in adult stroke.
Keywords: Pediatric stroke; Autologous cord blood stem cells; Prevalence; Brain plasticity; Brain development; Blood-brain barrier; Cerebral palsy; Perinatal brain damage; Neuroregeneration; Adult stroke
Abbreviations
AIS: Arterial Ischemic Stroke; CP: Cerebral Palsy; CPISR: Canadian Pediatric Ischemic Stroke Registry; CSVT: Cerebral Sinus Venous Thrombosis; CVA: Cerebral Vascular Accident; DMSO: Dimethylsufoxid; FA: Fractional Anisotropy; FLAIR: Fluid-Attenuated Inversion Recovery; FVL: Factor V Leiden; EGF/FGF: Endothelial Growth Factor/Fibroblast Growth Factor; G-CSF: Granulocyte-Colony Stimulating Factor; GMFCS: Gross Motor Function Classification System; HIE: Hypoxic–Ischemic Encephalopathy; Hucb: Human Umbilical Cord Blood; Hucbmnc: Human Umbilical Cord Blood Mononuclear Cells; ICA: Intra Cerebral Artery; ICH: Intracranial Hemorrhage; IP: Intra Peritoneally; IV: Intra Venous; MCA: Middle Cerebral Artery; MoA: Mode of Action; MRA: Magnetic Resonance Angiography; MRI: Magnetic Resonance Imaging; MRI-DTI: Magnetic Resonance Imaging-Diffusion-Tensor Imaging; MTHFR: 5,10 Methylenetetrahydrofolate Reductase; NGF/RA: Nerve Growth Factor/Retinoic Acid; NHDS: National Hospital Discharge Survey; PAIS: Perinatal Arterial Ischemic Stroke; PFO: Patent Foramen Ovale; PVHI: Periventricular Hemorrhagic Infarction; ROI: Regions Of Interest; SCD: Sickle Cell Disease; SPECT: Single Photon Emission Computed Tomography; SVT: Sinus Venous Thrombosis; TIA: Transient Ischemic Attack; Tpa: Tissue Plasmin Activator; UFH: Unfractionated Heparin and WMD: White matter damage.
Introduction
Pediatric brain damage of various etiologies, including stroke, and subsequent Cerebral Palsy (CP) are devastating and debilitating ailments with lifelong sequelae and high mortality. Unfortunately, there is no causative treatment beyond symptomatic and supportive care and the estimated lifetime costs per affected child amount to 800,000 Euro. The enormous number of 17 million people worldwide that live with cerebral palsy reflects the magnitude of unmet needs and the personal, medical, and global socioeconomic burden of this brain disorder.
But there is hope. Only recently the first successful cell-based treatment of arterial ischemic stroke in a child using autologous cord blood mononuclear cells (Jensen and Hamelmann, 2016 [1]) and of adult stroke using allogeneic cord blood mononuclear cells have been reported (Laskowitz et al., 2018 [2]). This renders a comprehensive review of the literature mandatory to explore safety and efficacy of cell-based treatment options for young and elderly patients alike.
Stroke is an entity with heterogenic etiologies including arterial ischemic stroke, hemorrhagic stroke, hemorrhagic infarction, and sinus venous thrombosis in newborns, infants, children, and adults. Hence, any attempt to examine care and non-cellular or cell-based therapies has to merit the specific characteristics of this heterogeneity as far as age, symptoms, diagnostics, pathophysiology, histopathology, and clinical course are concerned. This review describes the various etiologies of stroke stratified by age, i.e. Neonatal/Perinatal and childhood subsets of the pediatric population, as a basis for established non-cell-based and novel cell-based therapeutic approaches using cord blood mononuclear cells in the preclinical and clinical setting. Due to its fundamental importance for cell-based therapeutic strategies for stroke, an account of brain plasticity along with bloodbrain barrier function and the distinctions between the developing and the adult brain is provided that might explain in part as to why the efficacy of cell-based treatment may be different in childhood from that in adulthood.
Pediatric Stroke
Pediatric stroke is a subset of the general condition stroke. “The two periods of life in which the risk of having a stroke is highest are in the elderly and in the fetus or newborn infant. In both these periods, stroke is associated with heightened risk of death or, in survivors, of persisting neurological disability” (Nelson, 2007 [3]).
The condition Pediatric stroke, including Neonatal/Perinatal and Childhood stroke, is a cerebrovascular disorder in children which can be treated by autologous human mononuclear cells derived from cord blood (Jensen and Hamelmann, 2016 [1]). The condition is chronically debilitating and life threatening with a low prevalence. However, disorders of the cerebrovascular system are among the top 10 causes of death in children, with rates highest in the first year of life, the US mortality in 1998 attributable to stroke being 7.8/100,000 in children 0 to 1 year of age (Lynch et al., 2002 [4]).
Stroke in the pediatric population encompasses Neonatal/ Perinatal stroke (age 0 to <28 days) and Childhood stroke (age 28 days/1 month to <18 years). The reported outcome for stroke in children varies, but >50% of survivors develop neurologic or cognitive problems, about 33% have a recurrence of the disease, and 5% to 10% of affected children are bound to die (Lynch et al., 2002 [4]). Due to underlying differences in incidence, cause, and outcome, Neonatal/ Perinatal stroke is discussed separately from Childhood stroke.
Neonatal/Perinatal stroke
Neonatal/Perinatal stroke presented in the neonatal period includes a range of focal and multifocal ischemic, hemorrhagic, or venous sinus thrombosis cerebral tissue injuries, and to avoid confusion the terms Neonatal stroke and Perinatal stroke that are sometimes used interchangeably, the condition is from now onwards referred to as Neonatal/Perinatal stroke (Rutherford et al., 2011 [5]).
Definition: Neonatal/Perinatal stroke is defined as “a group of heterogeneous conditions in which there is focal disruption of cerebral blood flow secondary to arterial (Perinatal Arterial Ischemic Stroke (PAIS)) or cerebral venous thrombosis or embolization (Cerebral Sinus Venous Thrombosis (CSVT)), between 20 weeks of fetal life through the 28th postnatal day, confirmed by neuroimaging or neuropathologic studies” (Raju et al., 2007 [6]). This definition separates Neonatal/Perinatal stroke from neonatal Hypoxic-Ischemic Encephalopathy (HIE) (Tsuji et al., 2015 [7]; Rutherford et al., 2011 [4]).
Etiology:
Ischemic stroke: Risk factors for the development of Neonatal/ Perinatal PAIS and CSVT may be maternal and/or fetal and neonatal but both conditions are likely to result from a combination of predisposing factors (Rutherford et al., 2011 [5]). The reported causes of Neonatal/Perinatal stroke have included cardiac disorders, infection, blood abnormalities, and/or perinatal events, however, in a large number of cases the cause remains undetermined.
In Neonatal/Perinatal stroke prothrombotic factors are frequently found in studies of PAIS (range 42-78%) and CSVT (Chabrier et al., 2011 [8]). In 64% (27/42) of neonates with CSVT, not one had antithrombin, protein C or protein S deficiency but higher than expected rates of Factor V Leiden (FVL) and 5,10 Methylenetetrahydrofolate Reductase (MTHFR) mutations were found (Fitzgerald et al., 2006 [9]).
Mutation of FVL has been associated with thrombosis and other pregnancy disorders, including severe gestosis, fetal growth retardation, placental abruption, preterm delivery, placental infarction, spontaneous miscarriage, and fetal mortality (Lynch et al., 2002 [4]).
The most common discharge diagnoses reported in conjunction with Neonatal/Perinatal stroke included infection, cardiac disorders, and blood disorders, with <5% associated with “birth asphyxia”. Coagulation disorders, including the Factor V Leiden (FVL) and prothrombin mutation, protein C, protein S, and antithrombin III deficiencies have been identified in up to half of infants and children with cerebral thromboembolism (Bonduel et al., 1999 [10]; deVeber et al., 1998 [11]; Golomb et al., 2001 [12]). Phospholipids are important in the activation of protein C and the coagulation pathway. Antiphospholipid antibodies, including lupus anticoagulant and anticardiolipin, are directed against anticoagulant proteins, interfere with normal coagulation, and may be a risk factor for Neonatal/ Perinatal stroke. A study by Nelson et al. (1998) found elevated titers of antiphospholipid antibodies in children with cerebral palsy versus controls (Nelson et al., 1998 [13]).
Hereditary coagulation abnormalities may lead to adverse events via thrombosis at the maternal-placental interface. Maternal thrombosis may lead to preeclampsia, fetal growth restriction, abortion, or fetal mortality. Thrombosis in the fetal circulation is a possible source of emboli that can bypass the liver and the lungs to reach the fetal brain. Within the fetus or neonate thrombosis or embolism based on FVL mutation are always possible (Lynch et al., 2002 [4]).
Hemorrhagic stroke: A population-based study revealed that perinatal hemorrhagic stroke was typically unifocal (74%) and unilateral (83%) yielding a prevalence for perinatal hemorrhagic stroke of 6.2/100,000 live births while etiologies included thrombocytopenia and cavernous malformation; 75% were idiopathic, with evidence of encephalopathy (100%) and/or seizures (65%). Unlike birth weight, predictors of perinatal hemorrhagic stroke were male gender, fetal distress, emergency cesarean delivery, prematurity, and postmaturity (Armstrong-Wells et al., 2009 [14]). In a recent study, idiopathic hemorrhagic stroke was related to young maternal age, primiparity, difficult fetal transition, bradycardia, low Apgar scores, and fetal SGA. Recurrence is rare event, however, outcomes are often poor (Cole et al., 2017 [15]).
Sinus Venous Thrombosis (SVT): Neonatal sinovenous thrombosis is a multifactorial disease. An incidence of 1.4 to 12 per 100,000 term newborns has been reported. In term-born neonates, assisted or complicated delivery occurred in 62% of the cases and the majority presented seizures, some prothrombin mutation (FII G20210A). At follow-up, moderate to severe neurological sequelae were diagnosed in over one-third of the cases while 19% died (Berfelo et al., 2010 [16]).
Specific pathophysiological, Histopathological, Clinical characteristics:
Neonatal/Perinatal ischemic stroke: In Neonatal/Perinatal ischemic stroke the most common cause of arterial occlusion is septic embolisation associated with disseminated intravascular coagulation (Barmada et al., 1979 [17]).
Focal and multifocal ischemic brain necrosis of all cellular elements occur within the distribution of single or multiple cerebral vessel or vessels (Volpe, 2008 [18]). The cellular changes depend primarily on the time passed after the insult and anoxic neuronal change can be visualised by light microscopy after 18-24 hours. Then cells of the monocyte-macrophage type migrate from vessels entering the lesion as pleomorphic activated microglia, followed by astroglial proliferation over weeks and months forming a dense layer of glial fibrillary processes, i.e., a glial scar (Volpe, 2008 [18]; Jensen, 2014 [19]).
Neuropathology of 592 infants revealed an incidence of 5.4% for cerebral infarction that was related to gestational age (0 <28 weeks, 5% 28-32 weeks, 10% 32-37 weeks, and 15% between 37- 40 weeks gestation (Barmada et al., 1979 [17]). Involvement of the middle cerebral artery occurred in 50%. The topography of perinatal ischemic stroke/infarction is 75% unilateral, of these 65% involve the left middle cerebral artery, and 25% bilateral (Volpe, 2008 [18]).
Ischemic lesions of the preterm and term brain are of considerable importance to the clinician as they account for a high proportion of infants who present with mental retardation, seizures, or various forms of cerebral palsy, e.g., spasticity, choreoathetosis, and ataxia. In the term infant, ante partum and intra partum asphyxia account for 90% of the cases.
Neonatal/Perinatal hemorrhagic stroke: Hemorrhagic cerebral infarction results when the injured capillaries in an ischemic infarct are ruptured by release of an arterial obstruction (e.g., embolus) or an increase in venous pressure, or when small amounts of bleeding from injured capillaries are not controlled by an intact clotting system. Thus, hemorrhagic infarction is observed in the newborn primarily with [1] embolic arterial occlusion because of distal movement of an embolus, [2] venous thrombosis because of the increase in venous pressure proximally, and [3] arterial thrombosis (or perhaps vasospasm) that is partial (or intermittent) or accompanied by a disturbance of coagulation. Indeed, most examples of “intracerebral hemorrhages” or “grade IV” intraventricular hemorrhage (PIVH) in the term newborn probably represent hemorrhagic venous infarction (Volpe, 2008 [18]).
Neonatal/Perinatal cerebral venous thromboses involve in ~65% the superior sagittal sinus, and infarction is present in 40% to 60%. The infarcts characteristically are hemorrhagic and MRI often reveals hemorrhagic infarction of the parasagittal cortical regions bilaterally with sagittal sinus thrombosis. Intraventricular hemorrhage is present in 20% to 35% of the cases. When focal and multifocal necroses are associated with dissolution of tissue and cavitation then Porencephaly is used to describe the lesion (Volpe, 2008 [18]).
Primary venous infarction usually involves obstruction of the deep vein system. There is dilatation of the deep vein tributaries with surrounding hemorrhages and necrosis. There is no recognizable clinical pattern in live born infants with primary venous infarction; rupture of blood into the ventricles will produce the clinical picture of intraventricular hemorrhage (Pape et al., 1979 [20]; Berger et al., 1997 [21]; Jensen and Holmer et al., 2018 [22]). If an infant were to survive a significant venous infarct, severe mental retardation and a high incidence of cerebral palsy would be expected as a result of destruction of long fiber tracts in the paraventricular white matter (Jensen, Neuhäuser, Jensen, 2018 [23]). Cortical hemorrhage is seen in association with cortical necrosis, and it has been suggested that these lesions are often hemorrhagic arterial infarcts (Friede, 1975 [24]).
Histological changes in the early stages include shrinkage of neurons, with acidophilic cytoplasm and pyknosis or karyorrhexis of nuclei. Sometimes there is ballooning and vacuolation of the cytoplasm of the degenerating neurons. At a later stage, there is loss of neurons and infiltration with lipid-laden macrophages and development of gliosis with capillary proliferation. The changes are similar in all areas of cortical or nuclear damage and resemble those seen in ischemic lesions in the adult brain (Pape et al., 1979 [20]). The time period involved in the propensity to cavitation (e.g., Porencephaly) is from approximately the second trimester of gestation to the first postnatal week or months. The high water content (90%) of unmyelinated brain tissue predisposes to cavitation (Volpe, 2008 [18]).
There is no specific clinical sign with cortical or white matter hemorrhagic infarction. If the lesion develops in newborns with birth asphyxia, coma, seizures, abnormalities of tone and/or neonatal reflexes may occur alone or in combination. Unilateral cortical infarcts secondary to focal compression necrosis or emboli may produce contralateral neurological signs and ipsilateral EEG changes (Pape et al., 1979 [20]; Jensen and Hamelmann, 2016 [1]).
