
Review Article
J Stem Cell Res Transplant. 2021; 8(1): 1035.
Driver Gene Mutations and Risk of Lymph Node Metastasis in Non-Small Cell Lung Cancer
Feiyue Z1,2, Li Z1,2, Zhang Bing1, Chen Benchao1,2, Wang Shuting1,2, Du Yu1,2 and Gaofeng L2*
¹Kunming Medical University, Kunming, China
²Department of Thoracic Tumor Surgery, Yunnan Cancer Center, Kunming, China
*Corresponding author: Li Gaofeng, Department of Thoracic Tumor Surgery, Yunnan Cancer Center, Kunming, 650118, China
Received: April 14, 2021; Accepted: May 13, 2021; Published: May 20, 2021
Abstract
Non-Small Cell Lung Cancer (NSCLC) lymph node status is closely related to its diagnosis, treatment, and prognosis. The lymph node status is an important basis for formulating clinical treatment strategies of NSCLC, therefore comprehensive and profound understanding of risk factors for lymph node metastasis is essential. There are many known factors for lymph node metastasis in NSCLC, such as pathological subtypes, tumor size, tumor location. Meanwhile, whether the mutation of the driver gene affects the lymph node metastasis is still lacking enough research. This article aims to elaborate the relationship between NSCLC driver gene mutation and lymph node metastasis, from NSCLC lymph node metastasis-related risk factors, driver genes and lymph node metastasis, metastatic lymph node mutation status analysis and detection these several aspects to summarize the latest research progress in NSCLC driver gene mutation and lymph node metastasis risk. It also fully explained the correlation between driver gene mutations and NSCLC tumor biological behaviors such as lymph node metastasis.
Keywords: Non-small cell lung cancer; Lymph node metastasis; driver gene mutation; Heterogeneity; Analysis of tumor genetic characteristics
Background
The occurrence and development of cancer are driven by key mutations in driver genes. Furthermore, lymph node metastasis is related to driver gene mutations in Non-Small Cell Lung Cancer (NSCLC) [1], According to the protein encoded by mutant genes involved in the molecular pathways, these driver gene mutations were divided into different mutation subtypes (such as EGFR, MET, HER2, KRAS, BRAF, ROS1, ALK, RET, and wild type).Besides, many new potential oncogenes, such as PIK3CA mutations, FGFR1 amplification, and DDR2 mutations have been continuously identified [2-4]. Driver genes and targeted therapy have brought a revolution in the diagnosis and treatment of NSCLC [5]. The significance of studying the causes and mechanisms of NSCLC driver genes and lymph node metastasis cannot be underestimated.
Risk of Lymph Node Metastasis in NSCLC
Univariate and multivariate logistic regression analysis of factors showed that lymph node metastasis of NSCLC was closely related to age, tumor size, histology and differentiation, carcinoembryonic antigen level, vascular invasion (+), and pleural involvement (+) [6,7]. Tumor size has a great influence on lymph node metastasis. Besides tumor with micropapillary or solid components are associated with lymph node metastasis, while Ground-Glass Opacity (GGO) components are on the contrary [8,9]. Also, propensity score matching analysis shows that the risk of lymph node metastasis in lung adenocarcinoma is higher than that of lung Squamous Cell Carcinoma (SCC) [10]. Other studies suggest that patients with carcinoembryonic antigen levels greater than 5 ng/dL should be recommended for systemic lymph node dissection owing to the high risk of lymph node metastasis [11]. Far more than that, the central tumor location type and tumor long axis >2 cm but =3 cm are risk factors for lymph node metastasis [12]. Interestingly, studies have also shown that the tumor is located in the periphery, and the enlarged lymph nodes on CT are prone to lymph node metastasis [13]. Others, such as lymphatic invasion in tumors, are risk factors for lymph node metastasis in patients with NSCLC, and adjuvant therapy should be considered for such patients [14]. The role of driver gene mutations in NSCLC lymph node metastasis is still covered by a fascinating veil (Figure 1).
