
Research Article
J Stem Cell Res Transplant. 2019; 6(1): 1030.
Effect of Intramyocardial Administration of Endothelial Colony Forming Cells on Right Ventricle Function in a Model of Pulmonary Hypertension
Loisel F1,2, Guihaire J1,3, Boulate D1,4, Amsallem M1, Ghigna MR1,5, Issard J1, Communaux C5, Arouche N2, Noly PE1, Decante B1, Fadel E1,4,6, Uzan G2 and Olaf Mercier1,4,6*
1Research and Innovation Unit, Inserm UMR-S 999, Marie Lannelongue Hospital, Paris-Saclay University, Le Plessis Robinson, France
2Inserm 1197 Research Unit, 94807 Villejuif Cedex, France, Paris-Saclay University, France
3Department of Cardiac Surgery, Marie Lannelongue Hospital, Paris-Saclay University, Le Plessis Robinson, France
4Department of Thoracic and Vascular Surgery and Heart-Lung Transplantation, Marie Lannelongue Hospital, Paris-Saclay University, DHU TORINO, Le Plessis Robinson, France
5Department of Pathology, Marie Lannelongue Hospital, Paris-Saclay University, Le Plessis Robinson, France
6Paris-Saclay University, School of Medicine, Kremlin- Bicêtre, France
*Corresponding author: Olaf Mercier, Department of Thoracic and Vascular Surgery and Heart-Lung Transplantation, Research and Innovation Unit, Marie Lannelongue Hospital, 133 Avenue de la Resistance, 92350, Le Plessis Robinson, France
Received: November 25, 2019; Accepted: December 19, 2019; Published: December 26, 2019
Abstract
Right Ventricular (RV) failure is the main prognostic factor in Pulmonary Hypertension (PH), and ventricular capillary density has been reported to be a marker of RV maladaptive remodeling and failure. Our aim was to determine whether intramyocardial administration of Endothelial Colony Forming Cells (ECFCs) can improve RV function and capillary density in a piglet model of Chronic Thromboembolic Pulmonary Hypertension (CTEPH). We compared three groups: Sham (n=5), CTEPH (n=6), and CTEPH+ECFC (n=5). ECFCs were isolated from CTEPH+ECFC piglet peripheral blood samples at baseline. CTEPH and Sham groups underwent intramyocardial administration of saline, while the CTEPH+EPC group received ECFCs at six weeks (T6). RV function, pulmonary hemodynamics, and myocardial morphometry were investigated in the animals at ten weeks (T10). Additional experiment has been performed to evaluate engraftment kinetics of ECFCs over 3 weeks. Following ECFC administration, there were no significant changes in pulmonary or cardiac hemodynamic parameters (RVFAC, mPAP and TPR). However, CTEPH+ECFC piglets had increased localized capillary density and a reduction in myocardial fibrosis (CTEPH 13.15% [11.36-18.49] vs. CTEPH+ECFC 8.94 [5.25-10.98], p=0.045). ECFCs were found in the RV free wall at 24h and one week after injection but not beyond. ECFCs intramyocardial administration did not improve RV function in a model of CTEPH as it induced focalized capillary density improvement at injection site. Intramyocardial route may not be the most effective way to deliver cell therapy to RV in PH.
Keywords: Stem Cell Therapy; Right Ventricle in Pulmonary Hypertension; Intramyocardial Administration
Abbreviations
CTEPH: Chronic Thromboembolic Pulmonary Hypertension; EGM: Endothelial cell Growth Medium; ECFCs: Endothelial Colony Forming Cells; FGF: Fibroblast Growth Factor; PA: pulmonary artery; PAP: Pulmonary Arterial Pressure; PBMC: Peripheral Blood Mononuclear Cell; PH: Pulmonary Hypertension; RUL: Right Upper Lobe; RV: Right Ventricular; RVFAC: Right Ventricular Fractional Area Change; T10: 10 weeks; T6: 6 weeks; TAPSE: Tricuspid Annular Plane Systolic Excursion; TPR: Total Pulmonary Resistance
Introduction
Pulmonary Hypertension (PH) is characterized by the occlusion of the pulmonary vasculature, leading to an increase in pulmonary vascular resistance and resulting in a rise in Right Ventricle (RV) afterload. While the disease progresses, the RV shifts from an adaptive remodeling to a maladaptive one. Right ventricular function is the main prognostic factor in PH [1]. The maladaptive state is defined by a decreased capillary density observed in several animals models and in patients [2,3].
