
Research Article
J Stem Cell Res Transplant. 2021; 8(2): 1038.
NPS2143 Modulates the Phenotypic Switching of PASMCs by Inhibiting Autophagy in Hypoxia-Induced Pulmonary Hypertension
Wang L¹, Shao H², Che B³, Wang N³, Peng X²* and Wei C³*
1Department of Respiratory Medicine, The First Affiliated Hospital of Harbin Medical University, Harbin, 150001, China
2Department of Forensic Medicine, Harbin Medical University, Harbin, 150081, China
3Department of Pathophysiology, Harbin Medical University, Harbin, 150081, China
*Corresponding author: Xue Peng Department of Forensic Medicine, Harbin Medical University, Baojian Road, Harbin, 150081, China
Can Wei, Department of Pathophysiology, Harbin Medical University, Baojian Road, Harbin, 150081, China
Received: May 13, 2021; Accepted: June 09, 2021; Published: June 16, 2021
Abstract
Background and Objectives: Pulmonary Artery Hypertension (PAH) is considered as a malignant tumor in cardiovascular disease. Our previous study found that Calcium-Sensing Receptor (CaSR) is involved in pulmonary vascular remodeling in hypoxic pulmonary hypertension (HPH). However, the relationship of Pulmonary Artery Smooth Muscle Cell (PASMC) phenotypic switching, proliferation, and autophagy in CaSR-related HPH remain unclear. The purpose of this study was to detect the role of a CaSR antagonist, NPS2143, on the vascular remodeling by autophagy modulation under hypoxia.
Methods: Hypoxic rat PAH model were simulated in vivo. Meanwhile, mean Pulmonary Artery Pressure (mPAP) was measured while RVI, WT%, and WA% indices were calculated. Immunohistochemistry and Western blot were used to detect phenotypic switching and cell proliferation in pulmonary arteriole. Cell viability was determined in vitro by CCK8 and cell cycle. Cell proliferation, phenotypic switching, autophagy level and PI3K/Akt/mTOR pathways were investigated in human PASMCs through mRNA or Western blot methods.
Results: Rats with hypoxic-induced PAH had an increased mPAP, RVI, WT% and WA%. Moreover, expression of CaSR was significantly increased, followed by activation of autophagy (increased LC3b and decreased p62), phenotypic switching of PASMCs (reduced calponin, SMA-a and increased OPN) and pulmonary vascular remodeling. However, NPS2143 weakened these hypoxic effects. The results using hypoxic-induced human PASMCs confirmed that NPS2143 suppressed autophagy and reversed phenotypic switching in vitro by inhibiting PI3K/Akt/mTOR pathways.
Conclusions: Our study demonstrates that NPS2143 was conducive to inhibit the proliferation and reverse phenotypic switching of PASMCs by regulating autophagy levels in HPH and vascular remodeling.
Keywords: NPS2143; Phenotypic switching; Autophagy; Hypoxia pulmonary vascular remodeling
Introduction
Pulmonary Hypertension (PAH) is a life-threatening condition with no effective treatment at present. PAH is closely related to pulmonary vascular resistance and its irreversible remodeling. During the onset of PAH, excessive proliferation of pulmonary vascular smooth muscle cells (PASMCs) and decreased apoptosis accelerate the vascular remodeling [1]. In hypoxia-induced PAH, PASMCs undergo a contraction-synthesis phenotype transition, which is the main cause of pulmonary vascular remodeling [2]. However, the molecular mechanisms of pulmonary vascular remodeling in PAH are complex and still not fully understood.
Our previous study demonstrated that an increased CaSR expression involves phenotypic switching of PASMCs in small pulmonary artery in hypoxic rat [3]. NPS2143, a specific allosteric inhibitor of CaSR, is the first recognized CaSR antagonist [4,5]. NPS2143 has been reported to have a variety of biological properties, such as anti-cancer[6] and anti-inflammatory activities [7]. Recent studies also found that NPS2143 could inhibit the excessive proliferation of PASMCs in patients with idiopathic PAH [8]. Therefore, we hypothesize that interfering with NPS2143 can be a more effective method for treating hypoxic PAH.
Formation of hypoxic PAH involves many complex signaling and gene regulatory networks. Recent studies showed that autophagy acts as a self-protection mechanism for eukaryotic organisms to maintain intracellular environmental stability and participates in the occurrence and development of various types of PAHs [9]. Meanwhile, hypoxia can promote VSMC phenotype conversion and also be used as a stimulator of autophagy [10]. Therefore, we envisage that autophagy may be involved in hypoxia-promoting PASMCs phenotypic switching in the process of NPS2143 alleviating PAH. This study presents a more thorough understanding of the relationship between autophagy and phenotypic switching of PASMCs to provide a better therapeutic mechanism for the treatment of HPH with NPS2143.
