
Review Article
J Stem Cell Res Transplant. 2020; 7(1): 1031.
Bone Marrow/Stem Cell Transplantation for the Treatment of Patients with Malignant Diseases (Leukemia, Lymphoma or Myeloma)
Berhanu AD*, Abrha GT and Kasegn MM
Department of Biotechnology, Mekelle University, Ethiopia
*Corresponding author: Berhanu AD, Department of Biotechnology, Mekelle University, Ethiopia
Received: December 14, 2019; Accepted: January 13, 2020; Published: January 20, 2020
Abstract
A stem cell transplant is a procedure that replaces unhealthy blood-forming cells with healthy ones. The aim of this review is to provide detail information for the readers about the curative treatment option of bone marrow/stem cell transplantations for the treatment of patients with malignant diseases, mainly leukemia, lymphoma or myeloma and to familiarize with a relatively recent technique of bone marrow/stem cell transplants. Bone marrow/Stem cell transplantation procedures continue to be improved, making transplantation a treatment option for more patients. Certain other marrow disorders are also treated with bone marrow/stem cell transplantation. For some patients, this complex procedure offers a curative treatment option. Since blood and marrow are both good sources of stem cells for transplantation, the term “stem cell transplantation” has replaced “bone marrow transplantation” as the general term for this procedure. The abbreviation “BMT” is now used to represent blood and marrow transplantation. There are many terms for transplantation, including Bone Marrow Transplantation (BMT), marrow or umbilical cord blood transplantation, Hematopoietic Stem Cell Transplantation (HSCT). These are all different names for the same procedure. Today, the stem cells used for transplantation can come from marrow, peripheral blood or umbilical cord blood. Peripheral blood is now the most common source of stem cells for transplantation.
Keywords: Blood cells; Cancer; Marrow Disorders; Stem Cells; Sources of Stem Cells
Abbreviations
ALL: Acute Lymphoblastic Leukemia; BMT: Bone Marrow Transplantation; DLI: Donor Lymphocyte Infusion; GVHD: Graft- Versus-Host Disease; HLA: Human Leukocyte Antigens; HSCT: Hematopoietic Stem Cell Transplantation; HZV: Herpes Zoster Virus; NMDP: National Marrow Donor Program; Pbscs: Peripheral Blood Stem Cells; RIC: Reduced Intensity Conditioning Stem Cell Transplant; SCT: Stem Cell Transplantation; SLE: Systemic Lupus Erythematosus
Introduction
Bone marrow/Stem cell transplantation is a procedure that can restore marrow function for patients who have had severe marrow injury or abnormalities of the immune system. Marrow injury can occur because of primary marrow failure, destruction or replacement of marrow by disease, or intensive chemical or radiation exposure. The basis for stem cell transplantation is that all blood cells and immune cells arise from stem cells in marrow. At the turn of the 20th century, scientists began to formulate the idea that a small number of cells in the marrow might be responsible for the development of all blood cells. They began to refer to them as “stem cells.” The scientific exploration of marrow transplantation as a form of cancer treatment began at the end of World War II. Bone marrow transplantation and hematopoietic stem cell transplantation have been used with increasing frequency to treat numerous malignant and nonmalignant diseases forty years ago. Post-World War II “Cold War” fears of nuclear warfare stimulated interest in the effects of radiation on the human body. Early studies with animals quickly revealed that bone marrow was the organ most sensitive to the damaging effects of radiation. The reinfusion of marrow cells was subsequently used to rescue lethally irradiated animals. In the 1950s, patients were given lethal doses of radiation to treat leukemia. Although many had hematologic recovery following this treatment, all patients eventually succumbed to relapse of their malignancies or to infections. In the 1950s and 1960s, almost 200 allogeneic marrow transplants were performed in humans, with no long-term successes. However, during this time, transplantation using identical twin donors brought a fair amount of success and provided a crucial foundation to continued clinical research in the field [1].
In 1968, the first major landmark in bone marrow transplantation occurred with successful allogeneic transplantations performed for an infant with X-linked lymphopenic immune deficiency and for another with Wiskott-Aldrich syndrome [2,3]. These successes were followed by reports of effective transplantation for aplastic anemia and, later, for leukemia. Advances in histocompatibility testing and development of marrow donor registries, such as the National Marrow Donor Program (NMDP), have facilitated the use of unrelated donors, thus expanding the number of patients who can receive transplants [4].
In 1988, successful transplantation occurred in a young boy with Fanconi anemia using umbilical cord blood collected at the birth of his sibling [5]. The patient remains alive and well to this date. In 1992, a patient was successfully transplanted with cord blood instead of bone marrow for the treatment of leukemia. Over the past decade, the use of cord blood has rapidly expanded [6].
The 5-year leukemia-free survival rate in 503 children with Acute Lymphoblastic Leukemia (ALL) who received a transplant of umbilical cord blood that was mismatched for either one or two Human Leukocyte Antigens (HLA) was similar when compared with the survival rate of 282 children who received bone marrow transplants [7].
