Case Presentation
Austin Surg Case Rep. 2017; 2(1): 1016.
Gastric Adenocarcinoma with Bone Marrow Infiltration: Case Report and Review of the Literature
Siaperas P¹, Skarpas A¹*, Chorti M², Theanou A¹, Karanikas I¹ and Velimezis G¹
¹Surgical Department of Sismanoglion General Hospital, Athens, Greece
²Pathology Department of Sismanoglion General Hospital, Athens, Greece
*Corresponding author: Skarpas A, 2nd Surgical Department of Sismanoglion General Hospital, Athens, Greece
Received: June 01, 2017; Accepted: June 26, 2017; Published: July 03, 2017
Abstract
This case report describes a 77-year-old woman who initially was admitted in the ER department, complaining for diffuse abdominal pain, anorexia, weight loss and bone pain, while her blood tests showed coexistent anemia & severe thrombocytopenia. During her inpatient stay, she presented with hematemesis, hematochezia and petechiae, in the form of Diffuse Intravascular Coagulation (DIC).
Diagnostic work-up included upper gastrointestinal endoscopy and biopsy which revealed a diffuse gastric non-signet ring cell type adenocarcinoma, bone marrow biopsy which showed bone marrow infiltration from cancer cells with epithelial origin and diffuse necrosis, and bone scan which revealed multiple lytic bone lesions throughout the bony skeleton.
This case is significant, primarily for its importance in the differential diagnosis when approaching a patient with bone pain and lytic bone lesions and also because it points out an increasing incidence of diffuse type gastric cancer, and the severity of a coexisting infiltration of the bone marrow at the time of diagnosis.
Keywords: Gastric cancer; Bone marrow infiltration; Bone marrow metastasis; Lytic bone metastasis; Stomach cancer; Hematochezia; Diffuse type; Non-signet ring cell type; Chemotherapy
Case Presentation
This case report describes a 77-year-old woman, ex-smoker, with a history of high blood pressure and COPD, who presented in the ER department with diffuse abdominal pain, anorexia, fatigue, weight loss and diffuse bony pain, which started 15 days ago. She denied further symptoms, such as fever, night sweats, vomiting, hematochezia or melena. She also mentioned a history of bilateral fracture of the hip bones (1 & 4 years ago).
Physical examination revealed pale skin color and diffuse petechiae in the extremities and abdominal region, normal bowel movement and tenderness during palpation of the right upper quadrant of the abdomen. Rectal digital examination was negative for blood. Initial laboratory evaluation in the ER, showed anemia (Hgb 7.7g/dL - Ht 23,7%), MCH 27, 4pg, MCV 84, 3fl, MCHC 32, 5g/dL, low platelet count (42000K/μl), total bilirubin 1, 55mg/dl, indirect bilirubin 1, 10mg/dl, LDH 548 U/L and high alkaline phosphatase (1261).
Patient was admitted in Medical ward for further diagnostic evaluation. While being inpatient, a set of laboratory tests were performed: direct & indirect Coombs which were both negative, PT 14, 9sec, INR 1, 34, aPTT 35, 31sec, D-Dimmers >36581.8, fibrogen 1.7, serum protein electrophoresis, which returned positive for a small increase of the a1 band, haptoglobin 0.1g/l, Quantative determination of IgG/M/A was within normal range, Ca 19-9 >5000, PO4- 2, 9mg/ dl, Ca+ 9,1mg/dl, RET% 2, 83, ESR 30mm, FOBT positive. An x-ray of the skull, thorax and the upper extremities was also requested, which were normal.
Because of high D-Dimmers, low fibrogen and low PLT count, DIC syndrome was considered from the hematologists, as was, micro vascular hemolytic anemia of unknown origin. Differential diagnosis was suspicious of metastatic cancer or Paget’s disease. GI endoscopy was postponed initially due to low PLT count. CT scan imaging was performed, showing thickening of the gastric wall mainly located in the lesser curvature of the stomach and hyper-condensation formats, diffusely scattered in the vertebrae and bones. A bone scan was suggested to be performed when the patient was clinically stable. Hematologists proposed to treat DIC, with transfusion of fresh frozen plasma and blood transfusion when HGb<10.
