Case Report
Austin Surg Case Rep. 2016; 1(2): 1010.
Endobronchial Metastasis of Pulmonary Epithelioid Hemangioendothelioma
Ozturk A¹*, Aktas Z¹, Yilmaz A¹, Erdogan E² and Demirag F³
¹Department of Interventional Pulmonology, Ataturk Chest Diseases and Thoracic Surgery Training and Research Hospital, Turkey
²Department of Nuclear Medicine, Bezmialem Vakif University, Turkey
³Department of Pathology, Ataturk Chest Diseases and Thoracic Surgery Training and Research Hospital, Turkey
*Corresponding author: Ozturk A, Department of Interventional Pulmonology, Ataturk Chest Diseases and Thoracic Surgery Training and Research Hospital, Turkey
Received: August 11, 2016; Accepted: September 11, 2016; Published: September 19, 2016
Abstract
Background: Pulmonary Epithelioid Hemangioendothelioma (PEH) is a rare vascular tumor that has an epithelioid and histiocytoid appearance, originates from vascular endothelial or pre-endothelial cells and comprises less than 1% of all vascular tumors. Only a few cases have been reported in the literature to date.
Case Presentation: A 44-year-old female patient with hemoptysis was referred to our clinic for further investigation. Bilateral multiple parenchymalnodules and mediastinal lymph nodes were seen on chest-X-ray and Positron Emission Tomography (PET/CT). A necrotic mixed lesion tend to bleeding in the right upper lobe entry and medial wall of distal left bronchus with narrowed upper left and intermediate bronchus by tumoral infiltration were seen on her initial fiberoptic bronchoscopy. Because oftendency to bleeding of the lesions, she was underwent rigid bronchoscopy and cryobiyopsy was received for diagnose. Immunohistochemically, the tumor cells were stained for CD31 and the patient was diagnosed as PEH. Because there is no standard treatment for PEH she was followed for a year without treatment until the bone metastases were occured. Radiotherapy was begun as a palliative therapy. After fifteen months, she died as a result of PEH progression.
Conclusion: Pulmonary epithelioid hemangioendothelioma is a relatively rare entity. Additionally a case of PEH coexisting with endobronchial metastasis is extremely rare.
Keywords: Cryobiopsy; Hemoptysis: Pulmonary epithelioid hemangioendothelioma
Introduction
Pulmonary Epithelioid Hemangioendothelioma (PEH), formerly known as Intravascular Bronchioalveolar Tumor (IVBAT), is an uncommon tumor of vascular endothelial origin with an intermediate course between hemangioma and conventional angiosarcoma [1-3]. PEH can arise from various organs, including lung, liver and bone, simultaneously or sequentially; however, no case of endobronchial metastases were found in the literature so far.
Herein, we were presented an extremely rare case of a patient with endobrochial metastasis of PEH. This is a rare described case of PEH coexisting with endobronchial metastasis, pulmonary nodules and mediastinal lymphnodes, so far.
Case Presentation
A 44–year-old, non-smoker, female patient had a moderate dyspnea and intermittent hemoptysis for two months was referred to our hospital for further investigation with malignant mesenchymal bronchoscopic findings in addition to a normal Positron Emission Tomography (PET/CT) images. She had no prior medical or occupational histoy. There was no family history of cancer. Only slightly decreased breath sounds were found onphysical examination. Laboratory values and pulmonary function test were normal except increased erythrocyte sedimentation rate. PET/CT scan demonstrated hypermetabolic mass in the hilum of right lung and multiple hypermetabolic lymph nodes in the mediastinum with low density (SUVmax: 3.5) and bilateral multiple parenchymal nodules. In the mediastinum, hypermetabolic lymph nodes were seen in the right paratracheal, subcarinal and bilateral hilar region with SUVmax of 10 (Figures 1a and 1b). A necrotic mixed lession tend to bleeding in the right upper lobe entry and medial wall of distal left bronchus with narrowed upper left and intermediate bronchus by tumoral infiltration were seen on her initial fiberoptic bronchoscopy. Because of tendency to bleeding of the lesions, she was underwent rigid bronchoscopy and cryobiopsy through rigid bronchoscope under total intravenous anesthesia was performed for diagnose (Figures 2a and 2b).