In addition to bone marrow and cord blood, Peripheral Blood Stem Cells (PBSCs) have gained popularity as a source of stem cells since their initial introduction in the 1980s. The most important cell needed for successful transplantation is the hematopoietic stem cell. Currently, the major sources of stem cells for transplantation include bone marrow, peripheral blood, and cord blood [8].
Necessities of Bone Marrow/Stem Cell Transplantation
Bone marrow basics
Bone marrow is soft, spongy tissue found inside the bone, such as the hip and thighbones. All blood starts out as stem cells, “parent cells” produced in the bone marrow. Stem cells develop into one of the three types of mature blood cells. These are red blood cells, white blood cells and platelets and then enter into the bloodstream. Red blood cells carry oxygen throughout the body. White blood cells fight infection and platelets cause blood to clot. Bone marrow was the first source used for stem cell transplants because it has a rich supply of stem cells. The bones of the hip, chest (sternum/breastbone) and pelvis contain the largest amount of marrow and stem cells. For this reason, cells from these bones are used most often for a bone marrow/stem cell transplant. Enough marrow must be removed to collect a large number of healthy stem cells. For a bone marrow/stem cell transplant, the donor gets general anesthesia (drugs are used to put the patient into a deep sleep). Several large needle sticks are made through the skin into the back of the pelvic bone to remove marrow. The thick, liquid marrow is pulled out through the needle [9].
The harvested marrow is filtered, stored in a special solution in bags, and then frozen. When the marrow is to be used, it is thawed and then given just like a blood transfusion. The stem cells travel to the recipient’s bone marrow. There, they engraft or “take” over time and begin to make blood cells. Signs of the new blood cells usually can be measured in the patient’s blood tests in about 2 to 4 weeks [10].
Why Transplant Bone Marrow/Stem Cell?
The basic idea behind BMT/SCT is to allow high doses of chemotherapy and/or radiation therapy in order to kill rapidly dividing cells in the body and to make room for new healthy cells. Cancer cells, like other cells in the body divide rapidly. Though these treatments are among the most effective weapons against many forms of cancer, they don’t have precise aim and they cannot target only diseased cells. As a result, many normal rapidly dividing cells, including stem cells are also destroyed during the treatment. Therefore, “rescue” with transplanted bone marrow or stem cells enables the patient to produce new blood cells to replace those destroyed during treatment.
Types of Bone Marrow/Stem Cell Transplantations
There are two main types of bone marrow or stem cell transplants: these are autologous (the patient’s own bone marrow or stem cells are used) and allogeneic (a donor supplies the marrow or stem cells). A syngeneic transplant is a type of allogeneic transplant of marrow or stem cells from an identical twin.
Autologous bone marrow/stem cell transplantation
“Auto” means “self.” This type of transplantation involves the use of a patient’s own stem cells. Immature marrow cells (stem cells) are collected from the patient himself/herself before he/she receives high-dose chemotherapy or radiation treatment, and then frozen. The thawed cells are returned to the patient after he or she has received intensive chemotherapy and/or radiation therapy for his or her disease. This is called a “rescue” transplant [11]. The primary purpose of an autologous transplant is to allow the patient to be given high doses of chemotherapy with or without radiation that would otherwise be too toxic to tolerate because the marrow would be severely damaged. Such high doses of treatment can sometimes overcome resistance of the disease to standard doses of chemotherapy. Autologous bone marrow/stem cell transplantation is used mainly to treat people who have a blood cancer diagnosis, but may be used to treat patients who have some other types of cancer. Autologous transplantation requires that an individual have sufficient numbers of healthy stem cells in the marrow or blood. For example, in patients with acute leukemia, remission must be achieved before the patient’s marrow or blood is harvested and frozen for later use (Figure 1).
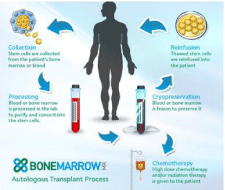
Figure 1: Autologous Transplantation.
Source: https://www.google.com/search?q=Bone+marrow+transplantation.
The diagram shown below explains the steps involved in autologous stem cell transplantation.
• The patient is treated to control the disease and to markedly decrease the number of cancer cells in marrow and blood.
• The stem cells from blood or marrow are then harvested. If blood is used as the source of stem cells, the patient is treated with a stem cell mobilizer after chemotherapy, which draws stem cells out of the marrow and into the blood. If the marrow is the source of stem cells, the marrow is removed under sterile conditions in the operating room while the patient is anesthetized.
• The cells are mixed with a cry protective agent so that they can be frozen and later thawed without injury.
• At a later time, when the patient is treated intensively with chemotherapy and/or total body radiation that destroys the marrow function, the frozen stem cell collection is thawed and infused into the patient so that blood cell production can be restored.
This procedure is also referred to as “autologous stem cell infusion,” because stem cells are not being transferred from one person to another. Since the stem cells are the recipient’s own, Graft-Versus-Host Disease (GVHD) is rarely a problem. However, the patient’s immune system does require time to recover after the procedure, and risk of relapse of the person’s disease may be higher.