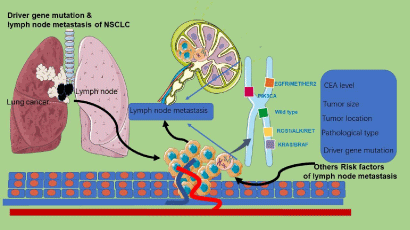
Figure 1: Driver gene mutation and risk of lymph node metastasis in non-small cell lung cancer. Which kind of driver gene mutation easily leading to the metastasis
of lymph nodes in NSCLC?.
Driver Gene Mutations and NSCLC Lymph Node Metastasis
The role of driver gene mutations in NSCLC lymph node metastasis
Different genotypes of NSCLC have a different propensity for lymph node metastasis. Studies have suggested that compared with other genotypes, fusion gene mutation cases have a higher risk of lymph node metastasis, and these patients should adopt more in-depth treatment or monitoring strategies [2]. Comparing the accumulated genetic changes between the primary tumor and lymph node metastasis, it shows that p53 and EGFR mutations usually precede lymph node metastasis [15]. Another study conducted a comprehensive genomic and transcriptomic analysis of primary lung adenocarcinoma and corresponding lymph node metastasis and found that TP53 mutations are more abundant in the tumors of patients with metastases. This is very important for elucidating the molecular pathogenesis of metastatic lung adenocarcinoma [16]. It was EGFR mutations rather than KRAS mutations more related to N2 skipping metastasis (N2 lymph node metastasis without N1) in patients with lung adenocarcinoma [17].
Apart from lung adenocarcinoma, an Array-based comparative genomic hybridization analysis shows that lymph node metastasis in patients with primary lung squamous cell carcinoma is related to specific genomic aberrations: loss of 4p and acquisition of 19q12 [18]. Different genome aberrations may be related to different biological characteristics. For example, FGFR gene mutation is an independent prognostic factor of lymph node metastasis in lung squamous cell carcinoma, and it is significantly related to the increased risk of lymph node metastasis in NSCLC patients with stage I to III [19].
Carcinogenic mutations that drive lymph node metastasis can also indicate metastatic disease in other organs or tissues [20]. In lung adenocarcinoma patients with EGFR mutations, compared with patients with wild-type EGFR, patients with EGFR mutations have a higher rate of including intrabronchial local infiltration rate including metastasis rate and pleural infiltration rate, although both have similar lymph node metastasis rates [21]. Another retrospective analysis showed that EGFR mutations, ALK gene fusion, and RET gene fusion in patients with advanced NSCLC play an active role as a driving gene in brain metastasis [22]. Besides, it was found that the frequency of EGFR mutations is reduced, while the incidence of ALK and ROS1 fusions are opposite by analyzing the relationship between the gene status of EGFR, ALK, ROS1, and RET in the resected specimens of patients with primary lung adenocarcinoma accompanied by N1-N2 lymph node metastasis [23]. Therefore, it is recommended to thoroughly evaluate the driver genes of lymph node metastasis after radical resection.
Mutational heterogeneity between primary tumor and metastatic lymph node of NSCLC
The existence of genetic heterogeneity affects the results of molecular detection in biopsy samples of primary or metastatic sites in patients with advanced NSCLC [24]. Evaluation of EGFR mutations in the surgically resected primary tumor and metastatic lymph nodes of NSCLC by DNA sequencing and heteroduplex analysis found that a significant difference in EGFR mutations between the primary tumor and metastatic lymph nodes of NSCLC [25]. The comparison of driver gene mutation maps can clarify and distinguish the origin of tumors between primary and metastatic tumors [26]. There are also many differences in the mutation profiles of key cancer-related genes in primary lung cancer and its associated lymph node metastasis [27]. Next-Generation Sequencing technology (NGS) can also be used to detect driver genes mutations in primary and metastatic tumors. Once it is confirmed that the gene mutations in lymph node metastasis are inconsistent, it may indicate the second primary cancer [28]. Unlike clinical and/or pathological diagnosis, cancer-specific mutations can be used as clonal markers to more accurately classify the pathology of lung cancer and its lymphatic metastasis. thereby which could improve the treatment options and effects based on the difference not only in the clinical/histopathological but also the genomic diagnosis [29]. Taking into account the difference in EGFR, KRAS, and BRAF mutation status between the primary tumor and the corresponding lymph node metastasis, different tyrosine kinase inhibitors ought to be selected for treatment correspondingly [30].