Neonatal/Perinatal sinus venous thrombosis stroke: Sinus Venous Thrombosis (SVT) in children affects primarily neonates and results in neurologic impairment or death in approximately half of the cases. SVT is diagnosed when venous blood flow in at least one major sinus is impaired. Parenchymal infarction secondary to impaired venous drainage, and therefore not in an arterial distribution, results in approximately 60% of the cases. Perinatal complications (51%), e.g., hypoxic encephalopathy, dehydration (30%), and prothrombotic state (20%) are most common (deVeber et al., 2001 [25]).
In neonates, cerebral parenchymal infarcts are present in 42%, and 35% are hemorrhagic infarcts. These lesions are adjacent to the thrombosed sinus, e.g., hemorrhagic infarction in the cortex and subcortical white matter with superior sagittal sinus thrombosis and thalamic hemorrhage from thrombus in the internal cerebral veins and straight sinus. There is dilation of the deep vein tributaries with surrounding hemorrhages and necrosis. Rupture of blood into the ventricles may cause internal hydrocephalus. If capillary veins rupture, fibrin thrombi are seen within the veins. In cases of massive hemorrhage, the branches of the terminal vein may be grossly congested and there may be hemorrhage or infarction affecting the related white matter. Neuropathologic changes are similar to those described above (Pape et al., 1979 [20]).
The most common symptoms at presentation in the neonates are seizures (57%) (Grunt et al., 2010 [26]), but sinus venous thrombosis can also present in all ages with fever, lethargy, or coma or in younger infants with a history of decreased oral intake or respiratory distress. Even dilated scalp veins, eyelid swelling, or a large anterior fontanel may be revealed by physical examination (Tsze et al., 2011[27]). The best available clinical estimate of outcome is that after a mean of 2.1 years, 77% percent of neonates are neurologically normal. The primary neurologic manifestations in the neonates are seizures and diffuse neurologic signs. Adverse neurologic outcomes include seizures (20%), and the presence of infarcts (non-hemorrhagic or hemorrhagic) (deVeber et al., 2001 [25]).
Incidence and prevalence: Neonatal/Perinatal stroke affects between 1 in 2,330 – 5,000 newborns. In the National Hospital Discharge Survey (NHDS) from 1980 through 1998, the prevalence of stroke for term infants <30 days of age was 26.4/100,000, that of hemorrhagic stroke was 6.7/100,000, and that of ischemic stroke was 17.8/100,000 live births per year. This results in a rate of Neonatal/ Perinatal of approx. 1/4,000 live births per year (Lynch et al., 2002 [4]).
In Europe unilateral Neonatal/Perinatal Arterial Ischemic Stroke (PAIS) was recognized in the neonatal period in 1/2,300 term infants (Schulzke et al., 2005 [28]) and also bilateral stroke is not uncommon (Nelson, 2007 [3]). In general, Neonatal/Perinatal stroke is underrecognized clinically, because some infants are neurologically asymptomatic in the newborn period, and motor impairment is recognized only at about 4 to 5 months of age (Lynch et al., 2002 [4]).
In one study of infants greater than 31 weeks gestation with neonatal seizures, 12% had underlying cerebral infarction (Estan et al., 1997 [29]). These results suggest a prevalence of Neonatal/ Perinatal stroke of 24.7/100,000 per year in infants greater than 31 weeks gestation and are similar to a published report of 28.6/100,000 by Perlman and coworkers (Perlman et al., 1994 [30]).
Arterial ischemic stroke: In the Swiss Registry, neonates with a diagnosis of Neonatal Arterial Ischemic Stroke (NAIS) were included. Follow-up was performed 2 years (mean 23.3 months, SD 4.3) after birth (Grunt et al., 2015 [31]) and 100 neonates (two-third boys) had a diagnosis of NAIS with seizures being the most common symptom (95%). Furthermore, 81% had unilateral (80% left-sided) and 19% had bilateral lesions, respectively. There were maternal risk conditions (32%), birth complications (68%), and neonatal comorbidities (54%). Two years after birth, 39% presented with cerebral palsy and 31% with mental delay (Grunt et al., 2015 [31]). Given the cases of symptomatic NAIS, the prevalence is 12.5/100,000 live births. In addition, the fraction of 40% of total NAIS that is asymptomatic has to be accounted for (Grunt et al., 2015 [31]).
Hemorrhagic stroke: There is no specific prevalence data for hemorrhagic Neonatal/Perinatal stroke in Europe. In the Northern California Kaiser Permanente Medical Care Program 17 cases of symptomatic Neonatal/Perinatal hemorrhagic stroke were identified, which yielded a population prevalence of 8.14/100,000 live births per year (Agrawal et al., 2009 [32]).
Venous sinus thrombosis: In the Swiss Registry, neonates with a diagnosis of Cerebral Sinus Venous Thrombosis (CSVT), symptomatic at presentation were included. Follow-up was performed 2 years after birth. The prevalence of symptomatic CSVT was 3.2/100,000 live births (Grunt et al., 2010 [26]).
Thus, the total prevalence of Neonatal/Perinatal stroke, i.e., stroke caused by arterial ischemia, hemorrhages, and venous thrombosis combined, is 32.1/100,000 live births. This is largely in agreement with data from the Canadian Pediatric Ischemic Stroke Registry (CPISR).
Management of Neonatal/Perinatal stroke: As mentioned, risk factors for the development of Neonatal/Perinatal PAIS and CSVT may be maternal and/or fetal and neonatal but both conditions are likely to result from a combination of predisposing factors (Rutherford et al., 2011 [5]). The reported causes of Neonatal/Perinatal stroke have included infection, blood abnormalities, perinatal events, and or cardiac disorders.
Prevention of infection (early intervention, antibiotics), blood abnormalties (treatment of thrombophilia), trauma (prospective risk management during labour, including large term-born infants (Jensen and Holmer, 2018 [22]), and perinatal events during birth (early intervention during asphyxia (Berger et al., 1997 [21]) are the most important means of preventing Neonatal/Perinatal stroke. Secondly, if prevention fails, follow professional management of perhaps infected, traumatized, and asphyxiated newborns along the guidelines (BNCM, 2009 [33]). Furthermore sentinel events during parturition particularly when combined with neonatal cephalic molding during birth and head deformity require further examination of the head by imaging for early diagnosis of neonatal brain damage (Jensen and Holmer, 2018 [22]). If Neonatal/Perinatal stroke is inevitable, symptomatic, and diagnosed by appropriate imaging techniques (Cranial ultrasound, CT, MRI), therapeutic cooling procedure within 6 hours after birth has become standard of care for more mature preterm (>=36 weeks gestation) and term newborn infants to reduce the adverse effects of encephalopathy (Gluckman et al., 2005 [34]).
Symptoms: Due to the fact that 40% of infants are clinically normal in spite of having a cerebral infarction, makes imaging, i.e., cranial ultrasound, CT, and particularly MRI imaging indispensable for a proper and timely diagnosis. As stated above, only 60% of the infants presenting with cerebral infarction are symptomatic, generally showing seizures, apnoe, or abnormal tone. Depending on the location and extent of the lesion the clinical signs vary considerably between inapparent, asymptomatic, and coma.
Diagnosis: Neuro-imaging is required to confirm the diagnosis of Neonatal/Perinatal stroke or Cerebral sinus venous thrombosis. Available techniques include Ultrasonography (transfontanel, power Doppler), CT, and MRI. The use of CT is discouraged because of excessive radiation dosage. Also, intravenous contrast may be required for detection of sinus thrombosis. Conventional T1-weighted and T2-weighted MRI sequences, Diffusion-Weighted Imaging (DWI) or diffusion tensor imaging, MR venography, and MR Angiography (MRA) should be included if necessary (Rutherford et al., 2004 [4]).
Non-cellular treatment: There is no curative treatment for Neonatal/Perinatal stroke hitherto. Treatment is symptomatic and supportive. However, children with Neonatal/Perinatal stroke should receive regular medical screenings to determine appropriate interventions. Acute treatment of neonatal AIS includes supportive measures, e.g., maintenance of normal hydration, electrolytes, hemoglobin, clotting factors, glucose, oxygen, and pH levels are important. Beyond anticonvulsants, antibiotics and/or antivirals should be given if sepsis is suspected. At present, therapeutic hypothermia in neonates with cerebral arterial ischemic stroke or sinus, venous thrombosis has not yet to been validated but hyperthermia is to be avoided. There are no randomised controlled trials addressing the acute or chronic treatment of Neonatal/ Perinatal stroke and the majority of recommendations are based on case series and expert consensus. Administration of Unfractionated Heparin (UFH) or Low Molecular Weight Heparin (LMWH) in neonates with a first AIS and an ongoing documented cardioembolic source has been recommended by the American College of Chest Physicians (ACCP). However, anticoagulation or antiplatelet therapy is not recommended since the risk of recurrent stroke is minimal. Administration of anticoagulants in neonatal CSVT is controversial because recurrent CSVT is also rare (Rutherford et al., 2004 [4]).
Therapeutic hypothermia (cooling): Infants with symptomatic Neonatal/Perinatal stroke (accounting for 10% of all Neonatal Encephalopathies (NE)) and infants with evidence for encephalopathy on the basis of perinatal events (asphyxia, 85% of all NE), infection, blood abnormalties, trauma, and/or seizures are often very sick with multi-organ failure requiring intensive care. Current evidence shows that mild hypothermia (33-34°C) can improve neurological outcome at 2 years and is now recommended as the standard of care. However, cooling should be part of a package of neurointensive care that enables appropriate investigation, treatment, and imaging to be undertaken prior to long term follow-up being arranged for surviving infants. Infants with Neonatal/Perinatal stroke and suspected HIE who meet the inclusion criteria should be considered for treatment with cooling (Walston et al., 2012 [35]).
Seizures may be apparent on clinical examination (abnormal rhythmic movement of limbs, lip smacking, etc.) but can sometimes be difficult to diagnose. The amplitude integrated EEG (aEEG) as recorded by a Cerebral Function Monitor (CFM) is able to detect most seizures including many seizures not apparent clinically - known as subclinical seizures. Although an aEEG should ideally be performed on all infants with moderate-severe HIE, it is not mandatory prior to starting cooling and may not be available in all units. But there are other evidence based provisions to consider, too, as for example the Recommendations Class 1-3 for Neonatal/Perinatal Stroke (Roach et al., 2008 [36]).
Outcome and prognosis: The outcome of children with Neonatal/ Perinatal stroke varies among studies because of differences in stroke type, duration of follow-up, specific outcomes, and population studied. Electroencephalography and neuro-imaging studies provide measures of long-term cognitive and motor outcome. Children with an abnormal electroencephalogram during the first week after stroke typically develop hemiplegia. Mercuri and colleagues (1999) found that infarction involving the internal capsule predicts the development of hemiplegia, whereas involvement of other regions is less reliable (Mercuri et al., 1999 [37]). These results are similar to a previous study of preterm children with hemorrhagic infarction. In reference to language function, Trauner and colleagues (1996) have found that children with early unilateral brain damage exhibit prosodoic deficits when compared with controls, and the side of the lesion seems to be related to affective comprehension (Trauner et al., 1996 [38]). In contrast to the relationship between infarct location and language deficits or hemiplegia, the location of the lesion does not adequately predict visual abnormalities.
The recurrence rate of neonatal stroke is much less than in childhood stroke. In the Canadian Pediatric Ischemic Stroke Registry (CPISR), the largest cohort of stroke in children, the recurrence rate of children with neonatal stroke was 3% to 5%, and in survivors outcome was normal in 33% (deVeber et al., 2000 [39]).
Childhood stroke
Definition: Childhood stroke is defined as a cerebrovascular event that occurs between >28 days and <18 years of age (Lynch et al., 2002 [4]). The diagnosis is based on Magnetic Resonance Angiography (MRA) or conventional angiography abnormalities.
Etiology:
Ischemic stroke: There are no widely accepted classification systems for the cause of ischemic stroke in children. However, the mechanisms by which ischemic stroke occurs in children include the following: thromboembolism from an intracranial or extracranial vessel, or the heart; acute, transient, or progressive arteriopathy; and other rare causes, but in a large percentage of cases the cause is undetermined (Al Sulaiman et al., 1999 [40]). The diagnosis of stroke attributable to cardiac or transcardiac embolism has varied among cohort studies. Children with stroke attributable to cardiogenic embolism typically have some evidence of an underlying heart disorder, with or without a recognized thrombus. However, a recent study of Childhood stroke categorized children with cardiac or transcardiac embolism even without a history of cardiac disease (Chabrier et al., 2011 [8]). Children with stroke attributable to angiopathy typically have some evidence based on angiographic, non-invasive, or other studies of arterial dissection, vasculitis, Moyamoya disease, transient cerebral angiopathy, or other vasculopathy (Chabrier et al., 2011 [8]).
The most frequently reported risk factors for ischemic stroke in children include arteriopathy coagulation disorders, Sickle Cell Disease (SCD), cardiac disorders, infection, Moyamoya disease, arterial dissection, and other rare genetic disorders. But there are differences as far as the geographical region is concerned (Mackay et al., 2011 [41]).
Cardiac disease, as previously stated, is a common cause of stroke in children, accounting for up to 50% of strokes in case series (Chabrier et al., 2011 [8]). In the CPISR, cardiac disease was identified in 25% of children with ischemic stroke. Several cardiac disorders are associated with stroke in children, including congenital heart disease, intracardiac defects, cardiac procedures, and acquired heart disease. Several acquired and genetic coagulation factor abnormalities have been associated with ischemic stroke in children. A study of cerebral thromboembolism that included 92 children from the Hospital for Sick Children in Toronto found coagulation abnormalities in 38% of children studied. Antiphospholipid antibodies have been reported in a quarter of patients with a first stroke. In a study by Angelini and co-workers (1994), 75% of children with cerebral ischemia were positive for antiphospholipid antibodies (Angelini et al., 1994 [42]). Sickle cell-related stroke is associated with large vessel stenosis of the proximal middle cerebral or distal internal carotid artery. The risk of stroke in children with Sickle Cell Disease (SCD) is 200 to 400 times that of children without SCD, the recurrence rate is extremely high (50% by 3 years), and in children with stroke aged 6 months to 10 years, up to 30% have post varicella angiopathy. Moyamoya, a chronic non-inflammatory occlusive intracranial vasculopathy of unknown cause, is primarily seen in the Japanese population and accounts for 10% to 20% of arterial infarcts in children. Extracranial arterial dissection, mostly caused by trauma, is a common cause of stroke in young adults and children while intracranial arterial dissection occurs almost as frequently as extracranial dissection in children (see Lynch et al., 2002 [4] for review).
Hemorrhagic stroke: Hemorrhagic stroke is less common than ischemic stroke in children and several risk factors for hemorrhagic stroke have been identified in children, including vascular malformations, malignancy, and trauma. Furthermore, primary and secondary coagulation disorders including hemophilia, thrombocytopenia, liver failure, leukemia, and warfarin therapy are associated with hemorrhagic stroke in children (Lynch et al., 2002 [4]).
