Case Report
Austin Surg Case Rep. 2016; 1(2): 1012.
Role of the Multidisciplinary Team and Parenchyma Sparing Hepatectomy in a Patient Presenting with Complex Synchronous Metastatic Colorectal Cancer
Caterina V¹, Lucio U², Riccardo B², Piero B4, Alessandro L4, Piero C5, Gabriella L3, Francesco F3, Maura C6, Piero B² and Gianluca M¹*
¹Medical Oncology Unit, Azienda Ospedaliero- Universitaria Pisana, Italy
²General Surgery Unit, Azienda Ospedaliero-Universitaria Pisana, Italy
³Anaesthesiology and Intensive Care Unit, Azienda Ospedaliero-Universitaria Pisana, Italy
4Radiology Unit, Azienda Ospedaliero-Universitaria Pisana, Italy
5Hepatology Unit, Azienda Ospedaliero-Universitaria Pisana, Italy
6Pathology Unit, Azienda Ospedaliero-Universitaria Pisana, Italy
*Corresponding author: Gianluca Masi, Oncologia Medica Universitaria, Azienda Ospedaliero-Universitaria Pisana, Italy
Received: November 01, 2016; Accepted: November 21, 2016; Published: November 22, 2016
Abstract
Patients who present with colorectal cancer and synchronous liver metastases are a major challenge for oncologists and surgeons. Indeed, in this setting, the treatment strategy can achieve long-term survival and sometimes a definitive cure of disease. To achieve the best results it is mandatory a dedicated multidisciplinary team that is crucial to set the overall strategy, the achievement of a significant tumor shrinkage with current systemic therapies and finally the adoption of modern surgical techniques. Here we present a case of a complex metastatic colorectal cancer who presented with synchronous unresectable liver metastases who achieved a particularly favorable outcome. Patients were successfully treated with a multimodal strategy of systemic therapies and liver sparing surgery with minor but complex liver resection.
Keywords: Colorectal cancer; Liver metastases; Parenchyma sparing surgery
Introduction
Treatment of metastatic colorectal cancer has rapidly evolved and the availability of more effective chemotherapy regimens and biological agents directed against Vascular Endothelial Growth Factor (VEGF) and the Epidermal Growth Factor Receptor (EGFR), together with the increased use of surgery on metastases, have improved median Overall Survival (OS) up to more than 30 months in recent trials, with a 10‑year OS of 20-25% for radically resected patients [1]. The majority of metastatic colorectal cancer patients have unresectable metastatic disease. However, even though in this subset immediate radicalsurgical resection is not possible, improvements in medical management of metastatic colorectal cancer have raised the possibility of achieving radical resectability of metastases in case of response to systemic treatment. The impact of secondary surgery has been definitively proved by the analysis of Adam, et al. who demonstrate that long-term survival of patients undergoing secondary resection after response to chemotherapy is similar to patients resected upfront [2]. The involvement of vital hepatic structures still remainsa major challenge for resectability. The introduction of the new concepts of the Parenchyma Sparing Hepatectomy (PSH) increases the chance of treatment [3,4] offering an oncological advantage [5]. We here present a case of a mCRC patient with unresectable disease presentation, which support the Multi Disciplinary Team (MDT) approach and the usefulness of PSH in the management of such a complex disease presentation.
Case Presentation
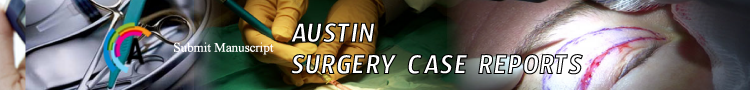
In February 2011, a 65 years-old man underwent abdominal ultrasonography that showed multiple liver lesions. A colonoscopy revealed a not stenotic lesion of the left colic flexure (Biopsy: adenocarcinoma). Computed Tomography (CT) of abdomen and chest showed concentric wall thickening (maximum 12 mm) charged to the proximal descending colon and extensive metastatic involvement ofthe 52% of the liver with the major lesion of 10 cm at the left hepatic lobe (Figure 1, Panel A). Baseline CEA was 680 ng/mL. Liver function tests were normal.
