
Review Article
J Stem Cell Res Transplant. 2021; 8(1): 1034.
Overview: Autologous Peripheral Blood Mononuclear Cells
Petrungaro A*, Scudo F and Quartarone E
Unit of Transfusion Medicine, Department of Services, University Hospital “G. Martino”, Via Consolare Valeria, 1 98100 Messina, Italy
*Corresponding author: Petrungaro Annamaria, Unit of Transfusion Medicine, Department of Services, University Hospital “G. Martino”, Via Consolare Valeria, 1 98100 Messina, Italy
Received: February 13, 2021; Accepted: March 09, 2021; Published: March 16, 2021
Keywords
Autologous Peripheral Blood Mononuclear Cells; Pall Celeris System; Wound healing Macrophages
Background
The implant of autologous mononuclear cells is an avant-garde method based on the use of a cell population within our body to regenerate tissues that have been damaged by various pathological events. The biological assumption is the richness and complexity of biochemical and cellular phenomena inherent in both organism response to damage and tissue regeneration. The key role is played by the mononuclear phagocyte system which regulates and modulates the activity of mesenchymal stem cells capable of differentiating and providing tissue repair. This system does not only have a traditional “scavenger” function within the immune system, but it is also of central importance in the modulation and activation of the response to tissue damage, be it traumatic, surgical, or degenerative. In this review we summarize the main features of this method and the most common uses in clinical practice where the interest is growing considering both the powerful, rapid and documented regenerative response of the various “target” tissues: vascular, cartilage, bone, muscle and tendon tissue.
Autologous Peripheral Blood Mononuclear Cells
Definition
Autologous Peripheral Blood Mononuclear Cells (PBMC) are an autologous cell concentrate (monocytes, macrophages, B-T lymphocytes, Natural Killer cells, CD34+ hematopoietic stem cells, KDR+ stem cells, Endothelial Progenitors Cells-EPC) of the latest generation with high angiogenic and regenerative capacity, taken from the patient’s peripheral blood [1].
Mechanism of action
Cell therapy with PBMNC is based on mimicking the physiological response of these cells to tissue and ischemic damage, increasing their speed and intensity of action through implantation into the damaged/ ischemic tissue. The angiogenic effect is fundamentally paracrine and consists of the release by monocytes of angiogenic cytokines (VEGF and bFGF) and growth factors [2,3]. Macrophages (MPs) are critical for tissue repair and regeneration but can also contribute to tissue damage and the genesis of fibrosis. MPs can have a variety of functional phenotypes in response to different stimuli; two of the best characterized in vitro phenotypes are a proinflammatory “M1” phenotype, produced by exposure to IFN-γ and TNF-a; and an antiinflammatory “M2a” phenotype, produced by the presence of IL-4 or IL-13.M2a Mp, often referred to as “wound healing” Mp, as it is involved in tissue repair [4]. MPs show a “classically activated” or M1 proinflammatory phenotype during the early stages of tissue regeneration in response to pathogens, IFN-γ and TNF-a, and later an M2a reparative phenotype [5-8]. However, phenotypic markers that appear to be coregulated, in cultures MPs can be expressed independently of each other in vivo.
It was initially thought that naïve MPs were activated upon exposure to bacterial components and that IFN-γ and TNF-a increased production of inflammatory cytokines and ROS by enhancing their killing ability. In contrast, the main effect of antiinflammatory cytokines on MPs was assumed to be deactivation, hence resolving the inflammation. However, Stein et al demonstrated that IL-4 is not only a “signal off” for immune activation of MPs as it also increases CD206 mannose receptor activity and enhances endocytosis of mannosylated ligands of murine MPs. Therefore, IL-4- treated MPs were defined as “alternatively” activated compared with “classical” activation by IFN-γ/TNF-a, and the concept of general deactivation thought to occur previously with IL-4 treatment was lacking [9].
Other studies have shown that stimuli previously considered MPs “deactivators” produce different phenotypes of macrophage activation in vitro. The term “alternative” activation has thus been coined to include these new phenotypes, now referred to as M2a/b/c, whereas “classical” activation is referred to as M1 [10].
The M2a phenotype is produced in vitro by exposure to IL-4 or IL-13, which act through the common IL-4Ra receptor with increased expression of CD206, arginase, and TGF-β [11-13]. The M2b phenotype is produced by exposure to a combination of IgG and LPS immune complexes, which increases IL-10 production and decreases IL-12 production, conferring potent anti-inflammatory properties [14,15]. In vitro exposure to IL-10 or glucocorticoids produces the M2c phenotype, which is characterized by high IL-10 and low IL-12 production [15] as well as the increased expression on the cell surface of the CD163 scavenger receptor [16,17]. The Mp phenotype can also be altered by phagocytosis of apoptotic or necrotic cells [18,19]. Together, these studies have shown that far from being simply activated or deactivated by proinflammatory versus anti-inflammatory cytokines, MPs can adopt a variety of different phenotypes in response to different stimuli.