Material and Methods
Animal models of HPH
A chronic hypoxia model was established as described in previous studies [11]. Male wistar rats (200-250 g) were randomly divided into 6 groups (n=5 per group), including control group, hypoxia 7d group, hypoxia 14d group, hypoxia 21d group, hypoxia 21d+NPS2143 group, control+NPS2143 group. Each hypoxia group rats were placed in a hypobaric chamber under normal pressure with a continuous flow of oxygen to stabilize its concentration in the chamber at (10±0.5)%, anoxic for 24 h per day. After hypoxia 7d, 14d, and 21d, the rats were removed from the chamber for subsequent experiments. The two NPS2143 groups were intraperitoneally injected with CaSR inhibitor, NPS2143 (5 mg/kg), every day.
All animal protocols used in this study were in accordance with the guidelines for the Care and Use of Laboratory Animals of the National Institutes of Health, and all animal experiments were approved by the Scientific Investigation Board of Harbin Medical University, Harbin, China.
Evaluation of PAH
The rats were anesthetized with 1% pentobarbital sodium intraperitoneally, a polyethylene catheter was inserted into the right external jugular vein to the pulmonary trunk, and the mean Pulmonary Artery Pressure (mPAP) was measured using a PowerLab Physiological Signal Processing System (AD Instruments Inc., Australia). Hearts were dissected from the thoracic cavity and fixed in 4% formaldehyde for 2 d. The weight of Right Ventricle (RV) and the left ventricle plus ventricular septum (LV+S) were measured separately to calculate the Right Ventricular Hypertrophy Index (RVHI), which reflects the changes in right ventricular hypertrophy.
Hematoxylin and Eosin (H&E) staining
Lung tissue of rat was fixed with neutral formalin, embedded in paraffin, and stained with HE. The walls of pulmonary arterioles were observed under microscope.
Weigert’s staining
Conventional paraffin section was dewaxed, dehydrated. Counterstained with prepared VG staining solution, 95% ethanol rapidly differentiates, quickly dehydrated by anhydrous ethanol and transparent xylene, and sealed with neutral gum.
Pulmonary vascular morphometry
After HE and Weigert’s staining, the inner and outer diameters of the pulmonary vessels (80-120 μm in diameter) were measured using Imagepro-plus 6.0 software, and the wall thickness, wall area, total pulmonary vascular area, wall thickness/vascular outer diameter (WT%), and wall area/vessel area/ (WA%) were calculated.
Transmission electron microscopy
The pulmonary arterioles of each group were fixed with 2.5% glutaraldehyde, dehydrated with gradient ethanol and acetone, embedded, oven-cured, sliced with ultra-thin slicer, with a thickness of 70nm, and double stained with 3% uranyl acetate-lead lead. Finally, the transmission electron microscope was used to observe and film.
Immunohistochemistry
Conventional paraffin sections were dewaxed in water, each slice was subjected to antigen retrieval. 0.3% H2O2 blocked endogenous peroxidase. Each section was incubated with anti-CaSR, p62, LC3b, OPN, calponin, SMA-a and GAPDH (purchased from Cell Signaling Technology, Boston, USA). Secondary antibody was selected based on primary antibody, DAB color. Finally, neutral gum was used to slice. The brown stained area on the vessel wall was observed under a light microscope to be positive.
Cell culture
Human Pulmonary Arteries Smooth Muscle Cells (HPASMCs) and medium SMCM were purchased from American ScienCell Company. HPASMCs cells were cultured in SMCM in complete medium (89% DMEM + 10% fetal calf Serum + 1% growth factor) with 100 μg/mL penicillin and 100 μg/mL streptomycin. The cells were cultured at 37°C, 21% O2 and 5% CO2. The second to fourth generation cells were used for subsequent experiments. When the subcultured HPASMCs were grown to 60% of the bottom of the flask, the serum-free medium was replaced for 24 h and the following experiments were performed.