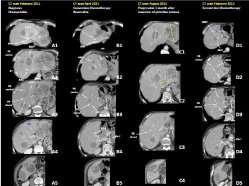
Figure 1: Computed Tomography (CT) scan of the case of multiple bilobar
unresectable synchronous Colorectal Liver Metastasis (CRLM) of a left
colonic adenocarcinoma. Panel A: CT-scan at the diagnosis: CRLMs
were unresectable due to the inflow and outflow involvement. A3: see the
involvement of the portal branch of the dorsal part of segment VIII (P8
dorsal). A4: Segments IVb and V were disease free and this was enough to
judge the patient potentially resectable. Panel B: CT-scan after 2 cycles of
chemotherapy documented a good response. B3: P8 dorsal was disengaged
from the CRLM. Panel C: CT-scan 1 month after the laparoscopic resection
of the primitive, documented disease progression (yellow arrows). Panel
D: computed tomography after 12 cycles of second line chemotherapy
documented a good responce. D1: The CRLM (blue arrow) in contact with
the Middle Hepatic Vein (MHV) to be treated by intraoperative radioablation.
D2: CRLM of segment VIII involved P8 ventral, MHV and RHV. D3: P8 dorsal
was completely disease free.
MHV: Middle Hepatic Vein; RHV: Right Hepatic Vein; P: Portal Branch.
The patient was in good general conditions, with an Eastern Cooperative Oncology Group (ECOG) Performance Status of 0 and didn’t have relevant past pathologic history to report.
Mutational analyses of the tumor biopsy resulted KRAS, NRAS, HRAS and BRAF wild type.
First MDT meeting
The patient was judged unresectable due to tumour involvement of the hepatic inflow and outflow, butpotentially resectable since segments IVb and V were disease-free (Figure 1, Panel A4). The patient was enrolled in first-line phase II trial where mCRC patients, wild type in RAS extended evaluation, were treated with panitumumab 6 mg/kg, 5-fluorouracil 2400 mg/sq continuous infusion 48 hours, lederfolin 200 mg/sq, irinote can 150 mg/sq and oxaliplatin 85 mg/sq, with cycles to be repeated every two weeks [6].
After the second cycle of chemotherapy, the patient experienced a Grade (G) 3 neutropenia, diarrhea and asthenia. As a consequence, the third cycle was delayed and dose of irinote can and 5-fluorouracil were reduced (120 mg/sq and 2000 mg/sq respectively) as per protocol. Nevertheless, the programmed control CT scantwo months after the diagnosis, was consistent with a partial response to the first cycles of chemotherapy (Figure 1, Panel B).
The patient achieved a good tolerance until the seventh cycle, when CT scan showed partial response with size reduction of more than 50% of the major liver lesion, measuring 4.1 cm.
Second MDT meeting
The result of the induction chemotherapy was evaluated and the patient was judged resectable, since the tumor shrinkage disengaged the portal branch for the dorsal part of segment VIII (i.e. compare P8 dorsalin) (Figure 1, Panel A3 and B3) and it was hypothesized a liver resection with preservation of segments IV, VIII-dorsal and V and reconstruction of middle Hepatic Vein (HV) appeared to be feasible. Considering the complexity of liver resection, the high tumor burden and the risk of microscopic disease outside the liver, MDT planned the surgical resection of the primary tumor first and then the liver surgery. This strategy, although not standardized, could be preferable in order to avoid the risk of an early progression of metastatic disease after a complex and demanding liver surgery and to assess the disease aggressiveness and biology.
On 12 July 2011 the patient underwent laparoscopic resection of the left colic flexure (Histology: poorly differentiated adenocarcinoma ypT3N0, 29 lymph nodes analyzed). The post-operative course was uneventful and the patient was discharged on the 6th post-operativeday.
A CT scan performed on August 2011 showed liver progression with major hepatic lesion measuring 8cm (Figure 1, Panel C1 and C2).
Third MDT meeting
Considering the rapid progression after the stop of chemotherapy it was decided to start asecond-line chemotherapy with FOLFOX and bevacizumab.
The CT scans performed after 6 and 12 cycles of chemotherapy showed again a partial response with the major liver lesion measuring 1.6 cm (Figure 1, Panel D).
Fourth MDT meeting
The response to second line chemotherapy was evaluated and the hepatic resection was deemed feasible and scheduled. At this time it was planned to manage the hepatic outflow with the tangential resection and reconstruction of right-HV and middle-HV (Figure 1, Panel D2) and with an intra-operative ablation of the little CRLM close to the middle-HV in segment IVa (blue arrow in Figure 1, Panel D1).
On April 2012 the patient underwent resection of segments I (paracaval portion and Spigel Lobe)-VIII ventral, II-III, VI-VII, metastasectomyof IVa (Figure 2, Panel A3), intraoperative radio frequency ablation of the lesion of segment IVa (Figure 2, Panel D2) (see the track ablation still evident 4 years after, blue arrow), tangential resection and reconstruction by direct suture of middle and right HVs. Surgery lasted 20 hours and 30 minutes; the cumulative pedicle clamping time was 350 minutes; the estimated intraoperative blood loss was 1600 ml and 3 blood units were transfused; the total volume of bleeding was caused by the resection and reconstruction of sovraepatic vessels close to hepato-caval confluence. The patient was discharged in the 20th post-operative day, with an uneventful post-operative course except for isolated iperbilirubinemia (up to 19 mg/dl), which spontaneously recovered. At histology 8 CRLMs were resected with an R0 resection; major diameter: 8 cm; satellitosis was present; Tumor Regression Grade according Rubbia-Brandt: 3.