Two days after admission to the medical ward, patient had an episode of hematemesis and an emergency gastroscopy was ordered, which revealed intense gastritis with no obvious source of bleeding. No biopsy was taken due to low platelet count. Three days later patient had massive melena. At that point surgical evaluation was done, which insisted in attempting again GI endoscopy and biopsies. Both upper and lower endoscopy was successfully performed, showing abnormal deformity of the gastric antrum, with no signs of active bleeding. Multiple biopsies were taken from the lesion. Colonoscopy was completely normal. Gastric biopsies showed gastric carcinoma, and since patient was continuously bleeding, having massive melenas, surgery was decided. Surgical operation was performed with upper midline incision, and findings included a prescribed antral mass, which was completely excised, performing Billroth-II gastrectomy with gastrojejunal anastomosis. Post surgical course was uneventful; however, the patient was still often needed blood and platelet transfusion, suggesting that disease was also infiltrating the bone marrow. Thus, bone marrow needle biopsy and bone marrow scan were also performed. Patient returned to the medical ward for aftercare and plan for further therapy.
The final pathologic review of the surgical specimen revealed a diffuse type (Laurent), gastric adenocarcinoma showing intense desmoplastic stroma with limited focal lymphocytic infiltration and extensive lymphocytic dispersion. Infiltration of the entire gastric wall was observed, while 5 out of 20 lymph nodes were positive. Immunohistochemistry straining was positive for CK-7, CEAp, e-Cadherin and CDX-2 and focal expression of CK20. Bone marrow specimen from needle biopsy was fully infiltrated from neoplasmatic cells with epithelial origin and diffuse necrosis. Bone scan revealed diffuse metastatic disease with lytic bone lesions throughout the bony part of the skeleton (Figure 1).
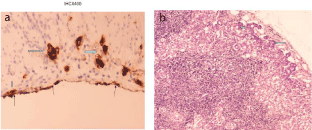
Figure 1: a. Gastric adenocarcinoma Expressed Immunohictochemixally
CEAp infiltrates subserosa (thick horizontal arrow) and serosa noticed with
thin arrows (thick vertical arrow).
b. Subcapsular local gastric lymph node infiltration by diffuse-type gastric
adenocarcinoma: H-EX100 (arrows).
Oncologists suggested palliative treatment because of the advanced stage of the disease. Patient died in the Medical ward 40 days post-gastrectomy (Figure 2).
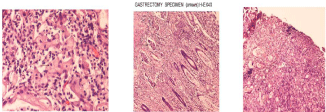
Figure 2: a. Gastric mucosa infiltration by signet ring type of adenocarcinoma (arrows), H-EX400.
b. Diffuse-type gastric adenocarcinoma infiltrates.
c. Gastric biopsy indicates infiltration by diffuse-type adenocarcinoma. H-EX40.
Discussion
Gastric cancer is the 4th most common cause of cancer worldwide and the 2nd leading cause of death from cancer [1]. It is more often associated with patients from East Asia and South America, but recent studies have confirmed a gradual increase in developing countries as well. In contrast, rates have been decreasing in the United States. Among developed countries, Japan and Korea have the highest rates of the disease [2], while especially in Korea it is considered the most prevalent malignancy and accounts for 18.3% of all solid tumors [3].
Since 1965, it has been divided according to Lauren Classification System, into intestinal-type and diffuse-type. Diffuse-type gastric cancer has usually a familial predisposition, an association with blood type A, suggesting a genetic cause and presents usually at a younger age, affecting women more often. It is often more advanced at presentation, particularly when compared to well or moderately differentiated intestinal type gastric cancer. It consists characteristically of small uniform poorly differentiated signet ring cells. It has a tendency to spread submucosally with less inflammatory infiltration comparing to the intestinal type, with early metastatic spread via transmural extension and lymphatic invasion. Intraperitoneal metastases are frequent and, in general prognosis is less favorable comparing patients with intestinal type cancer [2]. Intestinal variant typically arises in the setting of a recognizable precancerous condition, such as gastric atrophy or intestinal metaplasia. Men are more commonly affected in this group and the incidence increases with age. This cancer is typically well differentiated, with a tendency to form glands. Metastatic spread is generally hematogenous to distant organs. Finally it seems that the intestinal type is dominant in areas in which gastric cancer is epidemic, suggesting an environmental cause [2,4,5] (Table 1).
LAUREN CLASSIFICATION SYSTEM
INTESTINAL TYPE
DIFFUSE TYPE
Environmental
Familial
Gastric atrophy, intestinal metaplasia
Blood type A
Men> women
Women> men
Increasing incidence with age
Younger age group
Gland formation
Poorly differentiated, signet ring cell , without glands
Hematogenous spread
Transmural, lymphatic spread
microsatellite instability
Decreased E-cadherin
APC gene mutations
p53 , p16 inactivation
P53, p16 inactivation
(*APC: adenomatous polyposis coli)
Table 1: Lauren classification system.