Tandem transplants: Tandem transplant is an example of autologous transplant. In a tandem transplant, a patient gets 2 courses of high-dose chemo, each followed by a transplant of their own stem cells. All of the stem cells needed are collected before the first highdose chemo treatment, and half of them are used for each procedure. Most often both courses of chemo are given within 6 months, with the second one done after the patient recovers from the first one. A tandem transplant is also called a double autologous transplant. Tandem transplants are sometimes used to treat certain types of cancer, but doctors do not agree on when and how to use this type of transplant. For many people, the risk of serious outcomes is quite high. Tandem transplants are still being studied to find out when they might be best used [12].
Allogeneic bone marrow/stem cell transplantation
“Allo” means “other.” In allogeneic transplants, patients receive stem cells from their brother, sister, or parent i.e. a person who is not related to the patient called an unrelated donor [13]. With an allogeneic transplant, the stem cells come from a donor often a sibling but sometimes another volunteer whose cells are considered a “match” for the patient (Figure 2). The process of finding a match is called tissue typing or human leukocyte antigen typing. HLA is a protein on the surface of blood cells [14].
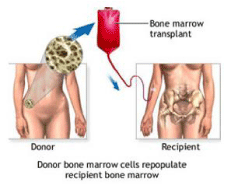
Figure 2: Allogenic Transplantation.
Source: https://www.google.com/search?q=Bone+marrow+transplantation.
Most times, the donor must have the same genetic makeup as the patient, so that their blood is a “match” to the patient’s. People have different sets of proteins (Human Leukocyte-Associated (HLA) antigens) on the surface of their cells. The set of proteins called the HLA type is identified by a special blood test [15].
The best-known HLA antigens are (HLA A, HLA B, HLA C, HLA DR, HLA DQ, and HLA DP). In most cases, the success of allogeneic transplantation depends in part, on how well the HLA antigens of the donor’s stem cells match those of the recipient’s stem cells. The higher the number of matching HLA antigens, the greater the chance that the patient’s body will accept the donor’s stem cells. In general, patients are less likely to develop a complication known as Graft-Versus-Host Disease (GVHD) if the stem cells of the donor and patient are closely matched. Today, fewer tests may be needed on siblings, since their cells vary less than an unrelated donor. But on unrelated donors, more than the basic 6 HLA antigens are often tested to reduce the risk of graft-versus-host disease [16].
Close relatives, especially brothers and sisters, are more likely than unrelated people to be HLA-matched. However, only 25 to 35 percent of patients have an HLA-matched sibling. The chances of obtaining HLA-matched stem cells from an unrelated donor are slightly better, approximately 50 percent. Among unrelated donors, HLA-matching is greatly improved when the donor and recipient have the same ethnic and racial background. Although the number of donors is increasing overall, individuals from certain ethnic and racial groups still have a lower chance of finding a matching donor.
Pros and Cons of allogeneic stem cell transplants
Pros of allogeneic stem cell transplant: The donor stem cells make their own immune cells, which could help destroy any cancer cells that remain after high-dose treatment. This is called the graftversus- cancer effect. Other advantage is that the stem cells from healthy donors are free of cancer cells.
Cons of allogeneic stem cell transplant: The transplant, also known as the graft, might not take that is, the donor cells could die or be destroyed by the patient’s body before settling in the bone marrow. Another risk is that the immune cells from the donor may not just attack the cancer cells – they could attack healthy cells in the patient’s body. This is called graft-versus- host disease.
Syngeneic stem cell transplant: This is a special kind of allogeneic transplant that can only be used when the recipient has an identical sibling (twin or triplet) who can donate - someone who will have the same tissue type. Identical twins/triplets have the same genes, they also have the same set of human leukocyte associated antigens. As a result, there is less chance of the transplant being rejected and graft-versus-host disease will not be a problem, this means the patient’s body will accept a transplant from an identical twin. Since identical twins represent a small number of all births, so syngeneic transplantation is rare [17].
A disadvantage is that because the new immune system is so much like the recipient’s immune system, there is no graft-versuscancer effect, either. Every effort must be made to destroy all the cancer cells before the transplant is done to help keep the cancer from relapsing (coming back).
Non-myeloablative allogenic transplants (mini-transplants): Some people have health conditions that would make it more risky to wipe out all of the bone marrow before a transplant. For those people, doctors can use a type of allogeneic transplant that is sometimes called a “mini-transplant.” Compared with a standard allogeneic transplant, this one uses less chemo and/or radiation to get the patient ready for the transplant. The doctor may refer to it as a non-myeloablative transplant or mention Reduced-Intensity Conditioning (RIC). The idea here is to kill some of the cancer cells, some of the bone marrow, and suppress the immune system just enough to allow donor stem cells to settle in the bone marrow [18].
Unlike the standard allogeneic transplant, cells from both the donor and the patient exist together in the patient’s body for some time after a mini-transplant. But slowly, over the course of months, the donor cells take over the bone marrow and replace the patient’s own bone marrow cells. These new cells can then develop an immune response to the cancer and help kill off the patient’s cancer cells - the graft-versus-cancer effect [19].