Endothelial Colony Forming Cells (ECFC) are good candidates for RV-targeted therapy since they promote vascular repair and angiogenesis [4,5]. Therefore, in a previous study, we administered ECFCs through the right coronary artery into the RV [6]. This study showed improved right heart function, increased capillary density and decreased cardiomyocyte’s hypertrophy after ECFC administration. However, this route of administration led to poor cell retention as ECFCs were not found in the RV 4 weeks after their administration. Intramyocardial administration is known to induce a better retention of transplanted cells than the intracoronary one and is often associated with a greater impact on the pathology [7].
As a result, we aimed at investigating the effect of ECFCs intramyocardial administration on RV function and capillary density in a piglet model of Chronic Thromboembolic Pulmonary Hypertension (CTEPH).
Materials and Methods
Chronic thromboembolic piglet model
Five-week-old large white piglets, weighing 20-22 kg, were included in the preliminary (n=1), the main study (n=18) and the additional one (n=1). The animals were randomly assigned to Sham or CTEPH groups. Briefly, the CTEPH model was established by ligaturing the left pulmonary artery followed by weekly embolization of the right pulmonary artery over a five-week period [8] (See supplemental data for detail).
A preliminary pilot study was undertaken using one CTEPH piglet in which ECFCs were administrated at six weeks (T6), and the animal was sacrificed 24h later. This preliminary study was undertaken to test the feasibility, to validate the injection protocol and to evaluate the ECFCs’ engraftment at 24h (Figure 1a). Eighteen piglets were included in the main study: 6 Sham, 6 CTEPH, and 6 CTEPH+ECFC (Figure 1a). At T6, the animals in the CTEPH groups received intramyocardial injections of saline, whereas the animals in the CTEPH+ECFC group each received 10.106 ECFCs. On the RV, four sites were marked by stitches (Figure 1b) and 10 injections of 25μl each were performed at each site, resulting in a total of 40 injections of 1ml of either saline or cell suspension. An additional experiment was set in place to evaluate the engraftment of the ECFCs after their administration. To do so, one Sham piglet received 2.5.106 ECFCs (previously transfected by lentivirus to express GFP, See supplemental methods for details) weekly during 3 weeks and was sacrificed at the end of the fourth week. Biopsies were collected at each site to search for the presence of ECFCs 1, 2 and 3 weeks post administration.
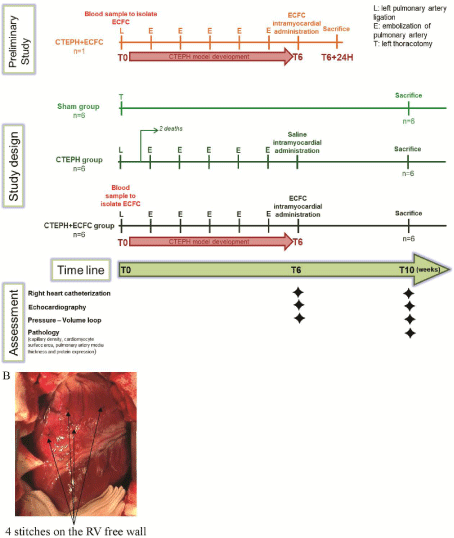
Figure 1: (A) Study design. (B) Stitches on RV free wall to mark the localization of intramyocardial administration.
No deaths related to infection of the surgical site were reported. There were no deaths in the CTEPH+ECFC group after ECFC administration, and two deaths in the CTEPH group after saline administration due to the severity of the disease. As a consequence, 2 additional animals have been enrolled in the study to have 6 piglets per group.