Hypoxia treatment of HPASMCs
A hypoxic environment was established by continuously mixing 95% N2, 1 % O2 and 5% CO2 in a hypoxic incubator. HPASMCs were first starved in serum-free DMEM for 24 h. Then they were exposed to one of the following 9 different experimental conditions for 24 h: (1) control: 21% O2 / 5% CO2 / balance N2; (2) hypoxia: 1 % O2 / 5% CO2/balance N2; (3) hypoxia with 1 μM NPS2143 (antagonist of CaSR); (4) normoxia with 1 μM NPS2143; (5) hypoxia with 5 mM 3-MA (inhibitor of autophagy); (6) normoxia with 5 mM 3-MA; (7) hypoxia with 5 μM R568 (an agonist of CaSR); (8) hypoxia with 10 μM NPS2143 and 5 μM R568; and (9) hypoxia with 50 nM NVPBEZ235 (a PI3K/mTOR dual inhibitors).
Cell proliferation assays
HPASMCs were seeded in a 48-well plate at 1×104 cells/well, and cultured at 37°C under 5% CO2 until the cells were 90% confluent, and then incubated with serum-free DMEM/F12 medium for 2 h to synchronize the cells. 10 μL of CCK-8 (purchased from Invitrogen, CA, USA) solution was added to each well. After the incubation, A450 was measured using a microplate reader.
Cell cycle assays
For cell cycle analysis, the HPASMCs to be tested were washed and centrifuged at 1000 r/min for 5 min. Supernatant was discarded and cells were resuspended in 80% ethanol or added propidium iodide. Cell cycle analysis was performed with a CytoFLEX S Flow Cytometer (manufactured by Beckman Coulter).
Western blotting
Pulmonary arterioles or HPASMCs were used to detect the target proteins. The following primary antibodies for rabbit anti-mouse CaSR, calponin, SMA-a, OPN, PCNA, Ki67, LC3b, p62, p-AKT, AKT, p-PI3K, PI3K, p-mTOR, mTOR, caspase-3, HIF-1a and GAPDH (purchased from Cell Signaling Technology, Boston, USA) were used. The resulting protein strip image was detected using Quality One software to analyze the image to GAPDH light density. The degree value is used as an internal parameter to correct the optical density value of the target protein.
Real-time fluorescence quantitative PCR
Total RNA was extracted by Trizol (DRR037A, Takara, Japan) method and its concentration and quality were measured. Reverse transcription of synthetic DNA was done according to the reverse transcription kit instructions (Takara, Japan). The expression levels of CaSR, PCNA, Ki67, LC3b, p62, calponin, SMA-a OPN, and β-actin were detected on a Takara fluorescence quantitative PCR instrument according to the SYBR Premix Ex TaqTMII (Tli RNase H Plus) protocol. Three repetitions were made for each concentration. Difference analysis of expression was performed on the qRT-PCR results using the 2-ΔΔCT method. The following primers were used:
CaSR: C T T T G T G C T G G G T G T C T T C A (forward), A A C A A G G A G C T G G A G A A G C A (reverse); PCNA: A C G C C G C C C G A A C T G A T (forward), C G T G C G T G A C A T T A A A G A G (reverse); Ki67: G G T G T C A A G C A C G A A T G (forward), G G G A C C T G G C A C G A A T A G A T (reverse); P62: T G A A G G C T A T T A C A G C C A G A G T C A A (forward), C C T T C A G T G A T G G C C T G G T C (reverse); LC3b: T T G A C C T C C C A A A G T G C (forward), T C C A A G C C T G T A A A C C C (reverse); calponin: G A A C T T G T C T G G G T C A T C T C G (forward), A C G C T G G T G G T C G T A T T T C T (reverse); SMA-a: A C C A T C G G G A A T G A A C G C T T (forward), T T G C G T T C T G G A G G A G C A A T (reverse); OPN: G C C G A G G T G A T A G C T T G G C T T A (forward), T T G A T A T C C T C A T C G G A C T C C T G (reverse); β-actin: C A A C C T T C T T G C A G C T C C T C (forward), C G G T G T C C C T T C T G A G T G T T (reverse).
Statistics
Data analysis was done using SPSS21.0 statistical software, all results were calculated according to the mean ±SEM, the comparison between the different groups was tested in One-way ANOVA, p < 0.05 was considered statistically significant.
Results
Hypoxia induces pulmonary vascular remodeling, the expressions of CaSR, autophay protein, HIF-1a, PASMCs proliferation and phenotypic switching in hypoxic PAH rats
We first established a hypoxic PAH model. It was found that the level of mPAP and RVI were increased at hypoxic 14d and 21d (Figure 1A and 1B). HE and Weigert’s elastic staining (Figure 1C) showed thickening of the pulmonary vascular wall and increased WT% and WA% (Figure 1D) of the pulmonary arterioles. Next, the expression of CaSR and HIF-1a in the pulmonary arterioles was significantly increased with the increased expression of LC3b and decreased expression of p62 in hypoxic 14d and 21d, compared to control rats (Figure 1E-1F). In addition, we used TEM to observe the formation of autophagosomes in pulmonary arterioles in hypoxic 21d (Figure 1G). These results suggested that there may be some association between CaSR and autophagy in HPH rats.