Product Requirements, Collection Methods and Clinical Use
PBMNCs are autologous blood components; thus, they are collected, prepared, and stored in the same manner as allogeneic blood components; and are identified, stored, transported, and distributed separately from blood components for allogeneic use.
They are blood components for non-transfusion use and can be used according to the following uses established by law:
• Use on skin or mucous surfaces (topical use);
• Intra-tissue or intra-articular infiltration;
• As a material to be applied locally at surgical sites, alone or in combination with non-cellular biological material (e.g., bone bank tissue) or medical devices;
• As material to be used “in vitro”, in laboratory procedures, for approved clinical studies according to current regulations.
The production, identification, traceability, appropriateness, informed consent, and consent to the processing of personal data in the context of clinical trials, allocation, delivery, and hemovigilance of blood components to be used for non-transfusion use, the same regulatory provisions apply as for blood components for transfusion use, except as provided in paragraph 7 below [20]. Autologous blood components for non-transfusion use are produced within the Transfusion Services or their organizational units; the production may take place outside the Transfusion Services within the limits and in the manner specified in the SIMTI Recommendations [22,23]. They are obtained from a patient with no risk of bacteremia; biological validation tests must be performed at the beginning of a therapeutic cycle, with a maximum validity period of 30 days; biological validation tests can be omitted if sampling, production, and application are planned in a single session without any preservation of the product [23].
The collection of PBMNCs, to perform a proper cell therapy with high regenerative angiogenic capacity, involves the use of the Pall Celeris System, which is a Point-of-Care (POC) device based on selective filtration, designed to obtain a high concentration of Peripheral Blood Total Nucleated Cells (PB-TNC). The unique feature of the system is its ability to eliminate the contamination of highly pro-inflammatory granulocytes [21]. Spaltro et al. studied PBTNCs isolated from healthy donors by characterizing them using flow cytometry and functional tests, aimed at assessing migratory capacity, ability to form capillary structures, endothelial trans-differentiation, and paracrine cytokine secretion. Human PB-TNCs obtained with the Pall Celeris filtration system have been shown to secrete a panel of relevant angiogenic factors and to migrate in response to Vascular Endothelial Growth Factor (VEGF) and stromal-derived factor 1. Human PB-TNCs have induced neovascularization by increasing the number of collagen fibers, capillaries, and arterioles up to 7 days after ischemia in the murine model [22].
Selective filtration allows cell separation based on membrane electrical charge, with minimal contamination of the concentrate by red blood cells and neutrophils. The system also preserves cell membrane integrity and prevents the release of active molecules and premature cell activation. The cell populations eventually present in the concentrate were previously listed [1]. The volume of peripheral blood to be drawn from the patient to obtain PBMNCs cell concentrate varies from 60 to 120 ml with an average of 100ml. All blood samples were collected with ACD-A Anticoagulant and stored at room temperature until use. The developed kit is easy to use. The concentrate of selected mononuclear cells is characterized by high cell viability because the blood is not stressed but filtered; it is extremely liquid and therefore easily injectable and so far, has not presented any adverse reactions. The infusion should normally be performed within 24 hours of blood collection and under sterile conditions. PBMNCs are isolated using the Pall Celeris system (Pall Medical Corporation) according to the manufacturer’s instructions (Figure 1) [22].
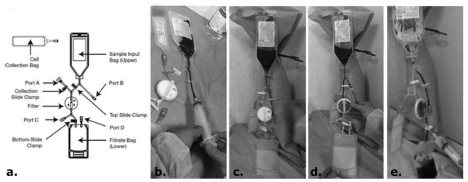
Figure 1: Pall Celeris system. A: Model of the Pall Celeris system; B-E: Images for Pall Celeris system use; B: Blood loading into the sample input bag through port
B; C and D: Filtration by gravity. E: Filter backflush to recover Total Nucleated Cells (TNCs) with the use of 14mL of saline solution through port C.
To date, the implant of PBMNCs is used for the already mentioned angiogenic and regenerative capabilities, especially in the treatment of patients with critical limb ischemia that cannot be revascularized or that have undergone revascularization [1,24,25] and in orthopedic pathologies such as angular defects of the lower limbs, fractures that have difficulty healing, in tendon or cartilage pathology [25].
Regarding the mode of application, dosage, frequency, and duration of treatment, please refer to the various specialist clinical protocols.
Conclusions
The implant of autologous peripheral blood mononuclear cells, thus belonging to the patient himself, is an innovative technique, sophisticated but at the same time simple and repeatable; through this procedure, it is possible to accelerate the process of tissue regeneration and facilitate the recovery from trauma, injury, ischemic, degenerative and surgical damage.
Declaration of Interest
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Authorship Contributions
All Authors contributed equally to the study.