ECFC harvesting
Peripheral blood samples of 100ml were collected from the CTEPH+ECFC piglets at baseline. An autologous cell-based therapy was established as the treated piglets received ECFCs isolated previously from their own blood. ECFCs were harvested by isolation of peripheral blood mononuclear cells (PBMC) by gradient centrifugation in Pancoll (Dutsher, Burmath, France), after which they were plated at a density of 50,000 cells/cm [9]. The PBMCs were maintained in endothelial basal medium-2 supplemented with EGM2 (Endothelial cell Growth Medium)-MV Single-Quots (containing hEGF, hydrocortisone, gentamicin, fetal bovine serum, VEGF, hFGF-B, R3-IGF-1, and ascorbic acid) (Lonza, Ermerainville, France) at 37°C in a 5% CO2 humidified incubator. After 8 to 15 days, cell colonies became visible and they were detached by trypsinization, followed by seeding at 10,000 cells/cm2 for expansion.
ECFC administration
Cell therapy administration was performed at T6 for the CTEPH+ECFC group. This time point was chosen because it has been proven in the study of Mercier et al. that chronic PH is developed in pigs 5 weeks after left PA ligature and weekly embolization [8]. Autologous ECFCs were resuspended in 1mL of saline solution in a syringe, and injected using 30G needle and a mesotherapy pistol to accurately deliver 25μl at each injection. Injection sites were marked beforehand in order to localize these areas at the time of RV biopsies sampling 4 weeks later.
Hemodynamic assessment
Heart rate, mean Pulmonary Artery Pressure (mPAP), cardiac output, and central blood saturation were measured at baseline (T0), 6 weeks (T6), and 10 weeks (T10). A pressure-volume loop analysis of the RV was performed at T6 and T10 with a conductance VentriCath 507 (Millar Instruments, Houston, TX, USA). (See supplemental methods for details).
Echocardiographic assessment
Transthoracic echocardiography (Vivid E9; GE Medical Systems Milwaukee, WI, USA) was performed at T0, T6, and T10. Right ventricular fractional area change (RVFAC) and the RV free wall strain were assessed.
Capillary density assessment in the RV
To assess the capillary density, RV samples were incubated with a rabbit anti-human CD31 (CD31 clone 1A10, 1:100, Diagomics, Blagnac, France) detected by ultraView Universal DAB Detection Kit (Ventana, California USA) staining with Benchmark GX (Roche, Boulogne-Billancourt, France). The capillary density was determined by the number of capillaries per mm2 in 10 fields of view for each animal at 400x (NIS-Element software, Nikon, Tokyo, Japan).
Cardiomyocyte area assessment in the RV
RV samples were incubated with wheat germ agglutinin (WGAAF488, Invitrogen, USA, 10μg/mL) and DAPI (1:10,000, Sigma- Aldrich, Missouri, USA). The cardiomyocyte surface was determined, as previously described(10) using Image software in 10 fields of view for each animal at 630x (NIS-Element software, Nikon).
Statistical analysis
Data from the three groups were compared with the Kruskal- Wallis test, and the groups were compared two by two with the Mann- Whitney test in case of a significant Kruskal-Wallis test. The results are presented as medians [Interquartile Range (IQR)]. Statistical analyses were performed using Prism 6 software (GraphPad® software, San Diego, California, USA).
Results
Preliminary study
The aim of the preliminary study was to evaluate the feasibility of right intramyocardial administration of ECFCs in piglets and to check that ECFCs were properly recovered in the RV tissue. A CTEPH piglet was sacrificed 24h after receiving 10.106 ECFCs through several intramyocardial injections. Immunohistological analysis revealed the presence of ECFCs in the RV myocardium (Figure 2a).
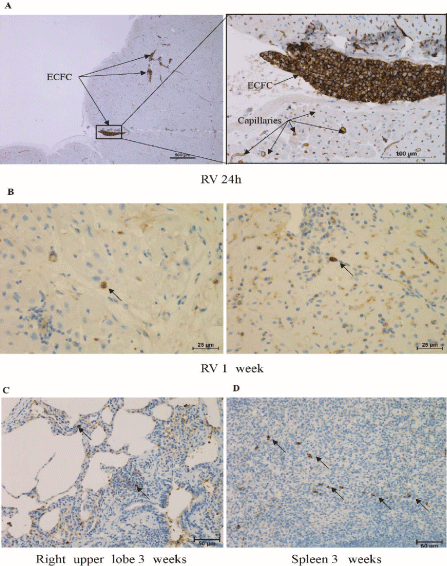
Figure 2: (A) ECFC found in the RV 24h after their intramyocardial administration (anti-CD31 labelling). (B) Isolated GFP-ECFCs found in the RV 1 weeks after
administration (anti-GFP labelling). GFP-ECFCs were also detected in the right upper lobe (C) and the spleen (D) 4 weeks after their administration.