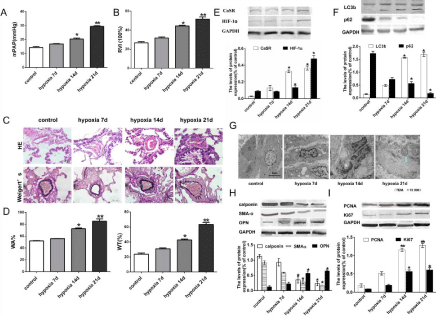
Figure 1: Hypoxia induces pulmonary vascular remodeling, the expressions of CaSR, autophay protein, HIF-1a, PASMCs proliferation and phenotypic switching
in hypoxic PAH rats. (A) Measurement and calculation of mPAP and RVI. (C) HE and Weigert’s staining (magnification 400×). (D) Calculation of WT% and WA%.
(E-F) The expression of protein CaSR, HIF-1a, LC3b and p62 were detected by Western blot (G) Transmission electron microscopy showed the effect of hypoxia
on autophagosomes of pulmonary arterioles in each group (magnification 15000×). (H-I) The expression of protein OPN, SMA-a, calponin, PCNA and Ki67 were
detected by Western blot. Data are presented as mean ± SEM. *P<0.05 compared to control group, **P<0.01 versus control group.
To further determine whether PASMCs was converted from contractile to synthetic phenotype and undergone cell proliferation under hypoxia, we next analyzed the expression levels of contractile marker SMA-a, calponin, synthetic marker OPN, proliferating factors PCNA and Ki67 in rat pulmonary arterioles at different intervals after hypoxia. The expression levels of SMA-a and calponin were significantly reduced, while the expressions of OPN, PCNA and Ki67 were significantly increased in hypoxic 14 d and 21 d, compared to control rats (Figure 1H and 1I). These results again confirmed that pulmonary arterial vascular remodeling during PAH formation is associated with PASMCs phenotypic switching and cell proliferation.
NPS2143 ameliorates rat PAH and vascular remodeling, inhibits autophagy and reverses phenotypic switching of pulmonary arterioles induced by hypoxia
We then injected NPS2143 during hypoxia in rats, and found that NPS2143 significantly reduced mPAP and ameliorated RVI in hypoxic 21d rats (Figure 2A and 2B). HE and Weigert’s elastic staining (Figure 2C) showed that NPS2143 thinned the pulmonary arteriolar wall, decreased the WT% and WA% (Figure 2D and 2E) compared to the hypoxic 21d group. Immunohistochemistry results found that the expression of CaSR was significantly increased in pulmonary arterioles in hypoxic 21d compared to the control group, which is associated with the elevated expression of autophagy-related protein LC3b and reduced expression of p62. Meanwhile, the expressions of SMA-a and calponin were obviously decreased, and the expression of OPN was remarkably increased (Figure 2F). But, NPS2143 reduced the effect of hypoxia on the autophagy protein and phenotype marker protein expressions. These results were confirmed at the mRNA level using qRT-PCR analysis (Figure 2G and 2H).
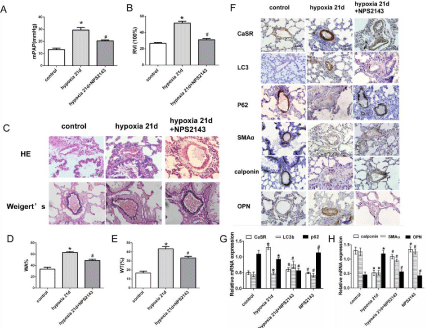
Figure 2: NPS2143 ameliorates rat PAH and vascular remodeling, inhibits autophagy and reverses phenotypic switching of pulmonary arterioles induced by
hypoxia. (A) Measurement and calculation of mPAP and RVI. (C) HE and Weigert’s staining (magnification 400×). (D-E) Calculation of WT% and WA%. (F)
Immunohistochemistry showed the expressions of CaSR, LC3b, p62, OPN, SMA-a, and calponin(magnification 400×). (G-H) Real-time RT-PCR data showed the
mRNA expression levels of CaSR, LC3b, p62 SMA-a, calponin and OPN. Data are presented as mean ± SEM. *P<0.05 versus the control group. #P<0.05 versus
the hypoxia 21d group.