The most common cause of hemorrhagic stroke in children are Arteriovenous Malformations (AVM) (Menovsky et al., 1997 [43]) resulting from failure in the formation of the capillary bed between cerebral primitive arteries and veins during the first trimester of gestation. The incidence of AVM in children is 1/100,000, of these approximately 10% to 20% will become symptomatic during childhood (Menovsky et al., 1997 [43]). The average probability of a first hemorrhage is 2% to 4% per year, with a recurrence risk as high as 25% by 5 years. Approximately 1% to 2% of aneurysms become symptomatic during childhood and are typically associated with other vascular lesions or chronic disorders. Also, cavernous malformations can lead to hemorrhagic stroke in children, one third being related to family history and have recently been linked to abnormalities in the long arm of chromosome 7. Magnetic Resonance (MR) imaging and angiography (MRA) confirm the diagnosis of AVM (see Lynch et al., 2002 [4] for review).
Sinus venous thrombosis (SVT): Data from the CPISR reveal that the incidence of SVT in children is 0.6/100 000, children per year and is highest in the first year of life (deVeber et al., 2001 [25]). A number of risk factors are linked to the development of SVT, including head and neck infections, dehydration, perinatal complications, and coagulation disorders. In one study, prothrombotic abnormalities were present 50% of the children with SVT and many of these children had multiple risk factors (deVeber et al., 1998 [11]).
Specific pathophysiological, histopathological, clinical characteristics:
Childhood ischemic stroke: Childhood ischemic stroke results in focal and multifocal ischemic brain necrosis of all cellular elements that occur within the distribution of single or multiple cerebral vessel or vessels (Volpe, 2008 [18]). As already mentioned, the cellular changes relate primarily to the time after theinsult and are accompanied with migration of cells of the monocyte-macrophage type that enter the lesion as activated microglia, followed by astroglial proliferation eventually forming a glial scar (Volpe, 2008 [18]).
Ischemic lesions of the brain during childhood are of considerable importance to the clinician as they account for a high proportion of infants who present as a focal neurologic deficit, e.g. hemiplegia (94%), or seizures (50%). Older children demonstrate more specific neurological defects similar to adults, e.g., hemiparesis, language (e.g., aphasia) and speech difficulties, visual deficits, and headache (Tsze et al., 2011 [27]).
Childhood hemorrhagic stroke: Hemorrhagic stroke is less common than ischemic stroke in children. The histopathological changes are those described above, are similar in all areas of cortical or nuclear damage, and resemble those seen in ischemic lesions in the adult brain (Pape et al., 1979 [20]).
Childhood hemorrhagic strokes usually present as headaches (59%), reduced consciousness (88%), or seizures (29%) and are, unlike arterial ischemic stroke, more likely to cause vomiting (48%). A subarachnoid hemorrhage can also present as irritability and a bulging fontanel in infants, but should be suspected in older children complaining of sudden acute onset headache, neck pain, meningisms, or photophobia. Dysarthria and bulbar dysfunction suggest lower brainstem involvement while aphasia points to involvement of the basal ganglia, thalamus, or cerebral hemispheres. The eyes look towards the lesion if the hemispheres, however, away from the lesion if the brainstem is involved (Tsze et al., 2011 [27]).
Childhood cerebral sinus venous thrombosis stroke (SVT): Data from the CPISR reveal that the incidence of Sinus Venous Thrombosis (SVT) in children is 0.6/100,000 per year and is highest in the first year of life (deVeber et al., 2001 [25]). A number of risk factors are linked to the development of Sinus Venous Thrombosis stroke (SVT), including head and neck infections, dehydration, perinatal complications, and coagulation disorders. In one study, prothrombotic abnormalities were present in half of children with SVT and many of these children had multiple risk factors (deVeber et al., 1998 [11]).
Venous infarction involves obstruction of the deep vein system. There is dilation of the deep vein tributaries with surrounding hemorrhages and necrosis. Rupture of blood into the ventricles may cause internal hydrocephalus. The histological changes largely resemble those described above (Pape et al., 1979 [20]).
Physical examination may reveal dilated scalp veins, eyelid swelling, or a large anterior fontanel whereas an older child would likely present with more slowly progressing signs, such as vomiting, headache, or any other signs of increased intracranial pressure. As mentioned above sinus venous thrombosis can present clinically in all ages with fever and lethargy or coma, but young infants can present with a history of decreased oral intake or respiratory distress (Tsze et al., 2011 [27]).
Incidence and prevalence: A stroke or Cerebral Vascular Accident (CVA) in children is considered to be a rare event. The prevalence of childhood stroke from population-based studies is estimated between 2-3/100,000 children in the United States and as high as 13/100,000 children in France (Kittner et al., 1996 [44]; Giroud et al., 1995 [45]). The CPISR reported ischemic stroke in 2.7/100,000 children <18 years of age per year, 40% being under 1 year of age, with a male to female (1.5:1) predominance. In the NHDS (1980 to 1998), for children 0 to 18 years, the prevalence of stroke was 13.5/100,000; that of hemorrhagic stroke was 2.9/100,000, and that of ischemic stroke was 7.8/100,000 children per year with a US mortality rate for stroke in children (1-15 years of age) of 0.6/100,000 children. Mortality was higher in males than females and in black compared with white individuals (see Lynch et al., 2002 [4] for review). About 10-25% of children with a stroke will die, up to 25% of children will have a recurrence, and up to 66% will have persistent neurological deficits, develop subsequent seizure disorders, and learning or developmental problems (Tsze et al., 2011 [27]).
Arterial ischemic stroke: In the Swiss Registry, 116 children age 1 month to 16 years with a diagnosis of Arterial Ischemic childhood Stroke (AIS) confirmed by neuroimaging were included. Follow-up information was obtained in 95/116 children (Simonetti et al., et al., 2015 [46]). Mortality was 14% (13/95) and was higher than in young adults. In 56% of the children followed, the outcome was favorable. The study comprised all childhood AIS cases in Switzerland with a prevalence of AIS of 0.17/100,000.
Hemorrhagic stroke: Arteriovenous Malformations (AVM) are the most common cause of hemorrhagic stroke in childhood and become symptomatic in 10-20% whereas only 1-2% of cerebral vascular aneurysms do so (Menovsky et al., 1997 [43]). The incidence of the previous (AVM) is reported to be 1/100,000 per year that of the latter (aneurysm) is not known. However, of a series of patients with suspected genetic disposition to development of Familial Intracranial Aneurysm (FIA), in which mutations on chromosome 11 and 6 may be involved, an annual rupture rate of 1.2% in undiagnosed aneurysms was determined (n=113, adults and children) which is 17 times higher than in subjects without a family history. At any rate, rupture of intracerebral aneurysms tend to occur in older ages rather than in childhood.
Venous sinus thrombosis: The Canadian study group CPISR determined an incidence of 0.6/100,000 CVST per year (deVeber et al., 2001 [25]). This is in general agreement with other populationbased studies (Agrawal et al., 2009 [32]. Thus, ignoring the miniscule fraction of cerebral ruptured vascular aneurysms in childhood, the total prevalence of childhood stroke amounts to 0.97/100,000 population.
Management of Childhood stroke: Employment of appropriate imaging techniques is crucial for diagnosis and hence for successful care provided. Non-contrast head Computed Tomography (CT) is sensitive for acute bleeding and should be obtained emergently to exclude a hemorrhagic cause of stroke. If a hemorrhagic stroke is identified, Magnetic Resonance Venography (MRV) should follow as 10% of hemorrhages in children is due to cerebral sinus venous thrombosis. For diagnosing Arterial Ischemic Stroke (AIS), noncontrast CT is also the initial study, however, within 12 hours from the insult CT may appear normal while MRI is more sensitive for early detection of an infarction. The most precise detail of vascular anatomy of all imaging modalities is provided by Catheter Angiography (CA) which is superior to MRA and Computed Tomography Angiography (CTA) and can be performed along with endovascular therapy (Tsze et al., 2011 [27]). Once the type of stroke is identified, treatment depends on etiology. Hemorrhagic strokes may require prevention of re-bleeding by recombinant factor VIIa, though adult studies have not confirmed improvement of survival or functional outcome. Surgical management of hemorrhagic stroke is considered highly controversial.
AIS management in childhood includes preventing subsequent ischemic events, e.g., by anticoagulation. Thrombolytic therapy in children with ischemic strokes must be carried out with caution, tPA may be considered in a selected group of children with CVST while management of stroke in Sickle cell disease requires exchange blood transfusion (Tsze et al., 2011 [27]).
There are guidelines for the general management of pediatric stroke covering presentation and diagnosis, investigations, acute care, secondary prevention (RCP Guidelines, 2004 [47]), and recommendations for children with sickle cell disease (Class 1-2), hypercoagulable states (Class 1-2), evaluation and treatment of hemorrhage in children (Class 1), treatment of cerebral venous sinus thrombosis (Class 1-2), and for stroke and heart disease (Class 1-3) (Roach et al., 2008 [36]).
Symptoms: There are some generalizations that can be made as to how stroke presents in children (Tsze et al., 2011 [27]). Arterial Ischemic Stroke (AIS) in childhood most often presents as a focal neurologic deficit. Hemiplegia is the most common focal manifestation, occurring in up to 94% of cases. Hemorrhagic stroke most commonly presents as headaches or altered level of consciousness, and are more likely to cause vomiting than AIS. Seizures are common in both ischemic- and hemorrhagic strokes. They occur in up to 50% of children with strokes, are not restricted to any group, and are not limited to any specific seizure type. There can be significant differences in the clinical presentation based on the child’s age. The younger the child, the more non-specific their symptoms may be. Neonatal/Perinatal strokes are more likely to initially present with focal seizures or lethargy in the first few days after birth. Older children demonstrate more specific neurological defects similar to adults. These include hemiparesis, language (e.g., aphasia) and speech difficulties, visual deficits, and headache (see Tsze et al., 2011 [27] for review).
Diagnosis: There are no published consensus guidelines on the evaluation of stroke in children, but, the evaluation should rule out other nonvascular causes and identify the cause of the stroke. Cranial ultrasound imaging is particularly useful for intraventricular and germinal matrix hemorrhages, but is less suitable for diagnosing ischemic stroke, in particular cortical or posterior infarcts. Transcranial doppler is useful in sickle cell-related stroke while CT is instrumental for identifying acute hemorrhages. Various MR techniques, including diffusion, perfusion, gradient echo, and FLAIR (Fluid-attenuated inversion recovery) imaging, are used to detect ischemic stroke in children. MR arteriography is helpful to diagnose arteriopathies as is computed tomography and MR venography for Sinus Venous Thrombosis (SVT). For children with suspected extracranial arterial dissection MR imaging should be considered and ultrasonography can detect abnormal flow patterns in >90% of patients. Functional MR is useful in monitoring changes over time during recovery. Conventional angiography may be considered when MR scanning has failed to identify a cause (see Lynch et al., 2002 [4] for review).
Also hematologic, metabolic, and angiographic studies have to be included in the work-up procedure, as recent evidence suggests that the identification of multiple risk factors predicts worse longterm outcome. Also, any history of head or neck trauma, unexplained fever or recent infection, drug ingestion, developmental delay, family history of bleeding problems, and associated headache are of importance (Lynch et al., 2002 [4]).
Treatment
Non-cellular treatment: There is no specific curative treatment for Childhood stroke hitherto. Treatment is symptomatic and supportive; however, children with Childhood stroke should receive regular medical screenings to determine appropriate interventions. In adults, Tissue Plasminogen Activator (TPA) is the only approved treatment for acute ischemic stroke, and has not been tested in children (Lynch et al., 2002 [4]). Once the type of stroke is identified, treatment depends on etiology. A goal specific to AIS management includes preventing a subsequent ischemic event, e.g., by anticoagulation. Thrombolytic therapy in children with ischemic strokes must be carried out carefully. Published guidelines suggest that tPA may be considered in a selected group of children with CVST. Management of stroke in Sickle Cell Disease (SCD) requires exchange blood transfusion (Tsze et al., 2011[27]).
The treatment for hemorrhagic stroke in children depends on the cause and the condition of the patient. In general, hemorrhagic strokes may require medical management beyond supportive measure, e.g., prevention of re-bleeding by recombinant factor VIIa, though adult studies lack demonstration of benefit. Although, surgical management of hemorrhagic stroke continues to be controversial, treatments for vascular malformations include surgery, endovascular embolization, and radiosurgery (Menovsky et al., 1997 [43]).
Outcome and prognosis: Depending on differences in functional measures, stroke type, and population studied, the outcome of children after stroke varies among studies. According to CPISR data including children with arterial ischemic stroke (n=402) and children with sinus venous thrombosis (n=160), revealed that 27% of children were neurologically normal, 61% were abnormal, 21.6% recurred, and 12% died (Lynch et al., 2002 [4]). Poor outcome in children with stroke is associated with certain neuroimaging abnormalities and seizures at presentation. Children with infarct volumes greater than 10% of intracranial volume have a worse outcome than children with less than 10% infarct volume and the recurrence rate is 20% to 40% (Lynch et al., 2002 [4]).
Cell-Based Therapy of Pediatric Stroke
Cellular characteristics of human autologous cord blood mononuclear cells
The first documented cell-based treatment of a girl presenting Neonatal/Perinatal birth traumatic arterial ischemic stroke with autologous cord blood mononuclear cells (hucbMNC) performed at five years of age dates back to 2009 (Jensen and Hamelmann, 2016 [1]). We deliberately used the whole range of unmanipulated mononuclear cells derived from autologous human umbilical cord blood including progenitor and stem cells. These include among other leukocytes (CD45+), lymphcytes (CD3+ T-cells, CD19+ B-cells, CD56+ NKcells), Monocytes (CD14+), Haemopoietic progenitor cells (CD34+), Mesenchymal stem cells (CD73+, CD90+, CD105+, CD13+, CD44+, CD29+), and Unrestricted Somatic Stem Cells (USSC)(CD45-).
Preclinical studies - Mode of action
Initially, in preclinical studies the mode of action was unclear. However, in cell culture, analysis of medium conditioned by human umbilical cord blood-derived Mononuclear Cells (hucbMNC) revealed substantial secretion of a number of cytokines, chemokines, and growth factors, induced by EGF/FGF-2 or NGF/RA treatment. Many of the secreted factors are well-known for their beneficial effects under inflammatory or brain-damaging conditions (Neuhoff et al., 2007 [49]) (Figure 1). “Thus, one potential mode of interaction was considered to be the release of neuroprotective, anti-inflammatory, or angiogenic factors by transplanted cells, thereby providing the basis for an indirect effect on host tissue” (Neuhoff et al., 2007 [49]). Hence, it was proposed that secondary mechanisms might be responsible for the beneficial outcome upon hucb mononuclear cell transplantation in a stroke-like lesion model (Meier et al., 2006 [52]). We used the Levine model in neonatal rats at PN7 (post-natal day 7) inducing hypoxic ischemia in the periventricular region by unilateral ligation of the (left) carotid artery in combination with 80 minutes respiration of hypoxic ambient air (8% O2) resulting in periventricular white matter damage, a porencephalic cyst, and contralateral (right) spastic hemiparesis, the key symptom of cerebral palsy subsequent to stroke affecting the internal capsule where long corticospinal tracts pass (Levine et al., 1960 [50]; Meier et al., 2006 [52]). In recent years, ischemic and hemorrhagic brain lesions have been successfully treated in juvenile and adult animal models of various species using human cord blood mononuclear cells (containing among other mesenchymal stem cells, MSC), i.e., in fetal sheep (Garnier et al., 2002 [51]), in newborn rats (Meier et al., 2006 [52]; Pimentel-Coehlo, 2010 [53]; Rosenkranz et al., 2010 [54]; Ahn et al., 2013 [55]), in newborn rabbits (Drobyshevsky et al., 2015 [56]), and in adult rats (Chen et al., 2001 [48]); Liao et al., 2009 [57]) (see Li et al., 2014 [58] for review).