ECFC engraftment
An additional experiment consisted in weekly administrations of ECFCs during 3 weeks to follow their engraftment week-by-week. Isolated GFP-ECFCs were found in the RV only one week after their administration (Figure 2b). These cells are in the RV myocardium. GFP-ECFCs were not detected in this tissue at 2, 3 and 4 weeks post administration. Some ECFCs were found in the spleen (Figure 2c) and lung right upper lobe (Figure 2d) 3 and 4 weeks after their administration.
Assessment at six weeks (T6)
There was no significant difference in terms of body weight, body surface area, and hemodynamic data (mPAP, TPR, cardiac output, RFVAC, RV free wall strain) between the CTEPH and CTEPH+ECFC groups at T6 (data not shown).
Events occurring during and after intramyocardial administration
During intramyocardial administration, one event of fibrillation occurred in a piglet receiving ECFCs and required intensive care. Furthermore, tamponade happened in one piglet after saline solution injection. In both cases, adverse events were rapidly identified and successfully managed. For ethical reasons, 2 piglets had to be sacrificed at 3 and 5 days after administration of saline solution because of the severity of the disease. They showed serious symptoms of right ventricular dysfunction and a strongly altered pulmonary hemodynamic (TPRi was 1488 dynes.s.cm-5 and 2074 dynes.s.cm-5; and mPAP 54 mmHg and 52 mmHg).
RV function and pulmonary hemodynamics
At T10, RVFAC was not significantly different between the three groups (Sham 35.37% [34.15-50.76], CTEPH 31.83% [29.1-55.86] and CTEPH+ECFC 37.87% [25.69-50.87]). There were no difference either for RV free wall strain (Sham -21.08 [-23.77- -18.19], CTEPH -25.54 [-31.44- -18.08] and CTEPH+ECFC -27.26 [-29.25 - -21.94]). In regard to pulmonary hemodynamics, both the mPAP and the TPR indexed to the body surface area remained significantly lower in the Sham group compared to both the CTEPH group and the CTEPH+ECFC group (mPAP: 13mmHg [8.25-15.5], 26mmHg [20.5-33.75], and 27mmHg [22.75-33.5], respectively, Sham vs. CTEPH p=0.0136 and Sham vs. CTEPH+ECFC p=0.0081 ; TPR: 280 dynes.s.cm-5 [204.1-347.6], 570.3 dynes.s.cm-5 [504.5-680.3], and 707.3 dynes.s.cm-5 [522-1246], respectively, Sham vs. CTEPH p=0.045 and Sham vs. CTEPH+ECFC p=0.0062). These hemodynamic parameters did not significantly differ between the CTEPH group and CTEPH+ECFC group (for mPAP: p>0.999 and for TPR: p>0.999) (data not shown).
RV and lung morphometry
The cardiomyocyte surface area was not different between the three groups (Sham 210.1μm² [200.6-268.4], CTEPH 235.5μm² [210.9-258.5] and CTEPH+ECFC 219.3 μm² [147.7-337.7]). The pulmonary artery media thickness in the RUL was greater in the CTEPH group (64.35% [58.51-66.51]) and the CTEPH+ECFC group (63.72% [53.97-69.78]) compared to the Sham group (25.11% [23-27.42]), (CTEPH vs. Sham p=0.0148, CTEPH+ECFC vs. Sham p=0.0074), while there was no significant difference between the CTEPH and the CTEPH+ECFC animals (p›0.999) (data not shown).