NPS2143 decreases proliferation, CaSR expression, autophagy level, and reverses phenotypic switching in HPASMCs under hypoxia
CCK8 and Cell cycle analysis both showed that NPS2143 inhibited HPASMC proliferation caused by hypoxia (Figure 3A and 3B). A similar finding suggested that NPS2143 also inhibited the increased expressions of protein and mRNA of PCNA and Ki67 caused by hypoxia (Figure 3C and 3D). Meanwhile, we found that the expression of CaSR was upregulated under hypoxia, with the significantly increased expression of LC3b and markedly reduced expression of p62 (Figure 3E and 3F). In addition, the expression of OPN was obviously increased, while the expressions of SMA-a and calponin were markedly reduced in HPASMCs under hypoxia (Figure 3G). NPS2143 abolished the above expressions. These results were confirmed at the mRNA level using qRT-PCR analysis (Figures 3H-3J).
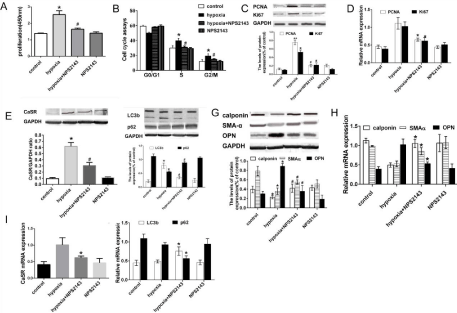
Figure 3: NPS2143 decreases proliferation, CaSR expression, autophagy level, and reverses phenotypic switching in HPASMCs under hypoxia. (A) HPASMCs
proliferation was measured by CCK8 assay. (B) Cell cycle analysis of HPASMCs by flow cytometry. (C) Expression of PCNA and Ki67 protein in HPASMCs under
hypoxia. (D) RT-PCR showed the expression of PCNA and Ki67 gene in HPASMCs under hypoxia. (E-G) Expression of CaSR, autophagy proteins and phenotypic
marker proteins in HPASMCs detected by Western blot. (H-J) RT-PCR showed the mRNA expression of phenotypic marker gene, CaSR and autophagy gene in
HPASMCs under hypoxia. Data are presented as mean ± SEM. *P<0.05 vs control group, **P<0.01 versus control group, #P<0.05 vs hypoxia group.
3-MA inhibits proliferation and reverse phenotypic switching in HPASMCs under hypoxia
When comparing to the hypoxia group, 3-MA (a selective autophagy inhibitor) reduced the expression of LC3b and increased p62 expression under hypoxia (Figure 4A). However, the expression of CaSR had no variation when added with 3-MA in hypoxia (Figure 4B). In addition, 3-MA reduced the proliferation and reversed the phenotypic switching of HPASMCs from synthetic phenotype to contractile phenotype at hypoxic 24 h (Figure 4C and 4D).
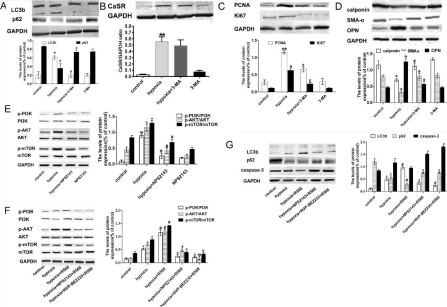
Figure 4: NPS2143 suppresses autophagy via inhibiting PI3K/Akt/mTOR pathways in HPASMCs under hypoxia. (A-D) Expression of autophagy proteins, CaSR,
PCNA, Ki67 and phenotypic marker proteins in HPASMCs detected by Western blot. (E) Expression of phosphorylated-PI3K/Akt/mTOR protein in HPASMCs under
hypoxia with 3-MA by Western blot. (F) Expression of phosphorylated-PI3K/Akt/mTOR protein in HPASMCs under hypoxia with NPS2143, R568 and PI3K/mTOR
dual inhibitor NVP-BEZ235 by Western blot. (G) Expression of autophagy protein LC3b, p62, and apoptosis protein capase-3 in HPASMCs under hypoxia with
NPS2143, R568, and PI3K/mTOR dual inhibitor NVP-BEZ235 by Western blot. Data are presented as mean ± SEM. *P<0.05 versus control group, ▢**P<0.01 versus
control group, #P<0.05 versus hypoxia group, ϶
P<0.05 versus hypoxia +R568 group, ϶
϶
P<0.01 versus hypoxia +R568 group.