The most common risk factors for gastric cancer are divided into nutritional, environmental, social, medical and genetic. The traditional risk factors do not play great role in diffuse-type gastric cancer, though studies have described the importance of familial syndromes such as hereditary diffuse gastric cancer [4,5,6]. Changes in certain environmental and social factors have led to a decrease in the predominant, intestinal-type gastric cancer. The major factors include reduced prevalence of Helicobacter pylori infection, decreased consumption of salted fish and meats, decreased consumption of smoked foods, and a decline in the use of tobacco products. In contrast, diffuse-type of gastric cancer has been increasing over the past 30 years [7,8]. This histologic shift from intestinal type gastric cancer to diffuse type will have important clinical implications [5,8] (Table 2).
NUTRITIONAL
Low fat or protein consumption
Salted meat or fish
High nitrate consumption
High complex carbohydrate consumption
ENVIROMENTAL
Poor food preparation (smoked, salted)
Lack of refrigeration
Poor drinking water (contaminated well water)
Smoking
SOCIAL
Poor social class
MEDICAL
Prior gastric surgery
H. Pylori infection
Gastric atrophy , gastritis
Adenomatous polyps
OTHER
Male gender
Table 2: The common risk factors for gastric cancer.
Carcinomatosis of the bones and the bone marrow more often originates from prostate, lung, breast, renal and bladder cancer [9- 18], and rarely from gastric carcinoma, more likely with primary cancer in the body of stomach, poorly differentiated adenocarcinoma and in cases with abundant lymph node metastasis in the vicinity. Metastasis to the bones and/or bone marrow is relatively uncommon with gastric carcinoma, although solid tumors are usually responsible for most cases of diffuse bone marrow metastasis and Disseminated Intravascular Coagulation (DIC) [19,20]. Bone metastasis often present with bone pain, pathologic fractures and spinal cord compression symptoms. In our case patient’s history involved diffuse bone pain. Pain management for these patients is very important and regarding pain it has been reported in the literature that while radiation therapy was effective in few cases [21], chemotherapy was not effective at all [22].
Most likely a primary gastric lesion spreads to the bone via lymphatic channels, although dissemination may also occur through portal [23]. As far as bone metastases in diffuse gastric cancer, most common sites involve ribs, scapula and pelvis, which were all found in our case [24]. Lesions are usually osteolytic in nature. Osteoblastic metastasis from gastric cancer has very rarely been reported [25]. Various laboratory abnormalities that suggest the possibility of bone metastases include elevated Alkaline Phosphate (ALP) – the levels were relatively related to the amount of bone metastases [26], increased LDH and anemia or/and thrombocytopenia. Our patient presented with all the above markers elevated [23,27].
Detection of bone metastases usually follows the diagnosis of gastric cancer. The reported incidence of bones metastases varies widely from as low as 1% in clinical practice to as high as 45% in screening studies, implying that many cases are asymptomatic [28]. When bone marrow metastases are found, more commonly involve signet-ring cell subtype gastric carcinoma and occur in younger patients [29,30], something that was not compatible with our case, as our patient was a 77-year-old woman and had a diffuse nonsignet ring cell gastric cancer. There has been described a tendency to occur in invasive cancer such as Borrmann type III or IV, in poorly differentiated adenocarcinomas and in cases with stroma being a scirrhous type, like in our patient [22,31,32,33].
In bone metastases from gastric cancer, cancer cells diffusely proliferate in the bone marrow and most of times, this can cause disseminated carcinomatosis; they also proliferate rapidly and thus induce bone destruction as well as hematological complications such as Disseminated Intravascular Coagulopathy (DIC), like in our case [29,34]. Once DIC develops, is an ominous sign with fast deterioration of the patient [35]. Yet the developmental mechanism of bone destruction has not yet been elucidated. More studies on this are required [29]. Prognosis when bone marrow is involved in cases of gastric adenocarcinoma is abysmal, because of a rapidly deteriorating clinical course that is often refractory to conventional treatment, with patient’s surviving an average of 44 days from the time of documented bone marrow involvement [27,28,36-38].