RV capillary density
After ECFC administration, localized increase in capillary density was revealed by CD31 immunohistochemical analysis (Figure 3a). Indeed, clusters with increased capillary density were observed. The comparison of the 3 groups revealed a higher capillary density (capillary/mm²) in the CTEPH group (189.7 [173.5-225.3]) and CTEPH+ECFC group (208.7 [181.6-253.3]) compared to the Sham group (134.6 [128.6-142]) (CTEPH vs. Sham p=0.0175 and CTEPH+ECFC vs. Sham p=0.0062) (Figure 3b). There was no significant difference between CTEPH+ECFC and CTEPH regarding the overall capillary density (p›0.999).
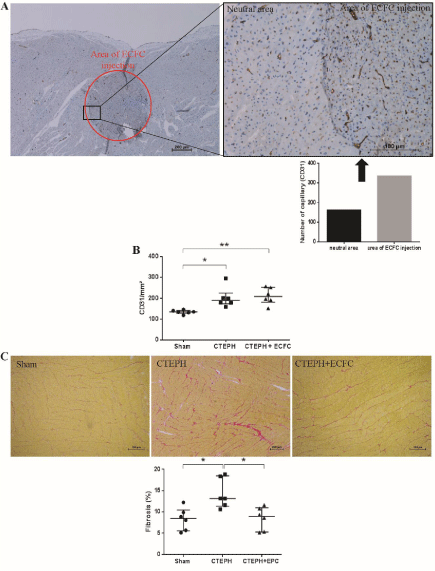
Figure 3: (A) Cluster of higher capillary density in the RV 4 weeks after ECFC administration. (B) CD31 labelling showed a higher capillary density in both CTEPH
and CTEPH+ECFC groups compared to the Sham group. (C) Red Sirius staining revealed an increased fibrosis in the RV of CTEPH piglets compared to the Sham
group. Fibrosis is decreased in the CTEPH+ECFC group.
RV fibrosis
Red Sirius analysis showed a general increase in fibrosis in the CTEPH group (13.15% [11.36-18.49]) compared to the Sham group (8.45% [5.55-10.46]) (p=0.0284). Interestingly, the fibrosis was lower in the CTEPH+ECFC group (8.94% [5.29-10.98]) compared to CTEPH group (p=0.045). There was no significant difference between the Sham group and the CTEPH+ECFC group (p=0.66) (Figure 3c). In this case, unlike capillary density, the decreased fibrosis in the CTEPH+EPC group is general and not present in the form of cluster.
Discussion
We first set in place a preliminary study to evaluate the safety and the feasibility of the method because it is an invasive one. This preliminary study allowed validation of our technic of administration. Indeed, we used it to ensure that ECFCs were well administered in the myocardium. After observing that ECFCs were injected into the RV, we began the main study. Four weeks after ECFC administration an increased capillary density in the area of injection and a general decrease of fibrosis were observed. However, no hemodynamic and RV function improvements were noticed.
In this study, 2 piglets were lost after intramyocardial injections due to the severity of the disease. Furthermore, 2 adverse events occurred during injections (ventricular fibrillation and tamponade). In a previous study we administered ECFCs in CTEPH piglets through the intracoronary route and had no adverse event reported [6]. Based on these results, we can state that intramyocardial administration has a higher risk and invasiveness than the intracoronary route of administration for the CTEPH piglet model. However, a clinical trial administering mesenchymal stromal cells through transendocardial injection in patients with severe ischaemic heart failure observed no side events and stated that this route of administration was safe [11]. Furthermore, in another study, van der Spoel et al. observed no differences in safety profiles between transendocardial and intracoronary cell injection [12]. However, transendocardial route cannot be directly compared to intramyocardial administration, because the first one is percutaneous and the second one requires an invasive surgical procedure. Transendocardial administration may be less invasive and less lethal than the intramyocardial one due to the percutaneous access. As a result, if an intramyocardial injection is intended, the transendocardial route seems less invasive and safer than the epicardial one. Nevertheless it is likely that this route of administration would not have improved our results, because the administration would still be localized in clusters.