The clinical value of metastatic lymph node mutation status
Epidermal Growth Factor Receptor (EGFR) and its downstream factors KRAS and BRAF have different frequencies of mutations in NSCLC, which can predict the clinical response to EGFR inhibitors. Therefore when these mutations are used to select patients for EGFRdirected tyrosine kinase inhibitor therapy, the mutation status differences between the primary tumor and the corresponding lymph node metastasis should be considered [30]. The EGFR mutation status of Metastatic Lymph Node (MLN) is a predictor of the response to EGFR-TKI treatment in NSCLC patients [31]. Also, compound mutation profiles can provide more accurate prognostic information for patients with stage II NSCLC [32].
Biopsy of NSCLC Metastatic Lymph Node Gene Mutation Status
Lymph node status is considered to be one of the most reliable indicators of the prognosis of lung cancer patients, and the histological evidence of metastasis spread and mutation analysis is also crucial for the best clinical stage and treatment [33]. The lymph nodes of NSCLC patients are usually staged using 18F-Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography (FDG-PET/CT). However, micrometastasis such as occult lymph node metastasis should be selected by mediastinoscopy or Transbronchial Needle Aspiration (EBUS-TBNA) guided by endobronchial ultrasound [34]. More importantly, the mutation status of multiple genes such as EGFR, KRAS, and p53 in metastatic lymph nodes could be obtained through EBUS-TBNA [35]. Not limited to EBUS-TBNA, ultrasoundguided supraclavicular lymph node biopsy is a reliable and safe method for detecting advanced lung cancer metastasis, histological subtypes, and identifying EGFR mutations. It can replace invasive lung biopsy as the initial tissue confirmation of advanced disease [36].
Problems and Prospects
As a result of the heterogeneity in the gene spectrum between the primary tumor and the metastatic lymph node of NSCLC, the mutation spectrum of the lymph node deserves more attention. Moreover, the related mechanism of gene mutations involved in cell proliferation, invasion, and migration and lymph node metastasis is not clear yet. Characterize and analyze the metastatic lymph node at the genome level, mRNA, and protein level is essential for a better understanding of tumor biology [37]. For example, MiR-199b in miRNAs can regulate tumor cell migration, invasion, and metastasis [38]. Besides, targeted therapies represented by EGFR-TKI have increasingly become an indispensable part of the field of NSCLC treatment [39]. More importantly, as the Cancer Genome Atlas (TCGA) applies high-throughput sequencing technology and other sophisticated technologies in the field of lung cancer, new targeted carcinogenesis in non-small cell lung cancer have been discovered one after another [40]. All of us should broaden our horizons and excavate the essential role of driver gene mutations in the tumor biological behavior, not limited to lymph node metastasis.
References
- Cheng Y, Wang S, Han L, et al. Concurrent somatic mutations in driver genes were significantly correlated with lymph node metastasis and pathological types in solid tumors. Oncotarget. 2017; 8: 68746-68757.
- Liu Z, Liang H, Lin J, et al. The incidence of lymph node metastasis in patients with different oncogenic driver mutations among T1 non-small-cell lung cancer. Lung cancer (Amsterdam, Netherlands). 2019; 134: 218-224.
- Castellanos E, Feld E, Horn L. Driven by Mutations: The Predictive Value of Mutation Subtype in EGFR-Mutated Non-Small Cell Lung Cancer. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2017; 12: 612-623.
- Oxnard GR, Binder A, Jänne PA. New targetable oncogenes in non-smallcell lung cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2013; 31: 1097-1104.
- Morgenstern D, Campo MJ, Dahlberg SE, et al. Molecularly targeted therapies in non-small-cell lung cancer annual update 2014. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2015; 10: S1-S63.
- Chen B, Xia W, Wang Z, et al. Risk analyses of N2 lymph-node metastases in patients with T1 non-small cell lung cancer: a multi-center real-world observational study in China. Journal of cancer research and clinical oncology. 2019; 145: 2771-2777.