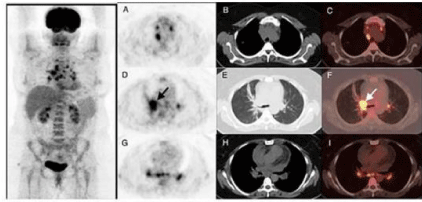
Figure 1: Maximum Intensity Projection (MIP) image of PET show hypermetabolic lesions in the bilateral hilum and mediastinum and axial slices of PET/ CT
showed intense FDG uptake of night hilar mass (D,E,F) (arrows) and hypermetabolic lymph nodes in the mediastinum.
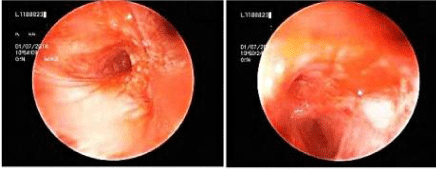
Figure 2: a. Tumoral infiltration of distal right main bronchus, upper lobe and secondary carina with narrowed intermediate bronchus caused by tumors. b. Necrotic
lesion on the medial wall of distal main left bronchus and narrowed left upper lobe.
Bronchoscopic cryobiopsy showed atypical cells in abundant eosinophilic stroma (Figure 3). Tumor cells have round and oval nuclei and intracellular vacuoles. Intracellular vacuoles are creating asignet ring appearance (Figure 4). Immunohistochemically, tumor cells were reactive for CD31, but no for cytokeratin AE1/AE3, thyroid transcription factor (TTF-1), Napsin A and CEA (Figure 5). In consideration of these findings, the patient was diagnosed with epithelioid hemangioendothelioma. Because there is no standard treatment for PEH she was followed for a year without treatment. Then the bone metastases (on ribs and vertebrae) were occured. Radiotherapy was begun as a palliative therapy for severe pain. She died as a result of PEH progression after fifteen months.
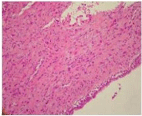
Figure 3: Tumor cells in abundant eosinophilic stroma (HEX100).
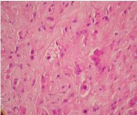
Figure 4: Tumor cells have cytoplasmic vacuoles intracytoplasmic lumina
(HEX400).
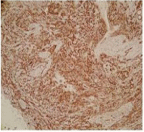
Figure 5: Showing positivity with CD31 in tumor cells (CD31X40).
Discussion
Pulmonary epithelioid hemangioendothelioma, originally described by Dail and Liebow, in 1975 as an IVBAT, is an uncommon tumor of vascular endothelial origin with an intermediate course between hemangioma and conventional angiosarcoma [1-3]. Immunohistochemical and electron microscopy studies revealed that IVBAT is of endothelial origin and the tumor was renamed PEH [4]. The 2004 World Health Organization classification of tumors regards PEH as a low-to intermediate-grade vascular neoplasm [5]. Therefore, some authors began to believe the hypothesis that PEH does not represent a distinct entity, but rather is an intermediate state of endothelial differentiation with a highly variable and unpredictable prognosis [6].
PEH is also of multicentric origin and extra pulmonary lesions arise from the liver, bone, thyroid, soft tissue and skin, simultaneously or sequentially. So far, about 120 cases of PEH have been reportedand none of them included endobronchial involvement. In light of this information this case may be the first report of endobronchial metastasis of PEH.
Patients with PEH are usuallyasymptomatic or have minor symptoms such as chest pain, cough, dyspnea rarely hemoptysis and weight loss. In our case, patient has moderate dyspnea and intermittent hemoptysis. PEH often occurs in middle-aged women, with a mean patient age of 40 years old (range 20 to 60 and is four times more common in women than men) [7]. The etiological factors are not well known.
Imaging of PEH is mainly characterized by multiple pulmonary nodules, rare solitary lesions and nodule diameters of 1-2cm (although a diameter of >5cm has also been reported) and sometimes present with lymph node metastases, pleural thickening or groundglass opacities. Calcifications and ossifications have occasionally been found in the lesions [3,8]. These can invade the pleura and cause pleural effusion or nodular thickening. As PEH shows multiple lung nodules on imaging, it is often misdiagnosed as peripheral lung cancer with lung metastasis. The differential diagnosis of PEH includes chronic granulomatous disease, amyloid nodules, hamartoma, primary or metastatic lung cancer, malignant mesothelioma and angiosarcoma. So PET/CT is considered to be an important tool for PEH diagnosis as the metabolic activity of the nodule and also for differential diagnosis. As shown in (Figures 1and 2), a significantly increased FDG uptake is right hilar and multiple mediastinal lymph nodes, while only mild or normal uptakes in nodules. Moreover, Cazzuffi reported three cases of PEH were falsely negative for PET/CT, suggesting that a negative PET/CT can not completely exclude PEH [9].