The histological analysis 24h after ECFC administration showed clusters of cells entering the myocardium along the needle route attesting that the administrations were localized in the tissue. Four weeks after the injections, localized areas of increased capillary density were observed in the RV. However, we did not observed a general increase in capillary density in the RV. It is important to highlight that even if there is no sustained ECFC engraftment, a localized effect was observed. In contrast, Kawamoto et al. observed an increased capillary density in the left ventricle after transendocardial administration in a pig model of myocardial ischemia [13]. Our observation may be explained by two hypothesis. First, we did not observe a general increase in capillary density because we had already an increased capillary density in this CTEPH model as compared to the Sham animals. The lack of a decreased capillary density in our model, like in the RV of PH patients, is one of the major limitation of our study. There may be a slight increase in capillary density after ECFC administration that we were not able to quantify. The second hypothesis is that we did not inject the optimal cell dose (i.e. number of cells/injection or number of injections). In this study 10.106 ECFCs were administered per piglet. The other studies that administered stem cells in the myocardium through direct intramyocardial injections used a highly variable number of cells (from 5.106 to 8.108) [14]. However, studies that used increasing doses of cells have come to different conclusions. Some showed a beneficial effect only when a high dose of cells was administered while others found a reverse doseresponse: the highest doses are the least effective [15,16]. It appeared that the concentration of the cells during administration rather than their number affected the cardiac tissue [17,15]. Finally, these studies were targeting the left ventricle whereas we worked on the RV. These two ventricles are slightly different and can lead to different results.
A decrease in fibrosis was noticed after ECFC administration. Other studies observed this effect after ECFC administration in different animal models of myocardial dysfunction [13,18,19]. However, none of them described the pathway through which ECFC succeeded in reversing myocardial fibrosis. Fibroblast Growth Factor (FGF) secreted by ECFCs may be an explanation [20]. Hu et al. observed that the treatment of mice with FGF16 attenuated interstitial fibrosis in response to myocardial infarction [21]. However, it seems that the effect of FGFs on cardiac fibrosis depends on the type of FGF and potentially the model of injury [22].
As stated above, we observed a localized increase in capillary density and a general decrease of fibrosis after ECFC intramyocardial administration. These two observations seem contradictory but we can hypothesize that the localized increased capillary density allowed a better diffusion of the factors secreted by the administered ECFC. These secreted factors could exert their effect in the tissue in a diffuse way, resulting in a general decrease of fibrosis.
The histological analysis 24h after ECFC administration revealed clusters of cells entering the myocardium which showed that the administration was localized in the tissue. In addition, we observed a localized improvement of capillary density. A clinical trial pointed that cells administered through transendocardic route had only an effect at the injection sites [23]. The right ventricular dysfunction in PH does not seem localized on specific areas of the RV and is rather a general dysfunction. In this study, it seems that the injections were too localized in view of the general dysfunction of the RV. That may be why no improvements had been observed in RV function. As a result, it seemed that the intramyocardial ECFC administration did not induce a significant improvement of the right ventricular function in CTEPH. However, this route of administration is beneficial in the case of localized dysfunction such as the ones that appeared in the left ventricle after myocardial infarction. Indeed, in this case, the intramyocardial administration of stem cells in the ischemic area or in the ischemic border can overcome the deleterious effects of the infarction [11].
After observing no significant effect on RV function, an additional study was performed to evaluate the ECFCs engraftement over time. Histological analysis reveal the presence of GFP-ECFCs only one week after their administration. None of the ECFCs injected were found in the RV beyond one week. Even if intramyocardial administration leads to a better cell retention than the intracoronary delivery, the retention remains low (7). Administering cells using a needle lead to a significant cell loss that could be attributed to different parameters : the trypsinization, the passage of the cells through the needle, the inflammation caused by the needle at the injection site and the damaged tissue where they are administered result in a low cell survival [24,25]. Furthermore, a study showed that cells are rapidly wash out from the myocardium through the venous system after intramyocardial administration [26]. This observation can explain the fact that after 4 weeks ECFCs were found in the RUL and spleen in our study.
In this study, as we verify that the ECFCs were well administered in the targeted RV, it seems that these cells triggered a local increase in capillary density. However this local effect was too weak to lead to a general improvement in RV function.