Acknowledgements
All Authors declare no organizations that funded our research, including grant numbers.
References
- Di Vieste G, Formenti I, Lodigiani S. Use of autologous peripheral blood mononuclear cells in a case of diabetic foot. In: Luca Monge, Associazione Medici Diabetologi, editor. The Journal of AMD. 2019; 22: 230-233.
- Gurevich DB, Severn CE, Twomey C, Greenhough A, Cash J, Toye AM, et al. Live imaging of wound angiogenesis reveals macrophage orchestrated vessel sprouting and regression. EMBO J. 2018; 37: e97786.
- Rehman J, Li J, Orschell CM, March KL. Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophagesand secrete angiogenic growth factors. Circulation. 2003; 107: 1164-1169.
- Moreira AP, Hogaboam CM. Macrophages in allergic asthma: fine-tuning their pro-andanti-inflammatory actions for disease resolution. J Interferon Cytokine Res. 2011; 31: 485-491.
- Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008; 8: 958-969.
- Heymann F, Trautwein C, Tacke F. Monocytes and macrophages as cellular targets in liver fibrosis. Inflamm Allergy Drug Targets. 2009; 8: 307-318.
- Ricardo SD, van Goor H, Eddy AA. Macrophage diversity in renal injury and repair. J Clin Invest. 2008; 118: 3522-3530.
- Stein M, Keshav S, Harris N, Gordon S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J Exp Med. 1992; 176: 287-292.
- Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004; 25: 677-686.
- Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003; 3: 23-35.
- Louis CA, Mody V, Henry WL, Reichner JS, Albina JE. Regulation of arginase isoforms I and II by IL-4 in cultured murine peritoneal macrophages. Am J Physiol. 1999; 276: R237-242.
- Song E, Ouyang N, Horbelt M, Antus B, Wang M, Exton MS. Influence of alternatively and classically activated macrophages on fibrogenic activities of human fibroblasts. Cell Immunol. 2000; 204: 19-28.
- Anderson CF, Gerber JS, Mosser DM. Modulating macrophage function with IgG immune complexes. J Endotoxin Res. 2002; 8: 477-481.
- Stout RD, Jiang C, Matta B, Tietzel I, Watkins SK, Suttles J. Macrophages sequentially change their functional phenotype in response to changes in microenvironmental influences. J Immunol. 2005; 175: 342-349.
- Buechler C, Ritter M, Orsó E, Langmann T, Klucken J, Schmitz G. Regulation of scavenger receptor CD163 expression in human monocytes and macrophages by pro- and antiinflammatory stimuli. J Leukoc Biol. 2000; 67: 97-103.
- Ambarus CA, Krausz S, van Eijk M, Hamann J, Radstake TRDJ, Reedquist KA, et al. Systematic validation of specific phenotypic markers for in vitro polarized human macrophages. J Immunol Methods. 2012; 375: 196-206.
- Ren Y, Savill J. Apoptosis: the importance of being eaten. Cell Death Differ. 1998; 5: 563-568.
- Fadok VA, McDonald PP, Bratton DL. Regulation of macrophage cytokine production by phagocytosis of apoptotic and post-apoptotic cells. Biochem Soc Trans. 1998; 26: 653-656.
- Bréchot N, Gomez E, Bignon M. Modulation of macrophage activation state protects tissue from necrosis during critical limb ischemia in thrombospondin- 1-deficient mice. PLoS ONE. 2008; 3: e3950.
- GazzettaUfficiale IT. Disposizioni relative ai requisiti di qualita’ e sicurezza del sangue e degli emocomponenti. 2015.
- Gabriella S, Stefania S, Elisa G. Characterization of the Pall Celeris system as a point-of-care device for therapeutic angiogenesis. Cytotherapy. 2015; 17: 1302-1313.
- Rigato M, Monami M, Fadini GP. Autologous Cell Therapy for Peripheral Arterial Disease: Systematic Review and Meta-Analysis of Randomized, Nonrandomized, and Noncontrolled Studies. Circ Res. 2017; 120: 1326-1340.
- SIMTI Raccomandazioni SIMTI sugli emocomponenti per uso non trasfusionale. 2012.
- Persiani F, Paolini A, Camilli D, Mascellari L, Platone A, Magenta A, et al. Peripheral Blood Mononuclear Cells Therapy for Treatment of Lower Limb Ischemia in Diabetic Patients: A Single- Center Experience. Ann Vasc Surg. 2018; 53: 190-196.
- Wei-Li Fu, Chun-Yan Zhou, Jia-Kuo Yu. A new source of mesenchymal stem cells for articular cartilage repair: MSCs derived from mobilized peripheral blood share similar biological characteristics in vitro and chondrogenesis in vivo as MSCs from bone marrow in a rabbit model. Am J Sports Med. 2014; 42: 592-601.