- Chen B, Wang X, Yu X, et al. Lymph node metastasis in Chinese patients with clinical T1 non-small cell lung cancer: A multicenter real-world observational study [J]. Thoracic Cancer. 2019; 10: 533-542.
- Yu X, Li Y, Shi C, et al. Risk factors of lymph node metastasis in patients with non-small cell lung cancer ≤ 2 cm in size: A monocentric population-based analysis. Thoracic Cancer. 2018; 9: 3-9.
- Wang L, Jiang W, Zhan C, et al. Lymph node metastasis in clinical stage IA peripheral lung cancer [J]. Lung cancer (Amsterdam, Netherlands). 2015; 90: 41-46.
- Deng Hy, Zeng M, Li G, et al. Lung Adenocarcinoma has a Higher Risk of Lymph Node Metastasis than Squamous Cell Carcinoma: A Propensity score-matched Analysis. World journal of surgery, 2019; 43: 955-962.
- Ye B, Cheng M, Li W, et al. Predictive factors for lymph node metastasis in clinical stage IA lung adenocarcinoma. The Annals of thoracic surgery. 2014; 98: 217-223.
- Huang L, Li W, Zhao L, et al. Risk factors of lymph node metastasis in lung squamous cell carcinoma of 3 cm or less in diameter. Medicine. 2017; 96: e7563.
- Zhang C, Pang G, Ma C, et al. Preoperative Risk Assessment of Lymph Node Metastasis in cT1 Lung Cancer: A Retrospective Study from Eastern China. Journal of immunology research. 2019; 2019: 6263249.
- Molla Y, Gradistanac T, Wittekind C, et al. Predictive risk factors for lymph node metastasis in patients with resected non-small-cell lung cancer: a casecontrol study. Journal of cardiothoracic surgery. 2019; 14: 11.
- Chang YL, Wu CT, Shih JY, et al. Comparison of p53 and epidermal growth factor receptor gene status between primary tumors and lymph node metastases in non-small cell lung cancers. Annals of surgical oncology. 2011; 18: 543-550.
- Wu K, Zhang X, Li F, et al. Frequent alterations in cytoskeleton remodeling genes in primary and metastatic lung adenocarcinomas. Nature communications. 2015; 6: 10131.
- Guerrera F, Renaud S, Tabbó F, et al. Epidermal growth factor receptor mutations are linked to skipping N2 lymph node metastasis in resected nonsmall- cell lung cancer adenocarcinomas. European journal of cardio-thoracic surgery: official journal of the European Association for Cardio-thoracic Surgery. 2017; 51: 680-688.
- Bolens Mc, Kok K, Van Der Vlies P, et al. Genomic aberrations in squamous cell lung carcinoma related to lymph node or distant metastasis. Lung cancer (Amsterdam, Netherlands). 2009; 66: 372-378.
- Li JJ, Yan S, Pan Y, et al. FGFR genes mutation is an independent prognostic factor and associated with lymph node metastasis in squamous non-small cell lung cancer. Cancer biology & therapy. 2018; 19: 1108-1116.
- Fan J, Wu J, Huang B, et al. Concomitant EGFR mutation and ALK rearrangement in multifocal lung adenocarcinoma: a case report. Diagnostic pathology. 2020; 15: 42.
- Nie Y, Gao W, Li N, et al. Relationship between EGFR gene mutation and local metastasis of resectable lung adenocarcinoma. World journal of surgical oncology. 2017; 15: 55.
- Sakai K, Takeda M, Hayashi H, et al. Clinical outcome of node-negative oligometastatic non-small cell lung cancer. Thoracic Cancer, 2016; 7: 670- 675.
- Zhang S, Yan B, Zheng J, et al. Gene status and clinicopathologic characteristics of lung adenocarcinomas with mediastinal lymph node metastasis. Oncotarget. 2016; 7: 63758-63766.
- Maw, Guo L, Shan L, et al. Homogeneity and High Concordance of ALK Translocation in Primary Lung Adenocarcinoma and Paired Lymph Node Metastasis. Scientific reports, 2017; 7: 10961.