One advantage of a mini-transplant is the lower doses of chemo and/or radiation. And because the stem cells are not all killed, blood cell counts do not drop as low while waiting for the new stem cells to start making blood. This makes it especially useful in older patients and those with other health problems who aren’t strong enough for a standard allogeneic stem cell transplant. It may rarely be used in patients who have already had a transplant [20].
Mini-transplants have been found to treat some diseases better than others. They may not work well for patients with a lot of cancer in their body at the time of transplant or those with fast-growing cancer. Also, the lowered immune response can still lead to graftversus- host disease [21].
This procedure is actively being studied, but it has only been in use since the late 1990s and long-term patient outcomes are not yet available. There are lower risks of complications, but the cancer may be more likely to return (relapse). Ways to improve the outcomes are still being studied [22].
Another future possibility is autologous transplant followed by an allogeneic mini-transplant. This is being tested in certain types of cancer, such as multiple myeloma. The autologous transplant can help decrease the amount of cancer present so that the lower doses of chemo given before the mini-transplant can work better. And the recipient still gets the benefit of the graft-versus-cancer effect of the allogeneic transplant [23].
Sources of Stem Cells for Transplantation
There are 3 possible sources of stem cells to use for transplants: bone marrow, the bloodstream (peripheral blood), and umbilical cord blood from newborns. Although bone marrow was the first source used in stem cell transplant, peripheral blood is used most often today.
Bone marrow stem cells
Bone marrow stem cells [24] are collected from the patient’s hip bone through a surgical procedure. The stem cells used in BMT come from the liquid center of the bone called the marrow [25,26]. In general, the procedure for obtaining bone marrow, which is called “harvesting,” is similar for all types of BMTs. The donor is given either general anesthesia, which puts the person to sleep during the procedure, or regional anesthesia, which causes loss of feeling below the waist. Needles are inserted through the skin over the pelvic bone or, in rare cases, the breastbone and into the bone marrow to draw the marrow out of the bone [27]. Harvesting the marrow takes about an hour. The harvested bone marrow is then processed to remove blood and bone fragments. Harvested bone marrow can be combined with a preservative and frozen to keep the stem cells alive until they are needed. This technique is known as cryopreservation [28]. Stem cells can be cryopreserved for many years.
Peripheral blood stem cells
Peripheral blood stem cells are now the most common source of stem cells for transplantation. They can be harvested from the donated blood [29]. Normally, few stem cells are found in the blood, but by giving hormone-like substances called growth factors to stem cell donors, the donors stem cells can grow faster and move from the bone marrow into the blood within a few days before the harvest carried out [30]. The stem cells are separated and collected and the rest of the blood is returned to the donor. The stem cells used in PBSCT come from the bloodstream. A process called apheresis or leukapheresis is used to obtain PBSCs for transplantation [31]. For 4 or 5 days before apheresis, the donor may be given a medication to increase the number of stem cells released into the bloodstream. In apheresis, blood is removed through a large vein in the arm or a central venous catheter in which a flexible tube that is placed in a large vein in the neck, chest, or groin area. The blood goes through a machine that removes the stem cells. The blood is then returned to the donor and the collected cells are stored. Apheresis typically takes 4 to 6 hours. The stem cells are then stored in bags, and frozen until they are given to the patient. After the patient is treated with chemo and/or radiation, the stem cells are given in an infusion much like a blood transfusion. The stem cells travel to the bone marrow, engraft, and then grow and make new, normal blood cells. The new cells are usually found in the patient’s blood a few days sooner than when bone marrow stem cells are used, usually in about 10 to 20 days [32].
Cord blood stem cells
The use of cord blood stem cells for transplantation is about two decades old. Compared to peripheral blood or marrow, cord blood is a relatively new source of cells, especially for adults. Cord blood stem cells are collected from a mother’s placenta immediately after a child is born [33]. Stem cells also may be retrieved from umbilical cord blood. After the baby is born and the umbilical cord has been cut, blood is retrieved from the umbilical cord and placenta. This process poses minimal health risk to the mother or the child. If the mother agrees, the umbilical cord blood is processed and frozen for storage by the cord blood bank. Only a small amount of blood can be retrieved from the umbilical cord and placenta, so the collected stem cells are typically used for children or small adults.
Not everyone who needs an allogeneic stem cell transplant can find a well-matched donor among the people who have signed up to donate. For these patients, umbilical cord blood may be a potential source of stem cells. Around 30% of unrelated hematopoietic stem cell transplants now come from cord blood. A large number of stem cells are normally found in the blood of newborn babies. After birth, the blood that is left behind in the placenta and umbilical cord (known as cord blood) can be taken, stored and frozen for later use in a stem cell transplant [34].