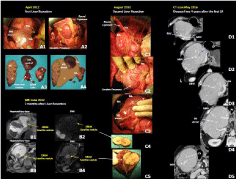
Figure 2: Intraoperative and radiology imaging of the case of multiple
bilobar unresectable synchronous Colorectal Liver Metastasis (CRLM) of a
left colonic adenocarcinoma. Panel A: Intraoperative field and specimens
of the first minor-but-complex liver resection. A1-2: the intraoperative field.
A3: Specimens of the resected liver. A4: Schematic representation of the
liver remnant: the absence of continuity between at least 3 adjacent resected
segments is evident. Panel B: Magnetic Resonance Imaging (MRI) 2 months
after the first liver resection documented the presence of 2 CRLMs. B1:
Hepatobiliary phase of the CRLM of segment IV (yellow arrow). B2: Diffusion
Weighted Imaging at MRI of the CRLM of segment IV (yellow arrow). B3:
Hepatobiliary phase of the CRLM of segment V (yellow arrow). B2: Diffusion
Weighted Imaging at MRI of the CRLM of segment V (yellow arrow). Panel
C: Intraoperative field and specimens of the second liver resection. C1-3: the
intraoperative field. C4: Specimens of the resected liver. Panel D: Computed
Tomography (CT) scan 4 years after the first liver resection. The patient was
disease free more than 2 years and half after chemotherapy withdrawal;
the whole liver is well perfused. D2: the track-ablation corresponding to the
intraoperative radioablation performed during the first liver resection is still
evident.
MHV: Middle Hepatic Vein; RHV: Right Hepatic Vein; P: Portal Branch; DWI:
Diffusion Weighted Imaging.
Figure 3: 3-D reconstruction at Computed Tomography (CT) scan of the case
of multiple bilobar unresectable synchronous Colorectal Liver Metastasis
(CRLM) of a left colonic adenocarcinoma. Panel A: CT-scan performed at
the diagnosis. Panel B: CT-scan performed 1 month after liver resection.
MHV: Middle Hepatic Vein; RHV: Right Hepatic Vein; LHV: Left Hepatic Vein.
The CT scan of May 2012 showed the patency of the reconstructed middle and right HVs. The calculated liver volume was 890 ml, representing the 66.5% of the estimated total liver volume (Figure 3). The CT scan showed also the growth of a 0.8 cmsatellite nodule in segment V adjacent to the liver cut surface.
An MRI, performed in June 2012, confirmed the growth of the satellite nodule of segment V and the presence of a second satellite nodule in segment IV aclose to the liver cut surface of segment IV (Figure 2, Panel B).
Fifth MDT meeting
Considering previous systemic treatments and the limited residual disease in the liver the surgical clearance of the 2 satellites was scheduled.
On August 2012, the patient underwent the second liver surgery. Intraoperative Ultrasound (IOUS) confirmed the presence of the 2 CRLMs without any other new lesion. The liver resection consisted in metastasectomy of segment V and of the CRLM of segment IVa (Figure 2, Panel C). Surgery lasted 8 hours and 10 minutes; the length of the intervention was also due to the presence of several intraabdominal adhesions; the cumulative pedicle clamping time was 49 minutes; the estimated intraoperative blood loss was 200 ml and 1 blood unit was transfused; the patient was discharged in the 9th postoperative day with an uneventful post-operative course. At histology (Figure 2, Panel C4) 2 CRLMs were resected with an R0 resection.
Sixth MDT meeting
In the absence of specific data about post-operative therapy after R0 resection, but considering the baseline characteristics of the case and the natural history of the disease, a post-operative chemotherapy with FOLFOX and bevacizumab for other 6 cycles was administered and the a maintenance therapy with bevacizumab until October 2013.
Seventy months after the diagnosis, the patient is still disease free and he has a normal quality of life.
Discussion
Colorectal liver metastases represent a major challenge for oncologists and surgeons. In fact, in this setting, the optimal treatment of patients can achieve a long-term survival and sometimes a definitive cure of disease. In recent years, improvements in both medical therapies and surgical approaches have led to an increased rate of patients considered amenable for surgery on liver.
In last years, different combination regimens have been prospectively evaluated and have shown interesting results in terms of conversion treatment and long-term overall survival.