Conclusion
Intramyocardial cell administration is invasive, requires a lot of stem cells and did not lead to an improved cardiac function in our study. As a result, the benefit/risk ratio of ECFCs intramyocardial administration in the RV of CTEPH piglets seems to be inefficient. Thus, this route of administration may not be optimal for RV in PH. The use of endothelial progenitor cells is promising but requires improvements of the route of administration. The use of biomaterial may be more efficient than intracoronary or intramyocardial ones and should be further studied.
Ethical Approval
This study was approved by Ministère De L’Éducation Nationale, De L’Enseignement Supérieur Et De La Recherche of France.
Statement of Human and Animal Rights
All procedures in this study were conducted in accordance with the Principles of Laboratory Animal Care formulated by the National Society for Medical Research and the Guide for the Care and Use of Laboratory Animals prepared by the Institute of Laboratory Animal Resources and published by the National Institutes of Health (NIH Publication No. 86-23, revised in 1996) and the guidelines from Directive 2010/63/EU of the European Parliament on the protection of animals used for scientific purposes (APAFIS#7649-2016- 112114519868v1).
Statement of Informed Consent
There are no human subjects in this article and informed consent is not applicable.
Funding, Acknowledgements
This work was supported by a public grant overseen by the French National Research Agency (ANR) as part of the second ‘Investissement d’Avenir’ program (reference: ANR-15-RHUS-0002).
References
- Ghio S, Gavazzi A, Campana C, Inserra C, Klersy C, Sebastiani R, et al. Independent and additive prognostic value of right ventricular systolic function and pulmonary artery pressure in patients with chronic heart failure. J Am Coll Cardiol. Janv. 2001; 37:183-188.
- Noly P-E, Dorfmüller P, Fadel E, Mercier O. Capillary density in right ventricular myocardium in congenital heart disease. J Heart Lung Transplant. 2018.
- Sutendra G, Dromparis P, Paulin R, Zervopoulos S, Haromy A, Nagendran J, et al. A metabolic remodeling in right ventricular hypertrophy is associated with decreased angiogenesis and a transition from a compensated to a decompensated state in pulmonary hypertension. J Mol Med. 2013; 91: 1315-1327.
- Murohara T, Ikeda H, Duan J, Shintani S, Sasaki K i, Eguchi H, et al. Transplanted cord blood-derived endothelial precursor cells augment postnatal neovascularization. J Clin Invest. 2000; 105: 1527-1536.
- Peng X-G, Bai Y, James JR, Shlapak DP, Ju S. Transplanted Endothelial Progenitor Cells Improve Ischemia Muscle Regeneration in Mice by Diffusion Tensor MR Imaging. Stem Cells Int. 2016; 2016: 3641401.
- Loisel F, Provost B, Guihaire J, Boulate D, Arouche N, Amsallem M, et al. Autologous Endothelial Progenitor Cell Therapy Improves Right Ventricular Function in a Model of Chronic Thromboembolic Pulmonary Hypertension. The Journal of Thoracic and Cardiovascular Surgery [Internet]. 2018.
- Vrtovec B, Poglajen G, Lezaic L, Sever M, Socan A, Domanovic D, et al. Comparison of transendocardial and intracoronary CD34+ cell transplantation in patients with nonischemic dilated cardiomyopathy. Circulation. 2013; 128: 42-49.
- Mercier O, Tivane A, Dorfmüller P, de Perrot M, Raoux F, Decante B, et al. Piglet model of chronic pulmonary hypertension. Pulm Circ. 2013; 3: 908-915.
- Vanneaux V, El-Ayoubi F, Delmau C, Driancourt C, Lecourt S, Grelier A, et al. In vitro and in vivo analysis of endothelial progenitor cells from cryopreserved umbilical cord blood: are we ready for clinical application? Cell Transplant. 2010; 19: 1143-1155.
- Bensley JG, De Matteo R, Harding R, Black MJ. Three-dimensional direct measurement of cardiomyocyte volume, nuclearity, and ploidy in thick histological sections. Sci Rep. 2016; 6: 23756.
- Mathiasen AB, Qayyum AA, Jørgensen E, Helqvist S, Fischer-Nielsen A, Kofoed KF, et al. Bone marrow-derived mesenchymal stromal cell treatment in patients with severe ischaemic heart failure: a randomized placebocontrolled trial (MSC-HF trial). Eur Heart J. 2015; 36: 1744-1753.