A possible drawback of cord blood is the smaller number of stem cells present. But this is partly balanced by the fact that each cord blood stem cell can form more blood cells than a stem cell from adult bone marrow. Still, it can take cord blood transplants longer to engraft and start working. To be safe, most cord blood transplants done so far have been in children and smaller adults. Researchers are now looking for ways to use cord blood for transplants in larger adults. One approach that is being taken is to find ways to increase the numbers of these cells in the lab before the transplant. Another approach is the use of the cord blood from 2 infants at the same time for one adult transplant, called a double-unit cord blood transplant [35].
How Does Bone Marrow/Stem Cell Transplantation Work?
Stem cells are predominately found in the bone marrow, but some occupy the bloodstream. These are called Peripheral Blood Stem Cells (PBSCs). If PBSCs are used in the transplant, it is referred to as Peripheral Blood Stem Cell Transplantation (PBSCT). Umbilical cord blood also contains stem cells that can be used in stem cell transplantation. When stem cells are transplanted, they enter the peripheral blood stream and travel to the bone marrow where they replace damaged stem cells and begin to make healthy blood cells. One advantage of using stem cells is that they can rapidly repopulate the full complement of blood and immune cells. Interestingly, it seems that the implanted stem cells find their own way to the bone marrow, as if they have a homing mechanism in place [36].
Types of Cancer (Malignant Diseases) Treated with BMT/SCT
BMT/SCT is most commonly used in the treatment of leukemia and lymphoma. They are most effective when the leukemia or lymphoma is in remission (the signs and symptoms of cancer have disappeared). BMT/SCT is also used to treat other cancers such as neuroblastoma (cancer that arises in immature nerve cells and affects mostly infants and children) and multiple myeloma. Researchers are evaluating BMT/SCT in clinical trials (research studies) for the treatment of various types of cancer [37].
How Can Bone Marrow/Stem Cell Transplantation Treat Cancer?
A bone marrow/stem cell transplant does not kill or destroy cancer cells. However, BMT/SCT allows patients to participate in treatment strategies that would otherwise be life threatening. These strategies include high-dose chemotherapy and/or radiation therapy. Chemotherapy and radiation therapy are designed to kill and/or slow the progression of cells that divide rapidly. As a result, these therapies are used to treat cancer because cancer cells divide quickly, without control or order. Stem cells and other immature cells throughout the body also divide frequently. This means that high-dose chemo and radiotherapy will not only destroy cancer cells, but they will also destroy a patient’s healthy bone marrow [38].
Without healthy bone marrow, the body is vulnerable to infection, devoid of oxygen, and prone to bleeding. A bone marrow transplant restores the stem cells that have been destroyed by chemotherapy and/or radiation therapy. These transplanted cells allow the bone marrow to produce blood cells once again [39].
Benefits and risks of stem cell transplantation
Benefits of stem cell transplantation: Advancements in stem cell therapies and tissue engineering hold great promise for regenerative medicine [40]. Stem cell transplants are used for treating patients whose stem cells have been damaged by disease or for treating the disease [41]. Stem cell transplants with kidney damage from pyelonephritis a type of urinary infection that has reached the kidney was found to improve kidney structure and function [42]. In various studies it’s proved that the patients remained free from Systemic Lupus Erythematosus (SLE) and improved continuously after highdose chemotherapy and hematopoietic stem-cell transplantation [43]. BMT and PBSCT are most commonly used in the treatment of leukemia and lymphoma. They are most effective when the leukemia or lymphoma is in remission i.e. the signs and symptoms of cancer have disappeared [44]. BMT and PBSCT are also used to treat other cancers such as neuroblastoma (cancer that arises in immature nerve cells) and affects mostly infants and children and multiple myeloma [45]. Stem cell transplantation may be an effective treatment for high risk myeloma patients with certain chromosomal abnormalities was proved recently. Autologous adult stem cell transplantation has been the latest tool in regenerative medical therapy and in cardiovascular diseases [46]. Autologous transplantation seems to be superior to both chemotherapy and allogeneic transplantation for treatment of multiple myeloma but Syngeneic transplantation appears to be as good as autologous transplantation and therefore it is used to perform in patients with the disease whenever there is an identical twin donor available [47].
Risks of stem cell transplantation: A stem cell transplant is a complex procedure with risks. Before a stem cell transplant, the patient receives chemotherapy and occasionally radiation therapy to destroy their unhealthy stem cells. This is called a preparative regimen. Some of the short-term side effects, such as nausea, vomiting, fatigue, loss of appetite, mouth sores, hair loss, and skin reactions may be due to the preparative regimen [48]. And the potential long-term risks include complications of the pretransplant chemotherapy and radiation therapy, such as infertility (the inability to produce children); cataracts (clouding of the lens of the eye, which causes loss of vision); secondary (new) cancers; and damage to the liver, kidneys, lungs, and/or heart.
Some other risks
Infection: After the transplant, before the new marrow has started to grow, the number of white blood cells is low and the immune system (how the body fights infection and stays healthy) is very weak. During this time, the body is susceptible to infections, sometimes from the bacteria that live in the patient’s own body. Therefore, infections that normally would not be harmful can be very serious, and patients can die of them. Bacterial, viral, and fungal infections are often seen following transplant. To prevent this, doctors may give the patient antibiotics to prevent or treat infection.