Gastric cancer remains a therapeutic challenge for medical oncologists, especially for those patients with bone marrow metastases. Chemotherapy for the treatment of advanced metastatic gastric carcinomas may be associated with better quality of life than symptomatic treatment alone. However, chemotherapy is not advised for patients with Eastern Cooperative Oncology Group score of 3 or higher and must be carefully utilized in a selective group of patients after extensive counseling. Those patients not responding should be recognized early [24]. Advanced signet ring cell carcinoma carries a poor prognosis compared to other types of gastric cancer [8]. Other factors associated with poor outcomes include micro invasion of vessels and tumor location; tumors involving the whole stomach understandably have worse prognosis compared to lesions localized in the antrum and the body [8]. Mean survival time is 4-5 months with bone metastases from gastric cancer and even less about 3 months with Leptomeningeal Carcinomatosis (LMC) [27,39,40] (Table 3).
GRADE
ECOG PERFORMANCE STATUS
0
Fully active, able to carry on all pre-disease performance without restriction
1
Restricted in physically strenuous activity but ambulatory and able to carry out work of a light or sedentary nature, e.g., light house work, office work
2
Ambulatory and capable of all self care but unable to carry out any work activities; up and about more than 50% of waking hours
3
Capable of only limited self care; confined to bed or chair more than 50% of waking hours
4
Completely disabled; cannot carry on any self care; totally confined to bed or chair
5
Dead
These scales and criteria are used by doctors and researchers to assess how a patient's disease is progressing, assess how the disease affects the daily living abilities of the patient, and determine appropriate treatment and prognosis. They are included here for health care professionals to access.
*As published in Am. J. Clin. Oncol.: Oken MM, Creech RH, Tormey DC, Horton J, Davis, TE, McFadden ET, Carbone PP. Toxicity And Response Criteria Of The Eastern Cooperative Oncology Group. Am J Clin Oncol.
Table 3: ECOG Performance Status. Developed by the Eastern Cooperative Oncology Group, Robert L. Comis, MD, Group Chair*.
Advanced Gastric Cancer (AGC) with distant metastases or recurrence remains incurable, with a median survival of 6-9 months. Systemic chemotherapy in gastric cancer patients with bone marrow metastases are limited. Chemotherapy is known to prolong survival as compared to Best Supportive Care (BSC), for the management of advanced gastric carcinoma. However, these reports in published literature were limited to patients with adequate bone marrow function; in other words, patients with bone marrow involvement were excluded [41]. The incidence of bone marrow metastases in gastric cancer patients is low; therefore, the clinical features and optimal treatment options for such patients has not yet been established. In the study by Kusumoto et al [15], the median survival was only 3 months in the chemotherapy group and 2 months in the BSC group, with borderline significance in gastric cancer patients with bone marrow metastasis. Kwon et al [16], published the largest case series in 2011. In that report, 15 patients received palliative chemotherapy and 10 patients received BSC. The median survival was 11 days in the BSC group compared to 121 days in the palliative chemotherapy group (P<0.001). A recent retrospective study by Park et al. reported a benefit for palliative chemotherapy in patients with bone metastasis: median overall survival was 167 days compared with 43 days for patients treated with radiotherapy and best supportive care. In that study, the chemotherapy regimens varied widely; most protocols consisted of a taxane, an anthracycline, a fluoropyrimidine, or a platinum agent [42].
Prognostic factors for poor survival were low performance status, multiple bone metastases, and elevated carcinoembryonic antigen. Patients with fewer than two of those adverse prognostic factors benefited from palliative chemotherapy. In the Park et al. Study, 85% of patients had multiple sites of metastasis, and therefore the true benefit of palliative chemotherapy in the setting of isolated bone metastasis remains unknown [42]. Because of the lack of prospective studies of gastric cancer patients with bone marrow metastases, an optimal chemotherapy regimen is still unknown. There has been though stated an usefulness of chemotherapy with MTX-5FU regimen even in critically ill patients with DIC, since bone marrow suppression is rare [43,44].
Conclusion
This case report highlights some important learning points associated with gastric cancer and bone marrow infiltration. Our case is significant, firstly because it underlines the importance of a broad differential diagnosis when approaching a patient with lytic bone lesions and back pain, since multiple rounded osteolytic lesions and pathologic fractures are often presumed to be multiple myeloma. Also, included in the differential are metastases from solid organs like prostate, breast, lung, thyroid, or kidney. However, it is prudent to consider gastric cancer as a potential primary site of disease even in the absence of overt gastrointestinal symptoms. Secondly our case is interestingly important because of the rarity of adenocarcinoma of diffuse type (Laurent), developing in an intense desmoplastic stroma with limited focal lymphocytic infiltration and extensive lymphatic dispersion, especially involving an old aged woman patient.