NPS2143 suppresses autophagy via inhibiting PI3K/Akt/ mTOR pathways in HPASMCs under hypoxia
Next, we intend to identify whether the decreasing level of autophagy regulated by NPS2143 was attributed to the change of PI3K/Akt/mTOR pathways. NPS2143, reduced the expressions of p-PI3K, p-Akt, and p-mTOR under hypoxia compared to control group (Figure 4E). NVP-BEZ235 (a specific PI3K/mTOR dual inhibitor) reduced the increased expressions of p-PI3K, p-AKT and p-mTOR caused by R568 (CaSR agonist) treatment under hypoxia (Figure 4F).
Moreover, we further detected the role of NVP-BEZ235 in the process of NPS2143-regulated autophagy. Figure 4G showed that the expression of LC3b was increased, and the p62 was decreased, while caspase-3 was also decreased under hypoxia, but there is no statistically significant for caspase-3 expression compared to the control group. R568 further enhanced the expression of LC3b and decreased p62. There was also no significant change in caspase-3 levels. However, NVP-BEZ235 significantly reduced the increased level of LC3b, decreased levels of p62 and caspase-3, The data indicate that NPS2143 inhibits the PI3K/Akt/mTOR pathways by reducing CaSR-enhanced autophagy.
Discussion
PAH is a serious and fatal circulatory disease, mainly caused by excessive proliferation of vascular wall cells, eventually occluding small pulmonary artery [12]. Although several studies have made progress in the treatment of PAH in recent years, the current treatment methods are still not satisfactory [13]. Therefore, a further exploration of pathogenesis of PAH and the selection of more effective treatments are imminent.
The over-proliferation of PASMCs plays a necessary role in hypoxic pulmonary vascular remodeling. Meanwhile, phenotypic switching of PASMCs, which is characterized by the conversion from contractile to synthetic phenotype proteins during hypoxia, contributes to the proliferation of PASMCs and vascular remodeling of PAH. Although the study of pathological changes in HPH has become clearer, the cellular mechanisms related to excessive and uncontrolled growth of vascular wall cells remain unclear.
Recently, we discovered that the expression of CaSR was increased which participated in the phenotypic switching of PASMCs in small pulmonary artery and vascular remodeling in hypoxic rat [3]. NPS2143 is the first reported small molecule CaSR allosteric inhibitor (calcilytics) with oral bioactivity [14]. NPS2143 can inhibit CaSR activity in the parathyroid gland and promote the secretion of endogenous Parathyroid Hormone (PTH). The main purpose of its early research is to develop oral drugs for the treatment of osteoporosis, but it still needs further clinical research and verification [15]. With the extensive study of the role of CaSR in various systems or diseases, the effects of calcimimetics and calcium inhibitors on disease have gradually been discovered. Yamamura [16] found that CaSR is involved in the pathological mechanism of PAH, while NPS2143 alleviates the occurrence and development of PAH and the formation of right heart hypertrophy. Guo [17] found that NPS2143 reduces Right Ventricular (RV) systolic blood pressure, right heart hypertrophy index (RV/LV+S ratio), and reverses right ventricular myocardial fibers in MCT-induced PAH rats and chronic hypoxiainduced PAH mice.
Our experiments showed that, with the prolongation of hypoxia, the mPAP and RVI were elevated with the thickened pulmonary arteriolar wall. At the same time, we also observed an increase in the expressions of the proliferation factors PCNA and Ki67, while the expressions of calponin and SMA-a were reduced, and the expression of the synthetic marker protein OPN was increased. These data indicate that the hypoxia pulmonary hypertension model was built and pulmonary vascular remodeling occurred. Hypoxia combined with NPS2143 significantly reduced the levels of mPAP and RVI, improved pulmonary vascular remodeling, inhibited the expressions of PCNA, Ki67, and OPN, increased the expressions of calponin and SMA-a. These data showed that NPS2143 can effectively inhibit the formation of hypoxic PAH and pulmonary vascular remodeling.
Autophagy is a highly conserved protein or organelle degradation process found in eukaryotes [18]. The relationship between autophagy and PAH is complex, and it can play multiple roles in the occurrence and development of different types of PAH. Proper autophagy can protect the vascular endothelium barrier, maintain normal vascular tone and relieve inflammation. However, autophagy loss or excessive autophagy can lead to excessive proliferation of pulmonary vascular wall cells, thereby aggravating pulmonary vascular remodeling and promoting PAH [19,20].