Bleeding: There is a risk from internal bleeding during the period before the platelet count recovers. This can be guarded against with platelet transfusions. The introduction of peripheral blood stem cell transplants has reduced the length of time the platelet count takes to recover to normal and so lessened the risk from bleeding. A growth factor that stimulates platelet production has been developed but results from clinical trials have not been promising and therefore this is not in routine use.
Anaemia: Inevitably, during the transplant procedure, the patient will fail to produce enough red blood cells and will therefore become anaemic. This is monitored by regular blood counts and is treated with blood transfusions.
Mucositis: Almost all transplant patients experience sores in their mouths and/or gut, called mucositis. These are caused when chemotherapy or radiotherapy damage the rapidly dividing cells that form the lining of the digestive tract. The more intensive the conditioning treatment is the more likely the patient is to develop mucositis, and the more likely it is to be severe.
Mucositis leaves the patient vulnerable to infection so antibacterial and antifungal preparations such as mouthwashes may be prescribed to reduce the risk.
Graft-versus-host disease (GVHD): Graft versus host disease (GVHD) is a common complication of allogeneic transplants. It occurs when the new stem cells (from the donor) do not recognize the patient’s cells and attacks them, leading to skin rashes, diarrhea, or liver abnormalities. This complication can develop within a few weeks of the transplant (acute GVHD) or much later (chronic GVHD) and range in severity from mild to moderate to severe. To prevent this complication, the patient may receive medications that suppress the immune system. Additionally, the donated stem cells can be treated to remove the white blood cells that cause GVHD in a process called “T-cell depletion.” Mild and moderate GVHD can be treated successfully with drugs and does not increase the risk of the patient dying. The most severe degree of GVHD is less frequent, but very serious, and patients can die of this complication. Generally, if GVHD develops, it can be very serious and is treated with steroids or other immunosuppressive agents. The severe GVHD can be difficult to treat, but some studies suggest that patients with leukemia who develop GVHD are less likely to have the cancer come back. Clinical trials are being conducted to find ways to prevent and treat GVHD [49].
Types of Graft Versus Host Disease
Acute graft versus host disease
When GvHD occurs within the first 100 days after transplant it is termed acute GvHD. This form typically causes a severe rash and may also attack cells in the liver and gut leading to nausea, vomiting, diarrhoea and jaundice.
Factors known to increase the risk of acute GvHD include: Increasing age of the patient or donor, a donor who had been pregnant in the past, any donor who had received a blood transfusion, reduced doses of immunosuppressive drugs post-transplant, less exact tissue match between donor and recipient.
Acute GvHD can be classified as grade I to grade IV on the extent and severity of the condition. Grade I may require no treatment, although some centers recommend treatment if the transplant is from an unrelated donor. Grade II or higher is considered moderate to severe and always requires treatment. Moderate to severe acute GvHD is a serious and potentially life-threatening complication of allogeneic stem cell transplantation.
Acute GvHD can be prevented by giving more intensive treatment to suppress the immune reaction post-transplant but this increases the risk of serious infections. Removal of a particular type of lymphocyte from the graft has also been shown to reduce the risk of acute GvHD but this increases the risk of graft rejection, serious infections and of relapse.
Once moderate to severe acute GvHD has been diagnosed the first choice of treatment is usually steroids (prednisolone) to increase the level of immunosuppression. If this is unsuccessful then a drug called Atgam™ (antithymocyte globulin) may be used to reduce the numbers of T lymphocytes which play a key role in causing and sustaining graft versus host disease.
Chronic graft versus host disease
Chronic GvHD can present in many different ways. In the majority of cases it affects the skin. It can also affect the lungs, liver and immune system. It may resemble certain auto-immune diseases, which may confuse the diagnosis. Chronic GvHD may present in patients in the absence of acute GvHD (‘de novo onset’), in patients who had acute GvHD which resolved fully (‘quiescent onset’), or following persistent acute GvHD (‘progressive onset’). It may develop at any time from three months post-transplant to six months after the end of immunosuppressive treatment.
Known factors to increase the risk of chronic GvHD include: Increasing age of the patient or donor, prior acute GvHD, Donor Lymphocyte Infusion (DLI), infection with the Herpes zoster virus (HZV), the cause of chickenpox and shingles.
The treatment of choice for most patients with chronic GvHD is with high dose steroids (prednisolone). If the patient has poor-risk features such as progressive onset chronic GvHD, low platelets, or jaundice then ciclosporine or a drug called tacrolimus may be used alongside prednisolone. Limited chronic GvHD (in skin, liver or both) has a good prognosis. If the condition is more widespread, the long-term outlook is poorer. The commonest cause of death related to chronic GvHD is infection and patients will usually receive multidrug antibiotic therapy in an attempt to prevent this complication.
Transplant rejection (Graft Failure)
Graft failure happens when the patient’s immune system attacks the new stem cells from the donor. This happens because the patient’s immune system thinks the new cells are harmful and need to be destroyed. If the immune system wins the fight, the old stem cells will come back, along with the disease (cancerous or aplastic anemia).