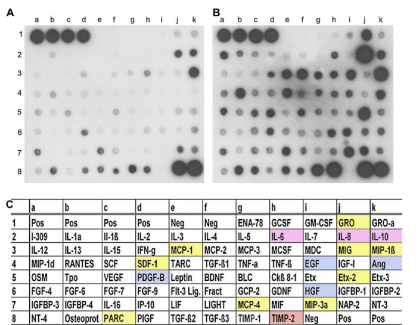
Figure 1: Protein antibody array detecting cytokines in human umbilical cord blood (hucb)-cell conditioned media. Representative examples of two antibody
array membranes, incubated with either nonconditioned culture medium (A) or culture medium conditioned by hUCB-derived mononuclear cells for 2 days (B).
Increasing intensity reveals increased secretion of the cytokines investigated. Dots a1 to d1 and j8 to k8 were positive controls (Pos); dots e1 to f1 and i8
were negative controls (Neg). (C) Overview of all proteins assayed on the membrane. Boxed factors are color-coded and refer to those proteins secreted at
significant levels. Colors designate interleukin proteins (purple), growth factors (blue), chemokines (yellow), as well as tissue inhibitors of metalloproteinase2
(TIMP-2) (orange). Ang: Angiogenin; BDNF: Brain-Derived Neurotrophic Factor; BLC: B-lymphocyte Chemoattractant; EGF: Epidermal Growth Factor; ENA-
78: Epithelial Neutrophil-Activating protein-78; Etx: eotaxin; FGF: Fibroblast Growth Factor; Fract: Fractalkine; GCP-2: Granulocyte Chemotactic Protein-2;
GCSF: Granulocyte Colony-Stimulating Factor; GDNF: Glial Cell-Derived Neurotrophic Factor; GM-CSF: Granulocyte Macrophage Colony-Stimulating Factor;
GRO: Growth-Regulated Oncogene; HGF: Hepatocyte Growth Factor; IFN-g: Interferon-G; IGF-1: Insulin-Like Growth Factor-1; IGFBP: Insulin-Like Growth
Factor Binding Protein; IL: Interleukin; IP-10: Interferon-Inducible Protein-10; LIF: Leukemia Inhibitory Factor; MCP: Monocyte Chemoattractant Protein; MCSF:
Macrophage Colony-Stimulating Factor; MDC: Macrophage Derived Chemokine; MIF: Macrophage Inhibitory Factor; MIG: Monokine Induced By G-Interferon; MIP:
Macrophage Inflammatory Protein; NAP-2: Neutrophil Activating Protein-2; NT: Neurotrophin; OSM: Oncostatin M; Osteoprot: osteoprotegrin; PARC: Pulmonary
and Activation-Regulated Chemokine; PDGF-B: Platelet-Derived Growth Factor-B; PIGF: Placenta Growth Factor; SCF: Stem Cell Factor; SDF-1: Stromal-Derived
Factor-1; TARC: Thymus Associated and Regulated Chemokine; TGF-b: Transforming Growth Factor-b; TNF: Tumor Necrosis Factor; TIMP: Tissue Inhibitor of
Melloproteinases; Tpo: Thrombopoietin; VEGF: Vascular Endothelial Growth Factor (Neuhoff et al., 2007 [49]).
‘Homing’ and neuroregeneration: As far as the mode of action of cord blood mononuclear cells for the treatment of stroke is concerned, the following mechanisms are to be considered. Neonatal/Perinatal stroke and childhood stroke, as well as ischemic and hemorrhagic brain damage of any etiology and origin cause activation of microglia within the damaged brain tissue, and (i.v. or i.p.) transplanted hucbMNC migrate actively into this region. This socalled ‘homing’ process is elicited by expression of Stromal Derived Factor (SDF)-1 binding to its receptor (CXCR4) on hucbMNC and leads to long-distance migration and eventually to release of antiinflammatory cytokines, growth factors, and chemokines in the damaged brain region. The resulting beneficial effects on functional neuro-regeneration after cerebral hypoxic ischemia in stroke include reduced spastic paresis and recovery of gross motor function, fine motor coordination, muscle strength, somatosensory cortical processing, and, as observed in children, recovery of cognition, vision, active and receptive speech competence (Meier et al., 2006 [52], Neuhoff et al., 2007 [49]; Rosenkranz et al., 2010 [54]; Geißler et al., 2011 [59] for review, Jensen, 2014 [19]) (Figure 2).
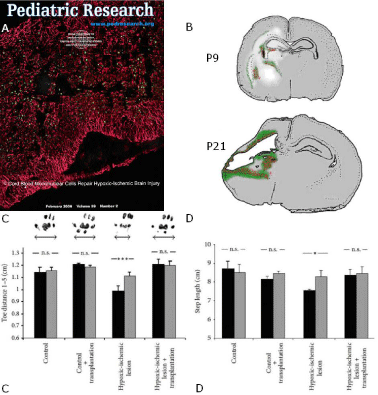
Figure 2: (A) Title page of ‘Pediatric Research’ February 2006, Volume 59, Number 2, referring to the publication of Meier et al. 2006 [52]: “Spastic paresis after
perinatal brain damage in rats is reduced by human cord blood mononuclear cells”. Please note, after hypoxic ischemia in neonatal rats at PN7 (Levine model)
and transplantation of human umbilical cord blood mononuclear cells (hucbMNC ) at PN8 there is ‘homing’ of the cells, i.e., targeted migration, into the damaged
brain area. The human HLA-DR-positive cord blood mononuclear cells (green) are located within a scaffold of GFAP-positive astrocytes (red) in the area of the
hypoxic ischemic lesion. This paper is available online at https://www.nature.com/articles/pr200649 (Meier et al., 2006 [52]). (B) After activation of microglia by
hypoxic ischemia, stromal dericed factor (SDF)-1 is expressed and binds to the CXCL4 receptor of the hucbMNC which in turn elicits long-distance migration
of the cells (‘homing’) into the damaged brain area. Location of Stromal derived factor-1 (SDF-1) immunoreactivity (red) with respect to the location of CD68
positive cells representing activated microglia (green). Schematic representation of the distribution of SDF-1 immunoreactivity (red) and CD68 immunopositive
cells (green) in hypoxic-ischemic rat brains at P9 and P21 (Rosenkranz et al., 2010 [54]). Figure note (B): The whitish areas on the lesioned side at P9, i.e., 2 days
after hypoxic ischemia, represent early stage periventricular white matter damage that is transformed in a porencephalic cyst at P21, i.e., 2 weeks after hypoxic
ischemia. (C) Spastic paresis after hypoxic ischemia is prevented by hucb mononuclear cells given systemically (Meier et al., 2006 [52]). At PN 21, i.e. 14 days
after hypoxic ischemia and 13 days after transplantation of human cord blood mononuclear cells at PN8, there was virtually no evidence of spastic paresis in the
hind paws (right) and (D) step length contralateral to the lesion site (left) in the transplanted newborns as compared with controls (black bars). Please note, this is
the first experimental demonstration of functional neuro-regeneration in newborn animals after stroke-like cerebral hypoxic ischemia caused by human cord blood
mononuclear cells. This paper is available online at https://www.nature.com/articles/pr200649 (Meier et al., 2006 [52]).
Beneficial effects were also observed in adult hemorrhagic stroke and postnatal intraventricular hemorrhage models in rats using mesenchymal stem cells derived from human cord and human cord blood, respectively (Liao et al., 2009 [57]; Ahn et al., 2013 [55]).
Recovery of neuroprocessing in the primary somatosensory cortex: Transplantation of human umbilical cord blood Mononuclear Cells (hucbMNC) into the peritoneal cavity has been shown to reduce sensorimotor deficits after hypoxic ischemic brain injury in neonatal rats (Geißler et al., 2011 [59]). In Figure 3 it is demonstrated by in vivo recordings that hucbMN cells have indeed the capacity to reduce injury-related impairment of neural processing in the primary somatosensory cortex.
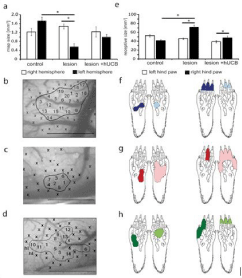
Figure 3: Effects of hypoxic-ischemic brain injury and hucbMNC treatment on Receptive Field (RF) and cortical map size (see [59]). (a): In lesioned rats the size of
the left cortical hindpaw (HP) representation was significantly reduced after hypoxic ischemic brain injury (HI) (versus controls, versus contralateral hemisphere).
Treatment with hucbMNC prevented map changes in the left cortical HP representation. (b-d) images of the cortical surface of the left hemisphere of a control
(b), lesioned (c), and hucbMNC treated rat (d). Numbers indicate penetration sites; x indicates noncutaneous responses. Hl: Hind Limb. Borders of the maps are
outlined. Scale bar 1mm. (e) In lesioned rats RF size of the right HP was increased (versus controls, versus HP ipsilateral to the lesion). hucbMNC treatment leads
to moderate RF increase, not significantly different from controls (h). Bars represent sem (f)-(h) examples of RFs on the right and left HP for a control (f), lesioned
(g), and hucbMNC treated rat (h). Number of rats used: control group, lesion group, and hucbMNC group . A total of 975 RFs were recorded (left hemisphere:
control 127: lesioned 97, treated 85; right hemisphere: control 192, lesioned 327, and treated 147) (Geißler et al., 2011 [59]). Figure note: a) In lesioned rats
the size of the left cortical hindpaw (HP) representation was significantly reduced after hypoxic ischemic brain injury (HI) (p= 0.005 vs. controls, p= 0.004 vs.
contralateral hemisphere). Treatment with hucbMNC prevented map changes in the left cortical HP representation. b)-d) images of the cortical surface of the
left hemisphere of a control (b), lesioned (c) and hucbMNC treated rat (d). Numbers indicate penetration sites, x indicate non-cutaneous responses, hl hindlimb.
Borders of the maps are outlined. Scale bar 1mm. e) In lesioned rats RF size of the right HP was increased (p= 0.007 vs. controls, p= 0.03 vs. HP ipsilateral to the
lesion). hucbMNC treatment lead to moderate RF increase, not significantly different from controls (p= 0.558). Bars hucbMNC represent s.e.m. f-h) examples of
RFs on the right and left HP for a control (f), lesioned (g) and treated rat (h). Number of rats used: control group n= 10, lesion group n= 17, hUCB group n= 6. A total
of 975 RFs were recorded (left hemisphere: control 127: lesioned 97, treated 85; right hemisphere: control 192, lesioned 327, treated 147) (Geißler et al., 2011 [59]).
Following hypoxic ischemia, the significantly altered dimensions of cortical maps and receptive fields, were largely restored after hucbMNC transplantation and the lesion-induced hyper excitability was no longer observed in hucbMNC treated animals. The pairedpulse behavior resembled that observed in control animals. Cortical processing of sensorimotor behavior almost completely recovered. Thus, hucbMN cells reconstitute “the way central neurons process information by normalizing inhibitory and excitatory processes” (Geißler et al., 2011 [59]) (Figure 3).
Human cell-based therapy of pediatric stroke
As outlined above, Neonatal/Perinatal- and Childhood stroke are well defined conditions in newborn infants and in childhood with specific pathophysiological, histopathological, and clinical clinical characteristics based on the extent and location within the brain of the infants. There is no curative treatment for these conditions hitherto. Due to the anatomical fact that long corticospinal nerve tracts originating from the motorcortex, which represents the whole body motor function, pass through the periventricular region and internal capsule, the neurologic sequelae match the injured tracts resulting in diplegia, hemiplegia, and quadriplegia depending on the extent of the lesion. Lesions more lateral to this region are less well defined.
In the human, autologous hucbMNC therapy for Pediatric brain damage leading to cerebral palsy, including hypoxic ischemia and stroke, Hypoxic-Ischemic Encephalopathy (HIE), Periventricular Leukomalacia (PVL), and cerebral hemorrhage (IVH/PIVH) is feasible, safe and efficacious (Jensen, 2009 [60]; Papadopoulos et al., 2011 [109]; Jensen, 2011 [61]; Lee et al., 2012 [68]; Sun et al., 2010 [62]; Jensen and Hamelmann, 2013 [63]; Cotten et al., 2014 [64]; Jensen and Hamelmann, 2016 [1]; Sun et al., 2017 [65]). Published clinical data on hucbMNC have largely been collected for safety and feasibility reasons, however, in all seven clinical trials hitherto published, including one of the most recent, in which a classical arterial ischemic stroke in a newborn was successfully treated, efficacy was demonstrated (Jensen and Hamelmann, 2016 [1]), provided a sufficient number of cells was available (Sun et al., 2017 [65]). This is also true for two randomised double-blind placebo controlled trials using allogeneic cord blood mononuclear cells (Min et al., 2013 [66]; Kang et al., 2015 [71]). The observations made are important clinically, particularly, since beneficial effects have been observed in an ailment for which there is no cure at present. These include improved survival, psychomotor development, cognition, vision, and speech competence (Jensen, 2014 [19]). Even structural improvements have been observed (MRI, DTI, FA values). MRI tractograpy analysis revealed an increased number and volume of brain tracts (all fibers passing mid pons) representing the formation of new connectomes six months after autologous hucbMNC therapy ([67], Figure 4).
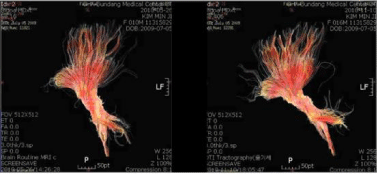
Figure 4: MRI tractography analysis before (A) and 6 months after (B) autologous cord blood transplantation in a child suffering from Cerebral palsy (Kwang
Yul Cha, [67]). The increased number and volume of brain tracts (all fibers passing mid pons) representing the formation of new connectomes after autologous
hucbMNC therapy is conspicuous and associated with significant improvement of psychomotor development (Min et al., 2013 [66]).