Our current findings revealed that autophagy is significantly activated in hypoxic-induced pulmonary arterioles of rats and HPASMCs. At the same time, the addition of NPS2143 showed to inhibit autophagy and alleviate hypoxia-induced pulmonary vascular remodeling and HPASMCs proliferation. The experimental results also showed that NPS2143 relieves hypoxic PAH and pulmonary vascular remodeling by regulating autophagy.
Meanwhile, we also found that hypoxia-induced activation of autophagy is related to the proliferation and phenotypic switching of PASMCs. Autophagy-specific inhibitor, 3-MA, can reduce proliferation and reverse the phenotypic switching of PASMCs, proposing that the activation of autophagy converted the PASMCs from contractile phenotype to synthetic phenotype and promote PASMCs proliferation under hypoxic condition, which can be an important factor in the progression of HPH disease and vascular remodeling. Concurrently, NPS2143 inhibits the autophagy induced by hypoxia. These results further verified that NPS2143 treatment can suppress the proliferation and reverse the phenotypic switching of HPASMCs through regulating autophagy.
The occurrence of autophagy is regulated by a variety of genes and signaling pathways [21]. The current study shows that at least 30 autophagy-related genes are involved in the dynamic regulation process [22,23]. To provide insight into the underlying mechanisms of NPS2143-regulated autophagy, we observed the classical PI3K/Akt/ mTOR signaling pathways. In our experiment, we found that the dual inhibitors of PI3K/mTOR effectively reduced CaSR agonist-enhanced autophagy levels in hypoxia-induced HPASMCs. The results suggest that NPS2143 inhibits autophagy and reduces hypoxic pulmonary vascular remodeling mainly through the PI3K/Akt/mTOR signaling pathway. Autophagy is a multistep and highly conserved cellular metabolic process. Enhanced expression of upstream autophagy or blockade of downstream degradation leads to aggregation of p62. Finally, p62 is incorporated into mature autophagy and degraded in autophagy, so there is a negative correlation between the expression of p62 in the whole cell and autophagy activity. Moreover, the level of p62 is often related to the ratio of LC3II/LC3I as the level of evaluation of autophagy. P62 is one of the marker proteins that reflect autophagy activity, and its content indirectly reflects the level of autophagosome clearance. The current results demonstrate that autophagy inhibition is associated with the elevated levels of p62, whereas autophagy activation is associated with decreased levels of p62, supporting its role in functional autophagy flux.
At the same time, NPS2143 also increased apoptosis in the hypoxic cell model. There is a multilevel and diversified relationship between autophagy and apoptosis, thus ensuring that cells can perfectly achieve a balance between life and death in response to various stress stimuli, and when this delicate balance is broken, various diseases start to occur [24].
In summary, our results demonstrated that NPS2143 regulates proliferation, cell cycle progression, and phenotypic switching in PASMCs caused by hypoxia, which relies on PI3K/Akt/mTOR signal pathway. Inhibition of this pathway prevents PASMCs proliferation and reverses HPVR. Our study reveals the mechanism of HPVR and provides more targets for the treatment of PAH.
Declarations
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Funding
This research was supported by the National Natural Science Foundation of China (No. 81800260, 81300200 and 81270273), and the Heilongjiang Provincial Committee of Health (No. 2019-005).
Authors’ Contributions
Lina Wang, Xue Peng and Can Wei designed the research and drafted the manuscript; Hongjiang Shao, Ningning Wang and Bingbing Che completed the experiment. All authors read and approved the final manuscript.
References
- Arora TK, Arora AK, Sachdeva MK, Rajput SK, Sharma AK. Pulmonary hypertension: molecular aspects of current therapeutic intervention and future direction. J Cell Physiol. 2018; 233: 3794-3804.
- Wang XY, Mo D, Tian W, Liu XX, Zhou YG, Sun Y, et al. Inhibition of RhoA/ ROCK signaling pathway ameliorates hypoxic pulmonary hypertension via HIF-1alpha-dependent functional TRPC channels. Toxicol Appl Pharmacol. 2019; 369: 60-72.
- Peng X, Li HX, Shao HJ, Li GW, Sun J, Xi YH, et al. Involvement of calciumsensing receptors in hypoxia-induced vascular remodeling and pulmonary hypertension by promoting phenotypic modulation of small pulmonary arteries. Mol Cell Biochem. 2014; 396: 87-98.
- Chavez-Abiega S, Mos I, Centeno PP, Elajnaf T, Schlattl W, Ward DT, et al. Sensing extracellular calcium - an insight into the structure and function of the calcium-sensing receptor (CaSR). Adv Exp Med Biol. 2020; 1131: 1031- 1063.