There are three types of rejection:
• Hyperacute rejection occurs a few minutes after the transplant, if the antigens are completely unmatched. The tissue must be removed right away so the recipient does not die. This type of rejection is seen when a recipient is given the wrong type of blood.
• Acute rejection may occur any time from the first week after the transplant to 3 months afterward. Everyone has some amount of acute rejection.
• Chronic rejection takes place over many years. The body’s constant immune response against the new cell slowly damages the transplanted stem cell.
Conclusions
From the overall of this review, it can be concluded that, prior to a transplant patients must receive a conditioning regimen of drugs and/or radiotherapy, in order to destroy the bone marrow cells. This is called myeloablation. In the case of a donor transplant, this is necessary to prevent rejection of the donor cells. For many patients who are older and/or have other illnesses this procedure is too stressful and therefore they are unable to receive a donor transplant. A Reduced Intensity Conditioning (RIC) stem cell transplant, sometimes known as a ‘mini-transplant’ or as ‘transplant-lite’, is a recent innovation and may offer an alternative to this group of patients because it uses lower doses of drugs and radiation to suppress the patient’s immune response sufficiently to allow donor cells to become established.
The major limitation of bone marrow/stem cell transplantation is the ability of the patient to withstand the high doses of chemotherapy and radiotherapy that are typically given before the transplant. Unfortunately, many of the conditions for which a donor stem cell transplant is the only curative option mainly affect older patients. Basically two types of transplants (autologous and allogeneic) are used for the treatment of patients with certain malignant diseases. One of the benefits of autologous transplantation is that there is no need to search for a related or unrelated donor, and therefore, autologous transplant is generally considered to be less dangerous than an allogeneic transplant.
Acknowledgements
The authors thank Dr. Hagos Abraha Ayder Comprehensive Specialized Hospital and Dr. Hagazi Fantay College of Veterinary Science, Mekelle University, for reviewing the manuscript and providing valuable suggestions.
References
- Bmtinfonet.org. 2019.
- Gatti RA, Meuwissen HJ, Allen HD. Immunological reconstitution of sexlinked lymphopenic immunological deficiency. LANCET. 1980; 2: 1366-1369.
- Bortin MM. A compendium of reported human bone marrow transplants. Transplantation. 1970; 9: 571-587.
- National Marrow Donor Program. 2019.
- Gluckman E, Broxmeyer HA, Auerbach AD. Hematopoietic reconstitution in a patient with Fanconi’s anemia by means of umbilical-cord blood from an HLAidentical sibling. N. ENGL. J. MED. 1989; 321: 1174-1178.
- Rubenstein P, Carrier C, Scaradavou A. Outcomes among 562 recipients of placental-blood transplants from unrelated donors. N. ENGL. J. MED. 1998; 339: 1565-1577.
- Eapen M, Rubinstein P, Zhang MJ. Outcomes of transplantation of unrelated donor umbilical cord blood and bone marrow in children with acute leukaemia: a comparison study. LANCET. 2007; 369: 1947-1954.
- Stem Cell Transplant for Cancer.
- Bone marrow Harvest. 2016.
- Engraftment syndrome following autologous hematopoietic stem cell transplantation: definition of diagnostic criteria.
- Mineishi S, Ferrara JLM. Autologous bone marrow transplantation. In: DeVita VT, Lawrence TS, Rosenberg SA, eds. Cancer: Principles and Practice of Oncology. 8th ed. Philadelphia, Pa: Welters’ Kluwer/Lippincott Williams & Wilkins. 2008; 2541-2548.
- University of Iowa Health Care Integrated Strategic Plan, 2017-2020.
- Cancer.org.
- Harring TR, Nguyen NT, Goss JA, O’ Mahony, CA. Human T-Cell Lymphoma Virus-Positive Allograft Used For Effective Orthotopic Liver Transplantation: A Case Report and Review of the Literature. J Transplant Technol Res. 2011; 1: 102.
- Dai W, Hale SL, Martin BJ, Kuang JQ, Dow JS. Allogeneic Mesenchymal Stem Cell Transplantation in Postinfarcted Rat Myocardium: Short- and Long- Term Effects. Circulation. 2005; 112: 214-223.
- UCDAVIS HEALTH.
- Gallardo D, de la Cámara R, Nieto JB, Espigado I, et al. Is mobilized peripheral blood comparable with bone marrow as a source of hematopoietic stem cells for allogeneic transplantation from HLA-identical sibling donors? A case-control study. Haematologica. 2009; 94: 1282-1288.
- Engman, CA, Hill, JM, Meehan, KR. Syngeneic Transplant in Mantle Cell Lymphoma: A Rare Event and Review of the Literature. Clin Adv Hematol Oncol. 2009; 7: 321-324.
- Carella AM, Beltrami G, Corsetti MT, Scalzulli P, Carella AM, Jr. & Musto P. A reduced intensity conditioning regimen for allografting following autografting is feasible and has strong antimyeloma activity. Haematologica. 2004; 89: 1534-1536.