Cell-based treatment of Neonatal traumatic arterial ischemic stroke: In a recent case report on an individual treatment, there was also a signal for a safe and efficacious use of autologous cord blood mononuclear cells to treat traumatic arterial ischemic stroke and cerebral palsy in a child presenting a remarkable recovery in spite of late transplantation (Jensen and Hamelmann, 2016 [1]) (Figure 5). Stroke resulted from birth traumatic molding and parietal depression of the head during delivery caused by functional cephalo-pelvic disproportion due to a “long pelvis” of the mother and was treated by infusion of 45.8ml autologous cord blood mononuclear cells (TNC 2.53x10e8, cryopreserved at birth) five years after the insult. Followup was at 3, 18, 34, and 57 months (Jensen and Hamelmann, 2016 [1]).
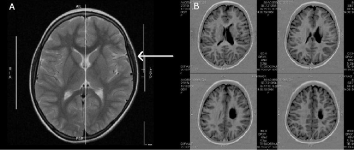
Figure 5: (A) MRT of the brain (M.H.) at 7 years of age (2011). Depression of the left temporal brain after traumatic molding by a “long pelvis” [1] during parturition
is still visible (arrow) (1.5-Tesla, head coil array, T2w TSE sequence). Please note, the volume of the left hemisphere is reduced in size, largely due to white
matter loss (-----). (B) MRT of the brain (M.H.) at 7 years of age (2011). Left arterial ischemic stroke in the central and periventricular white matter resulting in
a porencephalic cyst and enlarged lateral ventricle. The estimated total white matter loss amounted to approximately 20% (Jensen and Hamelmann, 2016 [1]).
The girl, who was born vital with a large head circumference, “normal Apgar scores, and normal umbilical arterial pH, presented a massive craniofacial deformity with depression of the left parietal bone and upper left jaw (maxilla). At six months, there were first neurologic anomalies. At 10 months right spastic hemiplegia was diagnosed and an arterial ischemic infarction with a porencephalic cyst in the central and paraventricular left white matter was confirmed by Magnetic Resonance Imaging (MRI). Regular physiotherapy and occupational therapy were commenced on a weekly basis. After autologous hucbMNC treatment, the girl recovered from hemiplegia to such an extent that she is now able to participate in 3km city runs without orthosis, obtained the gold badge lifeguard certificate in swimming and diving (DLRG), rides two-wheel bicycle, excelled at school, and plays piano using her affected right hand” (Jensen and Hamelmann, 2016 [1]).
Based on this case report, we reanalyzed our prospective cranial ultrasound screening data base in newborns. This analysis revealed that traumatic birth injury leading to cerebral white matter damage was associated with large head circumference >75th centile and cephalo-pelvic dysproportion in 4,725 term-born infants. Since these newborns are born in full vitality with normal Apgar scores and normal umbilical arterial blood pH, they usually are not examined by brain imaging. Therefore, they may constitute the ‘missing link’ between the insult escaping diagnosis and the development of unexpected cerebral palsy during childhood (Jensen and Holmer, 2018 [22]). In these largely male term-born infants the relative risk for brain damage and hence for lifelong sequelae may increase up to tenfold as compared to normal head circumference.
This demands for a change in obstetrical management of large for gestational age infants during delivery when prolonged or obstructed labor occurs to prevent brain damage by appropriate measures. Or at least, to consider umbilical cord blood collection and storage at birth as a prerequisite for autologous cell-based treatment (hucbMNC) in case white matter damage and/or stroke would be diagnosed upon imaging (Jensen and Holmer, 2018 [22]).
This obstetrical policy change is mandatory because pediatric stroke of any cause is a life-threatening and debilitating condition that is among the top ten causes of death in childhood with the highest mortality in the first 12 months of life and, importantly, unlike in large term-born infants, only rarely can be predicted (US Department of Health CDC, 2014 [70]). Approximately one third of all cases occur in children less than one year of age (Golomb et al., 2001 [12]). Furthermore, 50-85% of survivors of stroke will be left with long term problems which may include seizures, physical disability, speech or learning difficulties (Ganesan et al., 2000 [72]) and 20-40% of children have recurrent strokes (deVeber et al., 2000 [39]). “The burden of stroke in children is likely to be greater than in adults because children surviving stroke will have more years living with functional limitations and disability (YLDs)” (Vos et al., 2015 [73]) (see Table 1).
Total prevalence (×1000)
Total YLDs (×1000)
Prevalence by severity in 2013 (×1000)
1990
2013
1990
2013
Borderline
Mild
Moderate
Severe
Profound
Idiopathic intellectual disability
77 102·5 (65·24%)
94 680·0 (61·47%)
3822·3 (38·30%)
4666·7 (31·46%)
32 937·9 (21·38%)
38 873·1 (25·24%)
14 804·3 (9·61%)
5946·2 (3·86%)
2118·5 (1·38%)
Preterm birth complications
5913·0 (5·00%)
13 576·3 (8·81%)
558·1 (5·59%)
1450·4 (9·78%)
..
11 480·7 (7·45%)
1277·6 (0·83%)
535·2 (0·35%)
282·8 (0·18%)
Neonatal encephalopathy due to birth asphyxia and trauma
9520·3 (8·06%)
8947·4 (5·81%)
892·5 (8·94%)
1228·3 (8·28%)
..
7128·4 (4·63%)
833·1 (0·54%)
645·2 (0·42%)
340·8 (0·22%)
Ischemic stroke
2686·4 (2·27%)
4887·1 (3·17%)
967·9 (9·70%)
1764·2 (11·89%)
..
..
2978·2 (1·93%)
1248·5 (0·81%)
660·3 (0·43%)
Hemorrhagic stroke
1040·8 (0·88%)
1966·8 (1·28%)
386·6 (3·87%)
728·8 (4·91%)
..
..
1197·3 (0·78%)
503·4 (0·33%)
266·1 (0·17%)
Table 1: Prevalence and years lived with disability (YLDs), with percentage of total, for intellectual disability by cause in 1990 and 2013, and prevalence by severity in 2013 (Vos et al., 2015 [73]).
Cell-based treatment of global brain Hypoxic ischemia: Also, one child suffering from a prolonged global cerebral hypoxic-ischemic insult after cardiac arrest recovered substantially from quadriplegia and persistent vegetative state after hucbMNC treatment (Jensen and Hamelmann, 2013 [63]) (see Figure 6) as did 5/20 treated children presenting cerebral palsy (Lee et al., 2012 [68]).
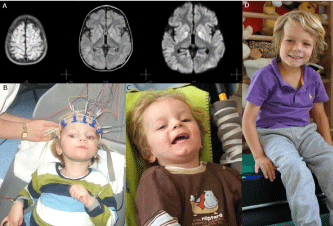
Figure 6: (A) Brain MRI of the patient (L. B.) 2 weeks after cardiac arrest for >25 minutes. Note, signs of severe global ischemia in cortical structures as evidenced
by (a) signal hyperintensity of gyri in almost entire cortex (FLAIR sequences) and basal ganglia (b), including caudate nucleus and putamen (c) (FLAIR DWI
sequences with contrast media) (see video documentation at https://stop-cp.org/videos/). (B) Before transplantation of hucbMNC. The patient L.B. (2.5. years old)
is quadriplegic and in a persistent vegetative state after global cerebral hypoxic ischemia. (C) At 7 weeks after autologous cord blood transplantation the boy is
laughing when played with, facing his mother, and showing social smiling (Jensen and Hamelmann, 2013 [63]; Webb et al., 2013 [69]). (D) At 4.5-year follow-up.
The boy has now entered primary school at the age of 7 and 4.5 years after transplantation of autologous cord blood after global hypoxic-ischemic brain damage
caused by cardiac arrest followed by a quadriplegic persistent vegetative state. He is still using a posterior gait trainer for ambulation. (Follow-up at 2 years see
video documentation at https://stop-cp.org/videos/(Jensen and Hamelmann, 2013 [63]; Jensen, 2014 [19])).
After autologous cord blood transplantation nine weeks after the insult there was a remarkable video documented recovery over the following months and years (Figure 6). Two weeks after cardiac arrest and ensuing arrest of cerebral blood flow and oxygen transport for >25 minutes, brain imaging (MRI) revealed signal hyper intensity in both the entire cortex and basal ganglia (Figure 6 A), and EEG rhythm and frequency were grossly disturbed showing transient isoelectric traces. On the day of transplantation, nine weeks after the insult, the boy still presented quadriplegic cerebral palsy (Figure 6 B) (Jensen and Hamelmann, 2013 [63]); his pupils were still dilated showing only minimal response to direct light. He was blind (cortical blindness), deaf, and whimpered permanently. One week after transplantation he stopped whimpering, at four weeks he executed simple tasks on demand, motor control improved, and spastic paresis was largely reduced (Jensen and Hamelmann, 2013 [63]). At seven weeks (Figure 6 C), EEG was normal and eyesight recovered in part; he started smiling when played with and was able to sit with support and to speak simple words (Ma-ma, Pa-pa). At one year after transplantation, spastic paresis was further reduced free sitting and walking with support were possible. After two years, there was independent eating, crawling, passive standing, and walking in a gait trainer (Jensen and Hamelmann, 2013 [63]). Fine motor control had improved to such an extent that the boy managed to steer a remote control car. After three years, receptive and expressive speech competence also improved significantly, and there was broad understanding (Jensen and Hamelmann, 2013 [63]). At the age of seven and 4.5 years after transplantation, the boy has entered primary school (Figure 6 C), though still using a posterior gait trainer for ambulation (Crocodile Retrowalker) (Jensen, 2014 [19]).
Cell-based treatment of Hypoxic-ischemic encephalopathy: There is first evidence from human newborns and children that treatment of hypoxic-ischemic encephalopathy, ischemic stroke, and global ischemic brain lesions by autologous human cord blood mononuclear cells is safe and efficacious (Cotten et al., 2014 [64], Sun et al., 2010 [62], Lee et al., 2012 [68], Jensen and Hamelmann, 2013 [63], Jensen and Hamelmann, 2016 [1]).
Autologous cord blood mononuclear cell treatment of preterm and term newborns suffering from hypoxic-ischemic encephalopathy, a condition that among other hypoxic neurologic damage may also encompass ischemic and hemorrhagic lesions, resulted in improved survival and improved psychomotor development as assessed by Bayley III scores in all three domains >=85 (Cotten et al., 2014 [64])
Randomized and non-randomized clinical trials
In children, there are three randomized placebo controlled clinical trials with various forms of brain injury leading to cerebral palsy in which autologous and allogeneic umbilical cord mononuclear cells have been transplanted, the latter after HLA-matching with and without immunosuppression, showing clear efficacy of the treatment, provided a sufficient number of cells is available (Min et al., 2013 [66]; Kang et al., 2015 [108]; Sun et al., 2017 [65]).
Furthermore, there is one non-randomized controlled trial in 46 newborns presenting hypoxic-ischemic Encephalopathy (HIE), one uncontrolled single arm trial in 20 children with CP (5/20 showing age-dependent improvement after HIE), and 3 case reports on 4 children with brain injury (one with stroke) in which - on this limited basis – efficacy of autologous cord blood mononuclear cells was shown. Taken together, approximately 400 infants worldwide have been treated with autologous mononuclear cells derived from cord blood without major adverse events. Thus, this cell-based treatment for pediatric brain damage and stroke is safe even though there still is a paucity of randomized placebo-controlled double blinded clinical trials (Cotten et al. 2014 [64], Jensen, 2009 [60]; Papadopoulos et al., 2011 [109], Jensen, 2011 [61]; Lee et al., 2012 [68]; Jensen and Hamelmann, 2013 [63]; Jensen and Hamelmann, 2016 [1]).
Brain Plasticity - Childhood Stroke versus Adult Stroke
A unique characteristic of the developing brain is its plasticity which is essential to shape the sensory, motor, limbic, and associative functions to become an adult brain and lasts approximately until 20 years of age as assessed by relative Gray Matter (GM) volume changes (Gogtay et al., 2004 [74]) (Figure 7) (Bhardwai et al., 2006 [75]; Blakemore et al., 2012 [76]).
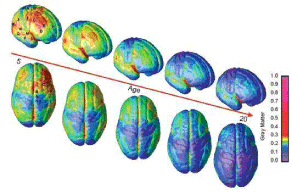
Figure 7: The right lateral and top view of the dynamic sequence of Gray Matter (GM) maturation over the cortical surface. The side bar shows a color representation
in units of GM volume. The initial frames depict regions of interest in the cortex as described for Figure 7. This sequence is available in Movies 1-4 in the supporting
information (Gogtay et al., 2011 [74]).
Plasticity in the developing brain is an extremely dynamic process of excess formation of synapses, dendrites and axons within minutes and an equally dynamic process of eliminating these neural structures (Marrs et al., 2001 [81]). Plasticity is maximal in the first two years of life. This explains the fact that children are able to recover from head injury much better than adults and the recovery of function is more complete. For example, if the entire left hemisphere is removed in a 4-year-old child, the child may still develop normal language functions whereas the same in an adult will lead to permanent loss of all language functions (Boatman et al., 1999 [82]). Furthermore, plasticity in the developing brain is intimately linked to the neuroregenerative effects of hucbMNC transplanted after hypoxic ischemic insults in newborn rats through glutamate induced activation of NMDA receptors causing expression of Brain-Derived Neurotrophic Factor (BDNF) and Vascular Endothelial Growth Factor (VEGF) (Rosenkranz et al., 2012 [83]). These factors have also been shown to be upregulated after treadmill exercise which as well results in increased brain plasticity in the hippocampus of juvenile rats (Figure 9 C,D,E) (Lou et al., 2008 [84]; Alsina et al., 2001 [85]).
Further evidence for brain plasticity interacting with cell-based therapy is provided by plastic remodelling at the level of neural processing with reduction of lesion-induced hyperexcitability indicated by a paired-pulse behavior paralleled by restoration of the dimensions of cortical maps and receptive fields after hucbMNC transplantation in newborn rats (Geißler et al., 2011 [59])
After stroke/hypoxic ischemia in the developing brain, neuroplasticity along with chemokines, anti-inflammatory cytokines, and growth factors expressed by hucbMNC recruit neural projections from surrounding areas to form alternative neural pathways that map around the lesion and eventually form the basis for new connectomes 6 months after treatment (Kwang Yul Cha, [67]) (Figure 4). This efficacious process based on plasticity is unique to the developing brain and unavailable to the adult brain.
During juvenile brain repair, e.g., after stroke, plastic changes are geared towards maximizing function by virtue of excess of synapses, dendrites, and axons in spite of the damaged brain area. Neuroplasticity is clearly age-dependent and largely confined to childhood as evidenced by pivotal observations in the optic tracts (Hubel and Wiesel, 1965 [86]; Blakemore, 1977 [87]) and the auditory system during development (Fallon et al., 2008 [88]). Visual blocking of the eye early during development causes cortical blindness while visual blocking in adulthood does not (Hubel and Wiesel, 1965 [86]). Also, auditory plasticity peaks at 3.5 years of age and deaf children can profit from cochlear implants. In contrast, genetically deaf children (cochlear) implanted after seven years of age exhibit incomplete maturation of cortical potentials (Fallon et al., 2008 [88]).