- van der Spoel TIG, Vrijsen KR, Koudstaal S, Sluijter JPG, Nijsen JFW, de Jong HW, et al. Transendocardial cell injection is not superior to intracoronary infusion in a porcine model of ischaemic cardiomyopathy: a study on delivery efficiency. J Cell Mol Med. 2012; 16: 2768-2776.
- Kawamoto A, Tkebuchava T, Yamaguchi J-I, Nishimura H, Yoon Y-S, Milliken C, et al. Intramyocardial transplantation of autologous endothelial progenitor cells for therapeutic neovascularization of myocardial ischemia. Circulation. 2003; 107: 461-468.
- Sheng CC, Zhou L, Hao J. Current stem cell delivery methods for myocardial repair. Biomed Res Int. 2013; 2013: 547902.
- Hare JM, Fishman JE, Gerstenblith G, DiFede Velazquez DL, Zambrano JP, Suncion VY, et al. Comparison of allogeneic vs autologous bone marrow– derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA. 2012; 308: 2369-2379.
- Wu R, Hu X, Wang J. Concise Review: Optimized Strategies for Stem Cell- Based Therapy in Myocardial Repair: Clinical Translatability and Potential Limitation. Stem Cells. 2018; 36: 482-500.
- Golpanian S, Schulman IH, Ebert RF, Heldman AW, DiFede DL, Yang PC, et al. Concise Review: Review and Perspective of Cell Dosage and Routes of Administration From Preclinical and Clinical Studies of Stem Cell Therapy for Heart Disease. Stem Cells Transl Med. 2016; 5: 186-191.
- Cheng Y, Guo S, Liu G, Feng Y, Yan B, Yu J, et al. Transplantation of bone marrow-derived endothelial progenitor cells attenuates myocardial interstitial fibrosis and cardiac dysfunction in streptozotocin-induced diabetic rats. Int J Mol Med. 2012; 30: 870-876.
- Gaffey AC, Chen MH, Venkataraman CM, Trubelja A, Rodell CB, Dinh PV, et al. Injectable shear-thinning hydrogels used to deliver endothelial progenitor cells, enhance cell engraftment, and improve ischemic myocardium. J Thorac Cardiovasc Surg. 2015; 150: 1268-1276.
- Urbich C, Aicher A, Heeschen C, Dernbach E, Hofmann WK, Zeiher AM, et al. Soluble factors released by endothelial progenitor cells promote migration of endothelial cells and cardiac resident progenitor cells. J Mol Cell Cardiol. 2005; 39: 733-742.
- Hu Y, Li L, Shen L, Gao H, Yu F, Yin W, et al. FGF-16 protects against adverse cardiac remodeling in the infarct diabetic heart. Am J Transl Res. 2017; 9: 1630-1640.
- Maddaluno L, Urwyler C, Werner S. Fibroblast growth factors: key players in regeneration and tissue repair. Development. 2017; 144: 4047-4060.
- Suncion VY, Ghersin E, Fishman JE, Zambrano JP, Karantalis V, Mandel N, et al. Does transendocardial injection of mesenchymal stem cells improve myocardial function locally or globally?: An analysis from the Percutaneous Stem Cell Injection Delivery Effects on Neomyogenesis (POSEIDON) randomized trial. Circ Res. 2014; 114: 1292-1301.
- Skuk D, Goulet M, Tremblay JP. Intramuscular transplantation of myogenic cells in primates: importance of needle size, cell number, and injection volume. Cell Transplant. janv 2014; 23: 13-25.
- Abdelwahid E, Kalvelyte A, Stulpinas A, de Carvalho KAT, Guarita-Souza LC, Foldes G. Stem cell death and survival in heart regeneration and repair. Apoptosis. mars 2016; 21: 252-268.
- van den Akker F, Feyen DAM, van den Hoogen P, van Laake LW, van Eeuwijk ECM, Hoefer I, et al. Intramyocardial stem cell injection: go(ne) with the flow. Eur Heart J. 2017; 38: 184-186.