The management of this asymptomatic adenocarcinoma of the left colonic flexure with multiple synchronous unresectable liver metastases well underlines the role of the MDT and of the knowledge of the PSH surgical technique. In fact, at the case presentation meeting the patient was judged unresectable due to the inflow and outflow liver involvement but, anyway, potentially resectable since segments V and IVb were disease-free (Figure 1, Panel A4).
This was one of our first cases of minor-but-complex liver resection and our learning curve was expressed mainly in the longer operative times, but not in a significant 90-day mortality and severe morbidity [2]. More than 4 liver segments were resected, but it was still a minor resection, since three adjacent liver segments were not resected. In fact, as shown in (Figure 2, Panel A), segments VIVII were resected, but segments VIII dorsal and V were preserved; segment VIII ventral, Spigel Lobe, the paracaval portion of segment I and segments II-III were resected, but the Caudate Processus and segment IV were preserved. The absence of continuity between at least 3 adjacent resected segments is well described in the schematic representation of the liver remnant in (Figure 2, Panel A4). The preservation of the “hepatic skeleton” determined the uneventful postoperative course in spite of the very long surgical times and the intraoperative bleeding which was compatible with the resection of both the HVs at the hepatocaval-confluence. (Figure 3) shows that 2 of 3 HVs have been preserved, even though both the middle and the right HVs were tangentially resected and reconstructed by direct suture.
It is important to remark that the initial decision in which the patient was judged unresectable was based not on the amount of the functioning liver representing the 60% of the estimated total liver volume. We can speculate that this PSH gave little regenerative stimuli since the calculated total liver volume 1 month after liver resection still remained the 66.5% of the estimated total liver volume.
Anyway, it is highly probable that the immunosuppression due to the liver resection contributed to the growth of two CRLMs not detectable by IOUS. At this point the patient posed a critical issue since he received full chemotherapy regimens and a recent really complex liver surgery. Taking into account that histology confirmed the presence of satellitosis, the 2 nodules were close to the surgical margins and the liver away from the margins was disease free, the MDT considered these two lesions assatellite nodules not identified by previously IOUS, which showed progression similarly to what happened after the resection of the primary. A repeated liver resection was considered the best approach to definitively clear the hepatic parenchyma.
The IOUS, performed during the re-resection, excluding the presence of other CRLM supported this hypothesis. The CRLM of segment V was easily resected and the CRLM of segment IVa was resected with a 1 mm margin. The post-operative course after this repeated liver resection was uneventful. More than 5 years after the mCRM diagnosis the patient is disease free, chemotherapy free and returned to his normal life.
This case clearly shows the important role of PSH to increase the therapeutic chance in mCRM patients. Moreover, this case highlights the essential role of the multidisciplinary board evaluation from the onset of the presentation and during treatment. This is the key element to maximizing the benefit of more intensive treatment modalities by adapting every therapeutic option to the behavior of the tumor biology.
References
- Cremolini C, Schirripa M, Antoniotti C, Moretto R, Salvatore L, Masi G, et al. First-line chemotherapy for mCRC-a review and evidence-based algorithm. Nat Rev Clin Oncol. 2015; 12: 607-619.
- Adam R, Wicherts DA, De Haas RJ, Ciacio O, Levi F, Paule B, et al. Patients with initially unresectable colorectal liver metastases: is there a possibility of cure? J Clin Oncol. 2009; 27: 1829-1835.
- Vigano L, Torzilli G, Cimino M, Imai K, Vibert E, Donadon M, et al. Drop-out between the two liver resections of two-stage hepatectomy. Patient selection or loss of chance? Eur J Surg Oncol. 2016; 42: 1385-1393.
- Urbani L, Masi G, Puccini M, Colombatto P, Vivaldi C, Balestri R, et al. Minor-but-Complex Liver Resection: An Alternative to Major Resections for Colorectal Liver Metastases Involving the Hepato-Caval Confluence. Medicine (Baltimore). 2015; 94.
- Mise Y, Aloia TA, Brudvik KW, Schwarz L, Vauthey JN, Conrad C. Parenchymal-sparing Hepatectomy in Colorectal Liver Metastasis Improves Salvageability and Survival. Ann Surg. 2016; 263: 146-152.
- Fornaro L, Lonardi S, Masi G, Loupakis F, Bergamo F, Salvatore L, et al. FOLFOXIRI in combination with panitumumab as first-line treatment in quadruple wild-type (KRAS, NRAS, HRAS, BRAF) metastatic colorectal cancer patients: a phase II trial by the Gruppo Oncologico Nord Ovest (GONO). Ann Oncol. 2013; 24: 2062-2067.