Cord blood cells enhance plasticity by BNDF and VEGF expression
Pharmacologically, plasticity of the developing brain is based on the pivotal role of Glutamate, an excitatory transmitter (Mundkur, 2005 [91]), which activates postsynaptic a-amino-3-hydroxy-5- methyl-4-isoxazolepropionic acid receptor (AMPA) and N-Methyl- D-Aspartate (NMDA) receptors (NMDAR1) causing depolarization and Ca++ influx and release of trophic factors, thus causing gene transcription and formation of new neural pathways and eventually of new connectomes. At birth a cortex neuron has 2,500 synapses and at 2-3 years of age the neuron has 15,000 synapses; this is twice that of the adult brain neuron.
Transplantation of hucbMNC and plasticity in the developing brain are intimately related causing neuronal Glutamate activation of NMDA and AMPA receptors to express BDNF and VEGF. This is the basis for neuroregeneration after hypoxic ischemic brain injury as demonstrated by our preclinical data (Rosenkranz et al., 2012 [83]). HucbMNC caused significant increases in both BDNF and VEGF in brain intact (sham operated, no hypoxic ischemia) newborn rats at PN9 (2 days after sham operation) (Figure 8 b, d) and maintained BDNF and significantly increased VEGF after hucbMNC treatment 2 days after hypoxic ischemia at PN9. In contrast, newborn rats at PN9 with hypoxic ischemia without hucbMNC treatment presented drastic decreases of BDNF and VEGF, respectively (Figure 8 a, c) (Rosenkranz et al., 2012 [83]).
Figure 8: Relative expression of BNDF and VEGF after hypoxic-ischemic lesion (lesion) and lesion followed by hucb cell transplantation (lesion+hucb). Quantitative
real time PCR analysis of BNDF (a, b) and VEGF (c, d) mRNA expression at P9 and P21 in left hemispheres of rat brains of the following experimental groups:
sham, lesion, lesion+hucb (a, c) and sham+hucb (b, d). Significant differences of p<0.05 are indicated by *** (Rosenkranz et al., 2012 [83]).
The function of NMDA receptors is enhanced postnatally, which explains in part the greater plasticity, and NMDA receptor activation can promote synaptic maturation by recruiting AMPA receptors causing Brain Derived Neurotrophic Factor (BDNF) formation (Mundkur, 2005 [91]; Hua et al., 2004 [92]). However, in adult non-human primates the expression levels of AMPA and NMDA glutamate receptor subunit proteins Glu2 and NMDR1 decreased significantly (Figure 9A) in many cortico-cortical projections as did dendritic spines (Figure 9B) in aged monkeys (9 years vs. 24 years old) reflecting diminished neural plasticity (Hof et al., 2004 [94], modified).
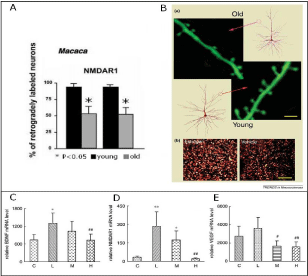
Figure 9: (A) NMDR1, an index of brain plasticity, decreased significantly in many cortico-cortical projections in aged monkeys (9 years vs. 24 years old) (Hof
et al., 2002 [93]). (B) Age-related alterations in dendritic morphology of cortico-cortical neurons in the neocortex of macaque monkeys. (a) Neurons forming long
cortico-cortical projections were retrogradely labeled to establish their connectivity, loaded with Lucifer yellow, and 3D reconstructed at very high resolution. The
upper neuron is from a 24-year-old animal; it has reduced dendritic complexity and a significant loss of dendritic spines compared with a neuron forming the same
projection in a 9-year-old animal (bottom). Overall, 20-35% loss of spine number and density was found in aged monkeys in both apical and basal dendrites. Scale
bar: 8μm for high magnifications of dendritic segments; 100μm for whole cell reconstructions. (b) Effect of estrogen replacement on spine number in layer 1 of
prefrontal cortex area 46 in rhesus monkey. Spinophilin immunoreactivity was used as a spine marker. Female monkeys were ovariectomized and treated with
either estradiol (left) or with vehicle (right). The estrogen-treated group had a 55% increase in spine density compared with the vehicle group, demonstrating that
early estrogen replacement has the capacity to restore spine numbers to normal levels. Scale bar, 5μm. (Hof et al., 2004 [94]). (C-E) Effects of exercise intensity
on expression of BDNF, NMDA R1, and VEGF mRNA levels in the hippocampus of juvenile SD rats. The glyceraldehyde-3-phosphate dehydrogenase (GAPDH)
gene was employed as an endogenous control for the quantitative real time PCR assay. The resulting corrected values were used to make group comparisons.
(C) Corrected values of BDNF mRNA levels. (D) Corrected values of NMDAR1 mRNA levels. (E) Corrected values of VEGF mRNA levels. *P<0.05, **P<0.01 vs.
control rats; #P<0.05, ##P<0.01 vs. low intensity exercise rats (one way ANOVA and LSD Test); C: control rats; L: low intensity exercise rats; M: Moderate intensity
exercise rats; H: High-intensity exercise rats (Lou et al., 2008 [84]).
Moreover, functional plasticity is augmented in juvenile brains, e.g., by treadmill exercise, which is reflected in NMDA receptor overexpression and improved learning capacity (Lou et al., 2008 [84]; Lou et al., 2006 [95]) (Figure 9 C-E). Interestingly, loss of synaptic plasticity causes memory loss that begins early in adulthood and then continues to worsen in an almost linear fashion, leading to a situation in which the magnitude of decline from 20 to 30 years is about as great as that from 70 to 80 years of age (Lynch et al., 2006 [89]).
Blood-brain barrier
Distinctions between juvenile and adult function of the Blood- Brain Barrier (BBB) are also to be considered with regard to neuroregenerative capacity. While the inflammatory response upon intrastriatal injection of interleukin-1ß (IL-1ß) in 2h old rat pups showed that the Blood-brain barrier is functional and more resistant to inflammatory stress at PN0 than at PN21 as assessed by IgG extravasation, the BBB breakdown happens to be more rapid upon chemokine expression (CINC-1; CXCL-1) at PN7 than at PN30, resulting in a massive Horse Reddish Peroxidase (HRP) extravasation in the previous whereas extravasation was minimal in the adult brain (Vexler et al., 2009 [96]; Anthony, et al., 1998 [97] (Figure10 A)).
Unlike the adult, the juvenile brain responded already after 4 h with breakdown of the BBB and leukocyte attraction and after 24 hours with HRP extravasation (Anthony et al., 1998 [97]). Given the fact that umbilical cord blood mononuclear cells transplanted in preterm fetal sheep 12h after an insult causing periventricular leukomalacia blunted microglial activation resulting in complete neuroprotection (unlike transplantation 5 days post-insult) (Li et al., 2016 [98]), the rapid BBB response in the juvenile brain at PN7 may have intact survival value as compared to the late BBB response in the old brain at PN30 (Anthony, et al., 1998 [97]) (Figure10 C-E).
Furthermore, comparative studies on juvenile (PN21) and adult rats (PN90) revealed a delayed breakdown of the adult BBB along with delayed and lower leukocyte recruitment by chemokines (CINC-1, i.e., CXCL1, and macrophage inflammatory protein (MIP)- 2, i.e., CXCL2) with virtually no horseradish peroxidase (HRP) extravasation as compared to juvenile brains (Anthony et al., 1998 [97]; Vexler et al., 2009 [96]; Anthony et al., 1997 [107]) (Figure 10). Finally, the brain of the adult rat is remarkably resistant to inflammation induced by interleukin IL-1ß and TNF-alpha and there is ‘a window of susceptitibility’ for polymorphonuclear neutrophil attraction in the juvenile brain (Anthony et al., 1997 [107]) (Figure 10). This may negatively affect neuroregeneration in the adult brain.
Figure 10: Blood–brain barrier breakdown induced by CXC chemokines in adult and juvenile rat brain measured by HRP extravasation. (A) Juvenile (3-week-old)
and (B) adult (3-month-old) rat brains 4h following an intra-striatal injection of 0.5μg rrCINC-1 (n = 3 rats per group). The extent of HRP extravasation in juvenile rat
brain 12 h following CINC-1 administration was similar to that at 4 h (Anthony et al., 1998 [97]). (C, D, E) Inflammation and blood-brain barrier breakdown induced
by CXC chemokines in adult and juvenile rat brain. Leukocyte recruitment to adult and juvenile striatum, measured at the intervals shown, following an injection
of (C) rrCINC-1 (0.5μg) or (D) rrMIP-2 (0.5μg). Polymorphonuclear neutrophils were identified using a rat polyclonal anti-neutrophil antibody (ANS). Macrophages
were identified using the monoclonal antibody ED1 (donated by the Dunn School of Pathology). (E) HRP extravasation in adult and juvenile brain 4h following an
injection of rrCINC-1 (0.5μg) or rrMIP-2 (0.5 μg). An asterisk denotes a significant difference between the respective adult and juvenile groups (mean ± SEM; n =
3 rats per group; t test, p < 0.05) (Anthony et al., 1998 [97]).
Brain plasticity is blocked during adult stroke
Unlike the developing brain, plasticity in the adult brain is minimal and confined to small areas of the Subventricular Zone (SVZ) and the Subgranular Zone (SGZ) of the dentate gyrus. However, the majority of the neuroblasts produced after stroke and attracted to the lesion by Stromal Derived Factor (SDF)-1 undergo apoptosis and only a few (10-20%) differentiate and survive in the long-term (Cui et al., 2013 [77]). This apoptosis is mediated by SDF-1 cleaved by Matrix Metalloproteinase-2 (MMP-2) into the cytotoxic truncated SDF-1(5- 67), which in turn activates the CXCR3 receptor causing cell death of the newborn neuron (Cui et al. 2013 [77]) (Figure 11). Furthermore, there is no significant neurogenesis in cortical areas of the adult brain outside the hippocampus (Bhardwai et al., 2006 [75]). This is one reason for poor outcome of brain injury in adults as compared to children (Kennard, 1940 [78]).
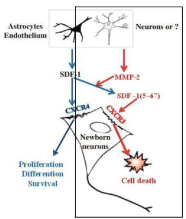
Figure 11: Active MMP-2 can cleave SDF-1 by proteolytic processing to remove the N-terminal tetrapeptide and generate SDF-1(5-67), which causes neuronal
death and inflammation by binding to the CXCR3 receptor (Cui et al. 2013 [77]).
Age-dependent genetic transcript changes reduce regenerative capacity: Subsequent to stroke there are age-dependent genetic transcript changes. Transient occlusion of the middle cerebral artery (MCAO) - a model that closely resembles human stroke - was employed to induce cerebral infarction in mice of 4 different ages (2, 9, 15, 24 months). By using Illumina cDNA microarrays and quantitative PCR the authors detected a distinct age-dependent response to stroke involving 350 differentially expressed genes. Their analyses also identified 327 differentially expressed genes that responded to stroke in an age-independent manner. Notably, gene transcripts that are involved in the regulation of synaptic plasticity and depressive disorders responded to stroke in a distinct age-dependent manner. Also, the transcripts involved in neurotransmitter release, Long- Term Potentiation (LTP), synapse formation, and axonal outgrowth were down regulated after stroke (except Thbs2 and Vamp3), whereby this down regulation was attenuated with increasing age (Sieber et al., 2014 [90]) (Figure 12,13).
Figure 12: Age-dependent (AD) and age-independent (AI) DEGs 2 and 7 days after stroke. (A) At day 2 of reperfusion, 118 AD DEGs were found to be upregulated,
whereas 57 DEGs were downregulated. At day 7 of reperfusion, 137 transcripts were found to be upregulated, whereas 10 were downregulated. Twenty-eight
upregulated DEGs at day 2 of reperfusion were still active at day 7. All AD DEGs were attenuated in their post-ischemic response in aged brains at day 2 as well
as at day 7 of reperfusion. (B) At day 2 of reperfusion, 56 AI DEGs were found to be upregulated, whereas 2 DEGs were downregulated. At day 7 of reperfusion,
172 transcripts were found to be upregulated, whereas 11 were downregulated. Eighty-six DEGs at day 2 of reperfusion were still active at day 7 (85 up- and 1
downregulated). Abbreviation: DEGs, differentially expressed genes (Sieber et al., 2014 [90]).
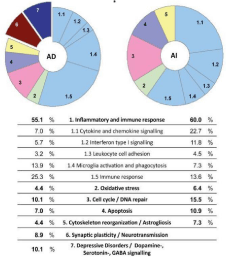
Figure 13: Functional gene categories of Age-Dependently (AD) and Age-Independently (AI) regulated transcrips. Most of the DEGs (~55% AD, ~60% AI) were
determined to be involved in different processes of the inflammatory and immune response. Stroke-regulated transcripts were also involved in oxidative stress, cell
cycle activation and/or DNA repair, apoptosis, and cytoskeleton reorganization and/or astrogliosis. DEGs involved in synaptic plasticity and/or neurotransmission
and depressive disorders and/or dopamine-, serotonin-, GABA-signaling were regulated in an exclusively AD manner. Abbreviation: DEGs, differentially expressed
genes (Sieber et al., 2014 [90]).
Most importantly, the transcript regulating micoglial activation (Cd68), the basis for ‘homing’ of hucbMNC and neuroregeneration in the developing brain, is downregulated in an age-dependent manner (Sieber et al., 2014 [90]). Furthermore, the post-ischemic expression level of almost all of the age related transcripts was significantly attenuated with age (Sieber et al., 2014 [90]). Also, the inflammatory response as reflected by the transcripts for expression of pro-inflammatory cytokine interleukin II1a is age-dependent (Sieber et al., 2014 [90]).
Inhibitory microenvironment for adult brain repair: Unlike the developing brain, plasticity is blocked genetically in the adult brain by PirB (Bochner et al., 2014 [79]) and Nogo receptor (Schwab et al., 2014 [80]) (Figure 14). Thus, the regenerative capacity of the adult brain after central nervous system injury is weak due to the absence of an enhancing microenvironment and presence of an inhibitory microenvironment for neuronal and axonal repair. Both the Nogo Receptor (NgR) and the Paired immunoglobulinlike receptor B (PirB), a recently discovered co-receptor of Nogo and myelin oligodendrocyte glycoprotein, are causing inhibition of axional regeneration after ischemic stroke (Liu et al., 2015 [99]). Post insult, in adult male mice stroke (MCAO), PirB mRNA expression increases after 2 hours, peaks at 24h, and remains elevated until 7 days (Liu et al., 2015 [99]). Concurrent blocking of NgR and PirB receptors almost completely eliminates the inhibition on axional regeneration (Liu et al., 2015 [99]) (Figure 14).