- Aschan J, Lonnqvist B, Ringden O, Kumlien G & Gahrton G. Graft-versuscancer effect. Lancet. 1996; 348 346.
- Lymphomation.org.
- Anderson LD, Savary CA, Mullen CA. Immunization of allogeneic bone marrow transplant recipients with tumor cell vaccines enhances graft-versustumor activity without exacerbating graft-versus-host disease. Blood. 2000; 95: 2426-2433.
- Lymphomation.org.
- The allogeneic graft-versus-cancer effect.
- Wilson A, Trumpp A. Bone-marrow haematopoieticstem- cell niches. Nat Rev Immunol. 2006; 6: 93-106.
- Gottlieb H, Klausen TW, Boegsted M, Olsen BS, Lausten GS. A Clinical Study of Circulating Cellular and Humoral Biomarkers Involved in Bone Regeneration Following Traumatic Lesions. J Stem Cell Res There. 2011; 1: 108.
- Hwang J, Lee S, Park H, Kim M. Autologous Bone Marrow Transplantation in Osteonecrosis of the Femoral Head. J Tissue Sci Eng. 2010; 2: 103.
- Argento MA, Manara LRB, Berni VC, Cortelazzo AL. Flapless Technique for Periodontal Bone Grafts in Treatment of Severe Periodontitis. Presentation and Long-Term Retrospective Study. J Microbial Biochem Technol. 2010; 2: 107-110.
- Ito J. What contributes to the success of in vitro fertilization using cryopreserved spermatozoa in rodents? J Fertiliz In Vitro. 2011; 1: 102.
- Stem cells: What they are and what they do.
- Medicinenet.com.
- Weaver CH, Buckner CD, Longin K, Appelbaum FR, Rowley S. Syngeneic Transplantation with Peripheral Blood Mononuclear Cells Collected After the Administration of Recombinant Human Granulocyte Colony- Stimulating Factor. Blood. 1993; 82: 1981-1984.
- “Peripheral Blood Stem Cell Harvest”. 2016.
- Stem Cell Transplant for Cancer.
- Marrow.org.
- Majhail NS, Brunstein CG, Wagner JE. Double umbilical cord blood transplantation. CURR OPIN IMMUNOL. 2006; 18: 571-575.
- 43 Supplements Exposed: Which Ones to Consider, Which Ones to Avoid.
- Bhatia S, Bhatia R. Transplantation-Related Malignancies. In: DeVita VT, Lawrence TS, Rosenberg SA, eds. Cancer: Principles and Practice of Oncology, 8th ed. Philadelphia, Pa: Wolters Kluwer/Lippincott Williams & Wilkins. 2008; 2417-2426.
- Bone Marrow Transplant.
- Irioda AC, Zocche L, Souza CMCO, Ferreira RJ, Aliprandini E. Pap Test as the First Step in Screening Genetic Stability in Cell- Based Therapy. J Stem Cell Res Ther. 2011; 1: 106.
- Harring TR, Kuten DA, Nguyen NT, Goss JA, O’Mahony CA. Orthotopic Liver Transplantation in Patients with Mixed Hepatocellular Carcinoma Cholangiocarcinoma. J Transplant Technol Res. 2011; 1: 104.
- A Traynor AE, Schroeder J, Rosa RM, Cheng D, Stefka J. Treatment of severe systemic lupus erythematosus with high-dose chemotherapy and haemopoietic stem-cell transplantation: a phase I study. Lancet. 2000; 356: 701-707.
- Verma D, Agarwal K, Wadhwa M, Shukla S, Prakash O. T-Cell Lymphoblastic Lymphoma/Leukemia of Tenon’s Capsule of Eye: An Unusual Presentation. J Clinic Experiment Ophthalmol. 2011; 2: 189.
- Avramidis D, Cruz M, Sidén Å, Tasat DR, Yakisich JS. Regrowth Concentration Zero (RC0) as Complementary Endpoint Parameter to Evaluate Compound Candidates During Preclinical Drug Development for Cancer Treatment. J Cancer Sci Ther. 2009; 1: 19-24.
- Jayashankar E, Roshinipaul T. Prognostication of Histomorphological Characteristics in Multiple Myeloma. J Cancer Sci Ther. 2010; 2: 153-156.
- Patel AN, Geffner L, Vina RF, Saslavsky J, Urschel HC. Surgical treatment for congestive heart failure with autologous adult stem cell transplantation: A prospective randomized study. J Thorac Cardiovasc Surg. 2005; 130: 1631- 1638.
- Gahrton G, Svensson H, Björkstrand B, Apperley J, Carlson K. Syngeneic transplantation in multiple myeloma - a case-matched comparison with autologous and allogeneic transplantation. Bone Marrow Transplant. 1999; 24: 741-745.
- About Cancer.
- Ludajic K, Balavarca Y, Bickeböller H. Minor ABO-mismatches are risk factors for acute graft-versus-host disease in hematopoietic stem cell transplant patients. Biol Blood Marrow Transplant. 2009; 15: 1400-1406.