The diagnosis of PEH is depending on immunopathological examination of the biopsy specimen. Bronchoscopy should be performed with PEH in patients presenting with hemoptysis despite usually normal bronchoscopic findings. Rarely, as in our case, there was an endobronchial involvement could be seen. The tumor cells of PEH are round with abundant eosinophilic cytoplasm and intracytoplasmic vacuolization having a signet ring-like appearance. Positive immunohistochemical staining for endothelial markers (CD31, factor VIII and CD34) is pivotal for the diagnosis of PEH and also in our case tumor cells were positive for CD 31while the markers for mesothelial cell, epithelial cell, and muscular differentiation were negative immunohistochemically (Figure 5).
There is no standard therapy for the PEH because of its rarity. Tumour resection is the most common treatment when the lesions of PEH inside one lung are relatively limited. Radiotherapy is ineffective due to radiobiological characteristics of PEH (slow growth). Good local control has been reported in a patient with exclusive PEH bone presentation treated by combining RT with bone surgery [10]. She had no therapy on following-up until multiple bone metastases were occurred in her ribs and vertebrae. Radiotherapy was begun locally as a palliative therapy for her severe pains.After fifteen month she died as a result of PEH progression.
In summary, we describe a very rare case of PEH coexisting with endobronchial metastasis presented with multiple bilateral lung nodules and mediastinal lymphnodes. She was diagnosed by bronchoscopic cryobiopsy. PET/CT scan was useful to differentiate the nodule which was PEH from the other nodules.
References
- Dail DH, Liebow AA. Intravascular bronchioloalveolar tumor. Am J Pathol. 1975; 78: 6-7.
- Dail DH, Liebow AA, Gmelich JT, Friedman PJ, Miyai K, Myer W, et al. Intravascular, Bronchiolar And Alveolar Tumor Of The Lung (IVBAT): an analysis of twenty cases of a peculiar sclerosing endothelial tumor. Cancer. 1983; 51: 452-464.
- Kitaichi M, Nagai S, Nishimura K, Itoh H, Asamoto H, Izumi T, et al. Pulmonary epithelioid haemangioendothelioma in 21 patients, including three with partial spontaneous regression. Eur Respir J. 1998; 12: 89-96.
- Weldon-Linne CM, Victor TA, Christ ML, Fry WA. Angiogenic nature of the “intravascular bronchioloalveolar tumor” of the lung: an electron microscopic study. Arch Pathol Lab Med. 1981; 105: 174-179.
- Travis WD, Brambilla E, Muller-Hermelink HK, Harris CC. World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of Lung, Pleura, Thymus and Heart. IARC Press; Lyon. 2004; 9.
- Liu Q, Miao J, Lian K, Huang L, Ding Z. Multicentric epithelioid hemangioendothelioma involving the same lower extremity: a case report and review of literature. Int J Med Sci. 2011; 8: 558-563.
- Amin RM, Hiroshima K, Kokubo T, Nishikawa M, Narita M, Kuroki M, et al. Risk factors and independent predictors of survival in patients with pulmonary epithelioid hemangioendothelioma. Review of the literature and a case report. Respirology. 2006; 11: 818-825.
- Xu JH, Chen LR. Pulmonary epithelioid hemangioendothelioma accompanied by bilateral multiple calcified nodules in lung. Diagn Pathol. 2011; 6: 21.
- Cazzuffi R, Calia N, Ravenna F, Pasquini C, Saturni S, Cavallesco GN, Quarantotto F, et al. Primary Pulmonary Epithelioid Hemangioendothelioma: A Rare Cause of PET-Negative Pulmonary Nodules. Case Rep in Med. 2011.
- Sardaro A, Bardoscia L, Angelelli G, Petruzzelli MF, Nikolaou A, Detti B, et al. Pulmonary epithelioid hemangioendothelioma presenting with vertebral metastases: a case report. Journal of Medical Case Reports. 2014; 201.