Figure 14: PirB-mediated growth cone collapse and axon growth inhibition. A classical axon inhibition-signaling pathway: Nogo on the oligodendrocyte surface
binds to the NgR-LINGO1-P75 receptor complex on the neuronal membrane. Cofilin is activated by RHOA-ROCK, resulting in actin depolymerization and axonal
inhibition. PirB-mediated signaling pathway: Nogo-66 binds to the PirB receptor on the neuronal membrane. (1) The Shroom 3-ROCK-Cofilin and LZK-JNK-Mysoin
IIA signaling pathways are activated by the POSH molecule; (2) SHP molecule recruitment leads to TrkB receptor dephosphorylation, PI3K and Akt activation,
and finally results in actin depolymerization and axonal inhibition. The figure is modified from Liu et al. 2014 [108]. PirB: Paired immunoglobulin-like receptor B;
NgR: Nogo Receptor; ROCK: Rho-associated Coiled coil-forming protein Kinase; JNK: c-Jun N-terminal kinase; SHP: Src Homology containing Protein tyrosine
phosphatase; TrkB: Tyrosine Receptor Kinase B; PI3K: Phosphatidylinositol 3-Kinase (Liu et al., 2015 [99]).
It is of note that the expression of NgR1 has been detected in microglia during development peaking at PN4 and found that NgR1 protein expression increased significantly in microglia with aging (Liu et al., 2015 [99]).
These inhibitory receptors (NgR, PirB) can recruit phosphatases, e.g., src homology 1-containing inositol 5’ phosphatase (SHP-1) and src homology 2-containing inositol 5’ phosphatise (SHP-2) to negatively regulate cell activation. Interestingly, we have shown previously that the expression of the inhibitory phosphatase (SHP-1), a protein associated with reduced astroglial proliferation, was reduced to control levels after initial elevation at PN9 upon transplantation of hucbMNC post-insult. Since astrocytes that upregulate SHP-1 after CNS injury do not enter the cell cycle this may have reduced reactive gliosis and glial scaring along with the observed reduced GFAP and CX43 tight junction protein expression in transplanted animals (Wasielewski et al., 2012 [100]).
Notably, stem cell transplantation for ischemic stroke in adults has so far not been shown to be efficacious according to the Cochrane Library (Boncoraglio et al., 2010 [101]). However, in a recent uncontrolled phase I trial on adult stroke treated by allogeneic umbilical cord blood mononuclear cells there was at least a signal of efficacy (Laskowitz et al., 2018 [2]). A recently completed clinical trial (NCT01884155, MinYoung Kim et al., 2017 [102]) on stroke in children, adults, and seniors treated by allogeneic umbilical cord blood mononuclear cells still awaits publication.
Also, head cooling, the standard of care in neonatal hypoxicischemic encephalopathy with high efficacy in subsets (which encompass stroke cases), is ineffective after stroke in adults (Harris et al., 2012 [103]), suggesting different mechanisms.
Cell dose needed for the adult brain
In experimental studies on stroke in adult animal models, there is some evidence for neurological improvement after transplantation of mononuclear cord blood cells but not in all (Mäkinen et al., 2006 [104]), however, it is evident that the number of mononuclear cells found in the lesion is far less than in juvenile models (Hess et al., 2008 [105]; Meier et al., 2006 [52]; Rosenkranz et al., 2010 [54], Wasielewski et al., 2012 [100]). In experimental adult stroke, it has hence been suggested that the neuroprotective effects observed may not be necessarily dependent on cells present in the lesion (Hess et al., 2008 [105]). However, the results suggest that the mitigation of Middle Cerebral Artery Occlusion (MCAO) induced deficits depend on the number of human umbilical cord blood cells injected directly or intravenously (Vendrame et al., 2004 [106], Hess et al., 2008 [105]).
In adult rats (175 grams body weight) significant beneficial effects after stroke were only noted with 10e6 or more mononuclear cord blood cells per animal. The results were not significant with 10e5 or 10e4 HUBCs. Therefore, the authors concluded that “…, one major obstacle will be attaining the number of cells needed for clinical benefit in man; our highest rat dose would translate into using the cells derived from >20 cords for just 1 dose of 10 7. In vitro expansion of the cells before infusion could resolve this issue” (Vendrame et al., 2004 [106]).
It is important to note, that autologous cord blood for separation of hucbMNC can only be collected once in a lifetime, i.e., at birth, and the number of mononuclear cells is hence limited. There is a mononuclear cell number per kilogram body weight (approx. 2 x 10e7/kg) assumed to be necessary for efficacy (Sun et al., 2017 [65]). For example, public cord blood banks engaged in allogeneic hematoonkologic cord blood treatments usually reject umbilical cord blood units containing <5 x 10e8 total nuclear cell count (TNC) (FDA regulation; https://www.fda.gov/downloads/BiologicsBloodVaccines/ GuidanceComplianceRegulatoryInformation/Guidances/ CellularandGeneTherapy/UCM357135.pdf, accessed on Nov 17, 2016 [110]).
Notwithstandig the fundamental neuropharmacologic treatmentrelated distinctions between the developing brain and the adult brain that may hamper cell-based treatment of stroke in the adult, the average weight of a European adult (72kg body weight) appears to be too high to reach an effective number of hucbMNC per kilogram body weight for systemic treatment of stroke – if autologous cord blood were available for the adult population. (https://ec.europa.eu/ health/ph_publication/eb_food_de.pdf; accessed Nov 17, 2016 [111]). However, treatment with pooled and HLA matched allogeneic cord blood monouclear cells may be feasible and safe as shown recently (Laskowitz et al., 2018 [2]).
Summary and Conclusion
In summary, there is a plethora of mechanistic, pharmacodynamics, and clinical evidence to support the view that autologous and allogeneic hucbMNC can be used effectively to treat patients with pediatric stroke, i.e., stroke in the developing brain, when plasticity is still present. However, cell-based treatment of stroke in the adult brain may continue to be a challenge that might perhaps be overcome in the future by a combination treatment of specific cord blood cell populations with concurrent blockage of Nogo and PirB receptor response. On the present balance of evidence it is concluded that the pathophysiological characteristics associated with plasticity and Blood-brain barrier function of the developing brain are closely linked to the pharmacological action of hucbMNC in such a way that cell-based treatment appears to be more efficacious in pediatric stroke than in adult stroke.
Acknowledgements
This study was supported by the non-profit philanthropic organization “STOP-CP in children! – Combat Infantile Cerebral Palsy!” e.V., Bochum Germany (https://stop-cp.org/).
References
- Jensen A, Hamelmann E. First autologous cord blood therapy for pediatric ischemic stroke and cerebral palsy caused by cephalic molding during birth - Individual treatment with mononuclear cells Case Reports in Transplantation. 2016; Article ID 1717426.
- Laskowitz DT, Bennett ER, Durham RJ, Volpi JJ, Wiese JR, Frankel M, et al. Allogeneic Umbilical Cord Blood Infusion for Adults with Ischemic Stroke: Clinical Outcomes from a Phase 1 Safety Study. Stem Cells Transl Med. 2018; 12.
- Nelson KB. Perinatal Ichemic Stroke. Stroke. 2007; 38: 742-745.
- Lynch JK, Hirtz DG, deVeber G, Nelson KB. Report of the National Institute of Neurological Disorders and Stroke. Workshop on Perinatal and Childhood Stroke Pediatrics. 2002; 109: 116-123.
- Rutherford MA, Ramenghi LA, Cowan FM. Neonatal Stroke Arch Dis Child Fetal Neonatal. 2012; 97: F377-384.
- Raju TN, Nelson KB, Ferriero D, Lynch JK, NICHD-NINDS Perinatal Stroke Workshop Participants. Ischemic perinatal stroke: summary of a workshop sponsored by the National Institute of Child Health and Human Development and the National Institute of Neurological Disorders and Stroke. Pediatrics. 2007; 120: 609-616.
- Tsuji M, Taguchi A, Ohshima M, Kasahara Y, Sato Y, Tsuda H, et al. Effects Of Intravenous Administration Of Umbilical Cord Blood Cd34+ Cells In A Mouse Model Of Neonatal Stroke. Neuroscience. 2014; 263: 148-158.
- Chabrier S, Husson B, Dinomais M, Landrieu P, Nguyen The Tich S. New insights (and new interrogations) in perinatal arterial ischemic stroke. Thromb Res. 2011; 127: 13-22.
- Fitzgerald KC, Williams LS, Garg BP, Carvalho KS, Golomb MR. Cerebral sinovenous thrombosis in the neonate. Arch Neurol. 2006; 63: 405-409.
- Bonduel M, Sciuccati G, Hepner M, Torres AF, Pieroni G, Frontroth JP. Prethrombotic disorders in children with arterial ischemic stroke and sinovenous thrombosis. Arch Neurol. 1999; 56: 967-971.
- deVeber G, Monagle P, Chan A, MacGregor D, Curtis R, Lee S, et al. Prothrombotic disorders in infants and children with cerebral thromboembolism. Arch Neurol. 1998; 55: 1539-1543.
- Golomb MR, MacGregor DL, Domi T, Armstrong DC, McCrindle BW, Mayank S, et al. Presumed pre- or perinatal arterial ischemic stroke: risk factors and outcomes. Ann Neurol. 2001; 50: 163-168.
- Nelson KB, Dambrosia JM, Grether JK, Phillips TM. Neonatal cytokines and coagulation factors in children with cerebral palsy. Ann Neurol. 1998; 44: 665-675.
- Armstrong-Wells J, Johnston SC, Wu YW, Sidney S, Fullerton HJ. Prevalence and predictors of perinatal hemorrhagic stroke: results from the Kaiser pediatric stroke study. Pediatrics. 2009; 123: 823-828.
- Cole L, Dewey D, Letourneau N, Kaplan BJ, Chaput K, Gallagher C, et al. Clinical Characteristics, Risk Factors, and Outcomes Associated With Neonatal Hemorrhagic Stroke: A Population-Based Case-Control Study. JAMA Pediatr. 2017; 171: 230-238.
- Berfelo FJ, Kersbergen KJ, van Ommen CH, Govaert P, van Straaten HL, Poll-The BT, et al. Neonatal cerebral sinovenous thrombosis from symptom to outcome. Stroke. 2010; 41: 1382-1388.
- Barmada MA, Moossy J, Shuman RM. Cerebral infarcts with arterial occlusion in neonates Ann Neurol. 1979; 6: 495-502.
- Volpe JJ. Neurology of the Newborn, 5th Edition, Saunders, Philadephia, USA, ISBN. 2008; 978: 3995-4002.
- Jensen A. Autologous Cord Blood Therapy for Infantile Cerebral Palsy: From Bench to Bedside. Obstetrics and Gynecology International. 2014; Article ID 976321.
- Pape KE, Wigglesworth JS, Haemorrhage, Ischaemia and the Perinatal Brain. Clinics in Developmental Medicine Nos. 69/79. Spastics International Medical Publications. The Lavenham Press Ltd., Lavenham, Suffolk. 1979.
- Berger R, Bender S, Sefkow S, Klingmüller V, Künzel W, Jensen A. Peri/intraventricular haemorrhage: a cranial ultrasound study on 5286 neonates. European Journal of Obstetrics Gynecology and Reproductive Biology. 1997; 75: 191-203.
- Jensen A, Holmer B. White Matter Damage in 4,725 Term-Born Infants is determined by Head Circumference at Birth: The Missing Link. Obstet Gynecol Int. 2018; 2018: 2120835.
- Jensen A, Neuhäuser G, Jensen K-O. Growth variables and brain damage at birth predict developmental disability at four years of age: a basis for individual preschool support. Obstet Gynecol Int. 2018.
- Friede RL. Developmental Neuropathology Vienna. Springer Verlag. 1975.
- deVeber G, Andrew M, Adams C, Bjornson B, Booth F, Buckley DJ, et al. Cerebral sinovenous thrombosis in children. N Engl J Med. 2001; 345: 417-423.
- Grunt S, Wingeier K, Wehrli E, Boltshauser E, Capone A, Fluss J, et al. Cerebral sinus venous thrombosis in Swiss children. Developmental Medicine & Child Neurology. 2010; 52: 1145-1150.
- Tsze DS, Valente JH. Pediatric Stroke: A Review Emergency Medicine International. 2011; Article ID 734506.
- Schulzke S, Weber P, Luetschg J, Fahnenstich H. Incidence and diagnosis of unilateral arterial cerebral infarction in newborn infants. J Perinat Med. 2005; 33: 170-175.
- Estan J, Hope P. Unilateral neonatal cerebral infarction in full term infants. Arch Dis Child Fetal Neonatal. 1997; 76: F88-F93.
- Perlman JM, Rollins NK, Evans D. Neonatal stroke: clinical characteristics and cerebral blood flow velocity measurements. Pediatr Neurol. 1994; 11: 281-284.
- Grunt S, Mazenauer L, Buerki SE, Boltshauser E, Mori AC, Datta AN, et al. Incidence and Outcomes of Symptomatic Neonatal Arterial Ischemic. Stroke Pediatrics. 2015; 135: e1220-1228.
- Agrawal N, Johnston SC, Wu YW, Sidney S, Fullerton HJ. Imaging Data Reveal a Higher Pediatric Stroke Incidence Than Prior US Estimates Stroke. 2009; 40: 3415-3421.
- Basic Neonatal Care Manual, Intergrowth-21st, International Fetal and Newborn Growth Standards for the 21st Century, The International Fetal and Newborn Growth consortium, University of Oxford UK, pp.1-49, September 2009.
- Gluckman PD, Wyatt JS, Azzopardi D, Ballard R, Edwards AD, Ferriero DM, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicenter randomised trial. Lancet. 2005; 365: 663-670.
- Walston F, Austin T, Clarke P, Shanmugalingam S Guidelines for Management of Infants with Suspected Hypoxic Ischaemic Encephalopathy (HIE). East of England Perinatal Networks, NHS 2012 Registration No: EOE-002-2012.
- Roach ES, Golomb MR, Adams R, Biller J, Daniels S, Deveber G, et al. Management of Stroke in Infants and Children A Scientific Statement From a Special Writing Group of the American Heart Association Stroke Council and the Council on Cardiovascular Disease in the Young. Stroke. 2008; 39: 2644-2691.
- Mercuri E, Rutherford M, Cowan F, Pennock J, Counsell S, Papadimitriou M, et al. Early prognostic indicators of outcome in infants with neonatal cerebral infarction: a clinical, electroencephalogram, and magnetic resonance imaging study. Pediatrics. 1999; 103: 39-46.
- Trauner DA, Ballantyne A, Friedland S, Chase C. Disorders of affective and linguistic prosody in children after early unilateral brain damage. Ann Neurol. 1996; 39: 361-367.
- deVeber G, Kirton A, Booth FA, Yager JY, Wirrell EC, Wood E, et al. Canadian Paediatric Ischemic Stroke Registry: Analysis of children with arterial ischemic stroke. Ann Neurol. 2000; 48: 526.
- Al Sulaiman A, Bademosi O, Ismail H, Magboll G. Stroke in Saudi children. J Child Neurol. 1999; 14: 295-298.