- Park S, Holmes-Tisch AJ, Cho EY, et al. Discordance of molecular biomarkers associated with epidermal growth factor receptor pathway between primary tumors and lymph node metastasis in non-small cell lung cancer. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2009; 4: 809-815.
- Goto T, Hirotsu Y, Mochizuki H, et al. Mutational analysis of multiple lung cancers: Discrimination between primary and metastatic lung cancers by genomic profile. Oncotarget. 2017; 8: 31133-31143.
- Chen Y, Mao B, Peng X, et al. A Comparative Study of Genetic Profiles of Key Oncogenesis-Related Genes between Primary Lesions and Matched Lymph Nodes Metastasis in Lung Cancer. Journal of Cancer, 2019; 10: 1642-1650.
- Tseng LH, De Marchi F, Pallavajjalla A, et al. Clinical Validation of Discordant Trunk Driver Mutations in Paired Primary and Metastatic Lung Cancer Specimens. American journal of clinical pathology. 2019; 152: 570-581.
- Higuchi R, Nakagomi T, Goto T, et al. Identification of Clonality through Genomic Profile Analysis in Multiple Lung Cancers. Journal of clinical medicine. 2020; 9: 573.
- Schmid K, Oehl N, Wrba F, et al. EGFR/KRAS/BRAF mutations in primary lung adenocarcinomas and corresponding locoregional lymph node metastases. Clinical cancer research: an official journal of the American Association for Cancer Research. 2009; 15: 4554-4560.
- Shimizu K, Yukawa T, Hirami Y, et al. Heterogeneity of the EGFR mutation status between the primary tumor and metastatic lymph node and the sensitivity to EGFR tyrosine kinase inhibitor in non-small cell lung cancer. Targeted Oncology. 2013; 8: 237-242.
- Sasatomi E, Finkelstein SD, Woods JD, et al. Comparison of accumulated allele loss between primary tumor and lymph node metastasis in stage II non-small cell lung carcinoma: implications for the timing of lymph node metastasis and prognostic value. Cancer Research, 2002; 62: 2681-9.
- Samurai H, Chansky K, Crowley J, et al. The International Association for the Study of Lung Cancer Staging Project: Proposals for the Revision of the N Descriptors in the Forthcoming 8th Edition of the TNM Classification for Lung Cancer. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2015; 10: 1675-1684.
- Kasa K, Asakura K, Kazama A, et al. Risk Factors for Predicting Occult Lymph Node Metastasis in Patients with Clinical Stage I Non-small Cell Lung Cancer Staged by Integrated Fluorodeoxyglucose Positron Emission Tomography/ Computed Tomography. World journal of surgery, 2016; 40: 2976-2983.
- Nakajima T, Yasufuku K, Nakagawara A, et al. Multigene mutation analysis of metastatic lymph nodes in non-small cell lung cancer diagnosed by endobronchial ultrasound-guided transbronchial needle aspiration. Chest. 2011; 140: 1319-1324.
- Choe J, Kim MY, Baek JH, et al. Ultrasonography-Guided Core Biopsy of Supraclavicular Lymph Nodes for Diagnosis of Metastasis and Identification of Epidermal Growth Factor Receptor (EGFR) Mutation in Advanced Lung Cancer. Medicine. 2015; 94: e1209.
- Gebauer F, Wicklein D, Tachezy M, et al. Establishment and Characterization of a Pair of Patient-derived Human Non-small Cell Lung Cancer Cell Lines from a Primary Tumor and Corresponding Lymph Node Metastasis. Anticancer Research. 2016; 36: 1507-1518.
- Shuting W, Gaofeng L, Minjun Z, et al. Research progress of MiR-199b in malignant tumors [J]. Chinese Cancer. 2020; 29: 367-372.
- Remon J, Ahn MJ, Girard N, et al. Advanced-Stage Non-Small Cell Lung Cancer: Advances in Thoracic Oncology 2018. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2019; 14: 1134-1155.
- Zhang Y, Zhang L, Li R, et al. Genetic variations in cancer-related significantly mutated genes and lung cancer susceptibility [J]. Annals of oncology: official journal of the European Society for Medical Oncology. 2017; 28: 1625-1630.