- Chen CY, Hour MJ, Lin WC, Wong KL, Shiao LR, Cheng KS, et al. Antagonism of Ca(2+)-sensing receptors by NPS 2143 is transiently masked by p38 activation in mouse brain bEND.3 endothelial cells. Naunyn Schmiedebergs Arch Pharmacol. 2019; 392: 823-832.
- Joeckel E, Haber T, Prawitt D, Junker K, Hampel C, Thüroff JW, et al. High calcium concentration in bones promotes bone metastasis in renal cell carcinomas expressing calcium-sensing receptor. Mol Cancer. 2014; 13: 42.
- Mine Y, Zhang H. Calcium-sensing receptor (CaSR)-mediated antiinflammatory effects of L-amino acids in intestinal epithelial cells. J Agric Food Chem. 2015; 63: 9987-9995.
- Yamamura A, Yagi S, Ohara N, Tsukamoto K. Calcilytics enhance sildenafilinduced antiproliferation in idiopathic pulmonary arterial hypertension. Eur J Pharmacol. 2016; 784: 15-21.
- Chen R, Jiang M, Li B, Zhong W, Wang Z, Yuan W, et al. The role of autophagy in pulmonary hypertension: a double-edge sword. Apoptosis. 2018; 23: 459-469.
- Yao Y, Li H, Da X, He Z, Tang B, Li Y, et al. SUMOylation of Vps34 by SUMO1 promotes phenotypic switching of vascular smooth muscle cells by activating autophagy in pulmonary arterial hypertension. Pulm Pharmacol Ther. 2019; 55: 38-49.
- Lahm T, Petrache I. LC3 as a potential therapeutic target in hypoxia-induced pulmonary hypertension. Autophagy. 2012; 8: 1146-1147.
- Zanatta E, Polito P, Famoso G, Larosa M, De Zorzi E, Scarpieri Eet al. Pulmonary arterial hypertension in connective tissue disorders: pathophysiology and treatment. Exp Biol Med (Maywood). 2019; 244: 120- 131.
- Liu HL, Chen XY, Li JR, Su SW, Ding T, Shi CX, et al. Efficacy and safety of pulmonary arterial hypertension-specific therapy in pulmonary arterial hypertension: a meta-analysis of randomized controlled trials. Chest. 2016; 150: 353-366.
- Nemeth EF, Goodman WG. Calcimimetic and calcilytic drugs: feats, flops, and futures. Calcif Tissue Int. 2016; 98: 341-358.
- Widler L. Calcilytics: antagonists of the calcium-sensing receptor for the treatment of osteoporosis. Future Med Chem. 2011; 3: 535-547.
- Yamamura A, Nayeem MJ, Al Mamun A, Takahashi R, Hayashi H, Sato M. Platelet-derived growth factor up-regulates Ca(2+)-sensing receptors in idiopathic pulmonary arterial hypertension. FASEB J. 2019; 33: 7363-7374.
- Guo Q, Huang JA, Yamamura A, Yamamura H, Zimnicka AM, Fernandez R, et al. Inhibition of the Ca(2+)-sensing receptor rescues pulmonary hypertension in rats and mice. Hypertens Res. 2014; 37: 116-124.
- Yang Z, Klionsky DJ. Eaten alive: a history of macroautophagy. Nat Cell Biol. 2010; 12: 814-822.
- Feng W, Wang J, Yan X, Zhai C, Shi W, Wang Q, et al. Paclitaxel alleviates monocrotaline-induced pulmonary arterial hypertension via inhibition of FoxO1-mediated autophagy. Naunyn Schmiedebergs Arch Pharmacol. 2019; 392: 605-613.
- Yamanaka R, Hoshino A, Fukai K, Urata R, Minami Y, Honda S, et al. TIGAR reduces smooth muscle cell autophagy to prevent pulmonary hypertension. Am J Physiol Heart Circ Physiol. 2020; 319: H1087-H1096.
- Zhang Q, Lai S, Hou X, Cao W, Zhang Y, Zhang Z. Protective effects of PI3K/ Akt signal pathway induced cell autophagy in rat knee joint cartilage injury. Am J Transl Res. 2018; 10: 762-770.
- Abada A, Elazar Z. Getting ready for building: signaling and autophagosome biogenesis. EMBO Rep. 2014; 15: 839-852.
- Walker SA, Ktistakis NT. Autophagosome biogenesis machinery. J Mol Biol. 2020; 432: 2449-2461.
- Bursch W. The autophagosomal-lysosomal compartment in programmed cell death. Cell Death Differ. 2001; 8: 569-581.