- Mackay MT, Wiznitzer M, Benedict SL, Lee KL, deVeber GA, Ganesan V, et al. Arterial Ischemic Stroke Risk Factors: The International Pediatric Stroke Study. Ann Neurol. 2011; 69: 130-140.
- Angelini L, Ravelli A, Caporali R, Rumi V, Nardocci N, Martini A. High prevalence of antiphospholipid antibodies in children with idiopathic cerebral ischemia. Pediatrics. 1994; 94: 500-503.
- Menovsky T, van Overbeeke JJ. Cerebral arteriovenous malformations in childhood: state of the art with special reference to treatment. Eur J Pediatr. 1997; 156: 741-746.
- Kittner SJ, Adams RJ. Stroke in children and young adults. Curr Opin Neurol. 1996; 9: 53-56.
- Giroud M, Lemesle M, Gouyon JB, Nivelon JL, Milan C, Dumas R. Cerebrovascular disease in children under 16 years of age in the city of Dijon, France: a study of incidence and clinical features from 1985 to 1993. J Clin Epidemiol. 1995: 48: 1343-1348.
- Simonetti BG, Cavelti A, Arnold M, Bigi, S, Regényi M, Mattle HP, et al. Long-term outcome after arterial ischemic stroke in children and young adults. Neurology. 2015; 84: 1-7.
- Paediatric Stroke Working Group, Royal College of Physicians of London. Stroke in childhood: clinical guidelines for diagnosis management and rehabilitation.
- Chen J, Sanberg P R, Li Y, Wang L, Lu M, Willing AE, et al. Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke. 2001; 32: 2682-2688.
- Neuhoff S, Moers J, Rieks M, Grunwald T, Jensen A, Dermietzel R, et al. Proliferation, differentiation, and cytokine secretion of human umbilical cord blood-derived mononuclear cells in vitro Hematology. 2007; 35:1119-1131.
- Levine S. Anoxic-ischemic encephalopathy in rats. Am J Pathol. 1960; 36: 1-17.
- Garnier Y, Vaihinger HM, Middelanis J, Brüstle O, Jensen A. Cerebral ischemia and umbilical stem cell transplantation in chronically prepared fetal sheep Proceedings 1st International Meeting of the Stem Cell Network North Rhine Westphalia, Düsseldorf, Germany. 2002.
- Meier C, Middelanis J, Wasielewski B, Neuhoff S, Roth-Haerer A, Gantert M, et al. Spastic paresis after perinatal brain damage in rats is reduced by human cord blood mononuclear cells. Pediatric Research. 2006; 59: 244-249.
- Pimentel-Coelho PM, Magalhaes ES, Lopes LM, Deazevedo LC, Santiago MF, Mendez-Otero R. Human cord blood transplantation in a neonatal rat model of hypoxic-ischemic brain damage: functional outcome related to neuroprotection in the striatum. Stem Cells and Development. 2010; 19: 351-358.
- Rosenkranz K, Kumbruch S, Lebermann K, Marschner K, Jensen A, Dermietzel R, et al. The chemokine stromal derived factor-1 (SDF-1) induces 'homing' of umbilical cord blood cells to a hypoxic-ischemic lesion in the rat brain. Journal of Neuroscience Research. 2010; 88:1223-1233.
- Ahn SY, Chang SY, Sung DK, Sung SI, Yoo HS, Lee JH, et al., Mesenchymal Stem Cells Prevent Hydrocephalus After Severe Intraventricular Hemorrhage. Stroke. 2013; 44: 497-504.
- Drobyshevsky A, Cotten CM, Shi Z, Luo K, Jiang R, Derrick M, et al. Human Umbilical Cord Blood Cells Ameliorate Motor Deficits in Rabbits in a Cerebral Palsy Model. Developmental Neuroscience. 2015; 37: 349-362.
- Liao W, Zhong J, Yu J, Xie J, Liu Y, Du L, et al. Therapeutic benefit of human umbilical cord derived mesenchymal stromal cells in intracerebral haemorrhage rat: implications of anti-inflammation and angiogenesis. Cell Physiol Biochem. 2009; 24: 307-316.
- Li J, McDonald CA, Fahey MC, Jenkin G, Miller SL. Could cord blood cell therapy reduce preterm brain injury. Front Neurol. 2014; 5: 200.
- Geißler M, Dinse HR, Neuhoff S, Kreikemeier K, Meier C. Human umbilical cord blood cells restore brain damage induced changes in rat somatosensory cortex. PLoS ONE. 2011; 6, Article ID e20194, 2011.
- Cord blood stem cells cure infantile brain damage Stammzellen aus Nabelschnurblut heilen kindlichen Hirnschaden. Top Magazin Ruhr, Wissenschaft - Medizin. 2009; 23: 80-81.
- Jensen A, First therapy of cerebral palsy caused by hypoxic-ischemic brain damage in a child after cardiac arrest? Individual treatment by transplantation of autologous cord blood stem cells. Regenerative Medizin. 2011; 4: 30-31.
- Sun, J. Allison, C. McLaughlin, Sledge L, Waters-Pick B, Wease S, et al. Differences in quality between privately and publicly banked umbilical cord blood units: a pilot study of autologous cord blood infusion in children with acquired neurologic disorders. Transfusion. 2010; 50: 1980-1987.
- Jensen A, Hamelmann E. First autologous cell therapy of cerebral palsy caused by hypoxic-ischemic brain damage in a child after cardiac arrest—individual treatment with cord blood. Case Reports in Transplantation. 2013; 2013: Article ID 951827.
- Cotten CM, MurthaAP, Goldberg RN, Grotegut CA, Smith PB, Goldstein RF, et al. Feasibility of autologous cord blood cells for infants with hypoxic-ischaemic encephalopathy. Journal of Pediatrics. 2014; 164: 973-979.
- Sun JM, Song AW, Case LE, Mikati MA, Gustafson KE, Simmons R, et al. Effect of Autologous Cord Blood Infusion on Motor Function and Brain Connectivity in Young Children with Cerebral Palsy: A Randomized, Placebo-Controlled Trial. Stem Cells Transl Med. 2017; 6: 2071-2078.
- Min K, Song J, Kang JY, Ko J, Ryu JS, Kang MS, et al. Umbilical cord blood therapy potentiated with erythropoietin for children with cerebral palsy: a double-blind, randomized, placebo-controlled trial. Stem Cells. 2013; 31: 581-591.
- Kwang Yul Cha. Power Point presentation based, CHA Stem Cell Institute. Stem Cells. 2012.
- -H. Lee, K. V. Choi, J. H. Moon, Jun HJ, Kang HR, Oh SI, et al. Safety and feasibility of countering neurological impairment by intravenous administration of autologous cord blood in cerebral palsy. Journal of Translational Medicine. 2012; 10: 58: 1-11.
- Webb J, Jensen A, Hamelmann E. Smiles All Round. International Day of Persons with Disabilities Biomedical Picture of the Day (BPoD). MRC Clinical Sciences Centre. 2013.
- Ten leading causes of death in Childhood. 2014.
- Golomb MR, Fullerton HJ, Nowak-Gottl U, deVeber G, International Pediatric Stroke Study Group. Male Predominance in Childhood Ischemic Stroke: Findings from the International Pediatric Stroke Study. Stroke. 2009; 40: 52-57.
- Ganesan V, Hogan A, Shack N, Gordon A, Isaacs E, Kirkham FJ. Outcome after ischaemic stroke in childhood. Dev Med Child Neurol. 2000; 42: 455-461.
- Vos T, Barber RM, Bell B, Bertozzi-Villa A, Biryukov S, Bolliger I, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. The Lancet. 2015; 386: 743-800.
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, et al. Dynamic mapping of human cortical development during childhood through early adulthood. PNAS. 2004; 10: 8174-8179.
- Bhardwaj RD, Curtis MA, Spalding KL, Buchholz BA, Fink D, Björk-Eriksson T, et al. Neocortical neurogenesis in humans is restricted to development. PNAS. 2006; 103.
- Blakemore SJ. Imaging brain development: The adolescent brain. Neuroimage. 2012; 61: 397-406.
- Cui L, Qua H, Xiaob T, Zhaod M, Jolkkonene J, Zhaoa C. Stromal cell-derived factor-1 and its receptor CXCR4 in adult neurogenesis after cerebral ischemia. Restorative Neurology and Neuroscience. 2013; 239-251.
- Kennard MA. Relation of Age to Motor Impairment in Man and in Subhuman Primates. Arch NeurPsych. 1940; 44: 377-397.
- Bochner DN, Sapp RW, Adelson JD, Zhang S, Lee H, Djurisic M, et al. Blocking PirB up-regulates spines and functional synapses to unlock visual cortical plasticity and facilitate recovery from amblyopia. Sci Transl Med. 2014; 6.
- Schwab ME, Strittmatter SM. Nogo limits neural plasticity and recovery from injury. Opin Neurobiol. 2014; 53-60.
- Marrs GS, Green SH, Dailey ME. Rapid formation and remodeling of postsynaptic densities in developing dendrites. Nature Neuroscience. 2001; 4: 1006-1013.
- Boatman D, Freeman J, Vining E, Pulsifer M, Miglioretti D, Minahan R, et al. Language recovery after left hemispherectomy in children with late-onset seizures. Ann. Neurol. 1999; 46: 579-586.
- Rosenkranz R, Kumbruch S, Tenbusch M, Marcus K, Marschner K, Dermietzel R, et al. Transplantation of human umbilical cord blood cells mediated beneficial effects on apoptosis, angiogenesis and neuronal survival after hypoxic-ischemic brain injury in rats. Cell and Tissue Research. 2012; 348: 429-438.
- Lou S-j, Liua J-y, Changa H, Chena P-j. Hippocampal neurogenesis and gene expression depend on exercise intensity in juvenile rats. Brain Research. 2008; 1210: 48-55.
- Alsina B, Vu T, Cohen-Cory S. Visualizing synapse formation in arborizing optic axons in vivo: dynamics and modulation by BDNF. Nature neuroscience. 2001; 4: 1093-1101.
- Hubel DH, Wiesel TN. Binocular Interaction In Striate Cortex Of Kittens Reared With Artificial Squint. Journal of Neurophysiology. 1965; 28: 1041-1059.
- Blakemore C. Genetic instructions and developmental plasticity in the kitten’s visual cortex. Phil. Trans. R. Soc. Lond. B. 1977; 278: 425-443.
- Fallon JB, Irvinea DRF, and Shepherd RK. Cochlear Implants and Brain Plasticity. Hear Res. 2008; 238: 110-117.
- Lynch G, Rex CS, Gall CM. Synaptic plasticity in early aging. Ageing Research Reviews. 2006; 5: 255-280.
- Sieber MW, Guenther M, Jaenisch N, Albrecht-Eckardt D, Kohl M, Witte OW, et al. Age-specific transcriptional response to stroke. Neurobiology of Aging. 2014; 35: 1744-1754.
- Mundkur N. Neuroplasticity in children Symposium on Developmental and Behavioral Disorders -1. Indian J Pediatr. 2005; 72: 855-857.
- Hua JY, Smith SJ. Neural activity and the dynamics of central nervous system development. Nature Neuroscience. 2004; 7: 327-332.
- Hof PR, Duan H, Tanya L, Page TL, Einstein M, Wicinski B, et al. Age-related changes in GluR2 and NMDAR1 glutamate receptor subunit protein immunoreactivity in corticocortically projecting neurons in macaque and patas monkeys. Brain Research. 2002; 928: 175-186.
- Hof PR, Morrison JH. The aging brain: morphomolecular senescence of cortical circuits. Trends in Neurosciences. 2004; 27: 607-613.
- Lou SJ , Liu JY, Yang RY, Chen PJ. Treadmill running enhances the ability of learning in young rats. Acta Physiologica Sinica. 2006; 58: 365-369.
- Vexler ZS, Yenari MA. Does inflammation after stroke affect the developing brain differently than adult brain? Dev Neurosci. 2009; 31: 378-393.
- Anthony D, Dempster R, Fearn S, Clements J, Wells G, Perry VH, et al. CXC chemokines generate age-related increases in neutrophilmediated brain inflammation and blood–brain barrier breakdown. Current Biology. 1998; 8: 923-926.
- Li J, Yawno T, Sutherland A, Loose J, Nitsos I, Bischof R, et al. Preterm white matter brain injury is prevented by early administration of umbilical cord blood cells. Experimental Neurology. 2016.
- Liu G , Ni J , Mao L , Yan M , Pang T , Liao H. Expression of Nogo receptor 1 in microglia during development and following traumatic brain injury. Brain Res. 2015; 1627: 41-51.
- Wasielewski, Jensen A, Roth-Härer A, Dermietzel R, Meier C. Neuroglial activation and Cx43 expression are reduced upon transplantation of human umbilical cord blood cells after perinatal hypoxic-ischemic injury. Brain Research. 2012; 1487: 39-53.
- Boncoraglio GB , Bersano A, Candelise L, Reynolds BA, Parati EA. Stem cell transplantation for ischemic stroke. Cochrane Database Syst Rev. 2010; 9.
- Kim M. NCT01884155 ClinicalTrials.Gov.
- Harris B, Andrews PJD, Murray GD, Forbes J, Moseley O. Systematic review of head cooling in adults after traumatic brain injury and stroke. Health Technology Assessment. 2012; 16.
- Mäkinen S, Kekarainen T, Nystedt J, Liimatainen T, Huhtala T, Narvanen A, et al. Human umbilical cord blood cells do not improve sensorimotor or cognitive outcome following transient middle cerebral artery occlusion in rats. Brain Res. 2006; 1123: 207-215.
- Hess DC, Borlongan CV. Stem cells and neurological diseases. Cell Prolif. 2008; 41: 94-114.
- Vendrame M, Cassady J, Newcomb J, Butler T, Pennypacker KR, Zigova T, et al. Infusion of human umbilical cord blood cells in a rat model of stroke dose-dependently rescues behavioral deficits and reduces infarct volume. Stroke. 2004; 35: 2390-2395.
- Anthony DC, Bolton SJ, Fearn S, Perry VH. Age-related effects of interleukin-1β on polymorphonuclear neutrophil-dependent increases in blood–brain barrier permeability in rats. Brain. 1997; 120: 435-444.
- Kang M, Min K, Jang J, Kim SC, Kang MS, Jang SJ, et al. Involvement of Immune Responses in the Efficacy of Cord Blood Cell Therapy for Cerebral Palsy. Stem cells and development. 2015.
- Papadopoulos KI, Low SSS, Aw TC, and Chantarojanasiri T. Safety and feasibility of autologous umbilical cord blood transfusion in 2 toddlers with cerebral palsy and the role of low dose granulocyte-colony stimulating factor injections. Restorative Neurology and Neuroscience. 2011; 29: 17-22.
- Guidance industry cord blood. 2018.
- European Commission‚ Gesundheit und Ernährung’ Eurobarometer Spezial, European Commission. 2018.