If signet ring cell morphology is found a lymphoma should also be included in the differential diagnosis. The prognosis is generally extremely poor. When patients are carefully selected, rarely they may benefit from palliative chemotherapy. Further studies may help us to determine the best treatment pathway for these difficult cases.
References
- Crivellari D, Carbone A, Sigon R, Buonadonna A, Cannizzaro R, Sorio R, et al. Gastric cancer with bone marrow invasion at presentation: case-report and review of the literature. Tumori. 1995; 81: 74-76.
- Townsend CM. Sabinston Textbook of Surgery. The Biological Basis of Modern Surgical Practice. 19th Edition.
- Shin HR, Jung KW, Won YJ, Park JG. 139 KCCR-affiliated Hospitals. 2002 annual report of the Korea Central Cancer Registry: based on registered data from 139 hospitals. Cancer Res Treat. 2004; 36: 103-114.
- Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. an attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965; 64: 31-49.
- Bamboat ZM, Tang LH, Vinuela E, et al. Stage-stratified prognosis of signet ring cell histology in patients undergoing curative resection for gastric adenocarcinoma. Ann Surg Oncol. 2014; 21: 1678-1685.
- Blair V, Martin I, Shaw D, Winship I, Kerr D, Arnold J, et al. Hereditary diffuse gastric cancer: diagnosis and management. Clin Gastroenterol Hepatol. 2006; 4: 262-275.
- Henson DE, Dittus C, Younes M. Differential trends in the intestinal and diffuse types of gastric carcinoma in the United States, 1973-2000: increase in the signet ring cell type. Arch Pathol Lab Med. 2004; 128: 765-770.
- Yokota Y, Kunii S, Teshima. Signet ring cell carcinoma of the stomach: a clinicopathological comparison with the other histological types. Tohoku Journal of Experimental Medicine. 1998; 186: 121-130.
- Anner RM, Drewinko B. Frequency and significance of bone marrow involvement by metastatic solid tumors. Cancer. 1977; 39: 1337-1344.
- Kiliçkap S, Erman M, Dinçer M, Aksoy S, Harputluoglu H, Yalçin S. Bone marrow metastasis of solid tumors: clinicopathological evaluation of 73 cases. Turk J Cancer. 2007; 37: 85-88.
- Mehdi SR, Bhatt ML. Metastasis of solid tumors in bone marrow: a study from northern India. Indian J Hematol Blood Transfus. 2011; 27: 93-95.
- Mohanty SK, Dash S. Bone marrow metastasis in solid tumors. Indian J Pathol Microbiol. 2003; 46: 613-616.
- Tasleem RA, Chowdhary ND, Kadri SM, Chowdhary QA. Metastasis of solid tumours in bone marrow: a study from Kashmir, India. J Clin Pathol. 2003; 56: 803.
- Frisch B, Bartl R, Burkhardt R. Bone marrow biopsy in clinical medicine: an overview. Haematologia (Budap). 1982; 15: 245-285.
- Penchansky L. Bone marrow biopsy in the metastatic work-up of solid tumors in children. Cancer. 1984; 54: 1447-1448.
- Wong KF, Chan JK, Ma SK. Solid tumour with initial presentation in the bone marrow- a clinic pathologic study of 25 adult cases. Hematol Oncol. 1993; 11: 35-42.
- Clifton JA, Philipp RJ, Ludovic E, Fowler WM. Bone marrow and carcinoma of the prostate. Am J Med Sci. 1952; 224: 121-130.
- Finklestein JZ, Ekert H, Isaacs H, Higgins G. Bone marrow metastases in children with solid tumors. Am J Dis Child. 1970; 119: 49-52.
- Pasquini E, Gianni L, Aitini E, Nicolini M, Fattori PP, Cavazzini G, et al. Acute disseminated intravascular coagulation syndrome in cancer patients. Oncology. 1995; 52: 505-508.
- Jarchow S. Diffusely infiltrative carcinoma: a hitherto undescribed correlation of several varieties of tumor metastasis. Arch Pathol. 1936; 22: 674-696.
- Murai N, Koga K, Nagamachi S, Nishikawa K, Matsuki K, Kusumoto S, et al. Radiotherapy in bone metastases- with special reference to its effect on relieving pain. Gan No Rinsho. 1989; 35: 1149-1152.
- Yoshikawa K, Kitaoka H. Bone metastasis of gastric cancer. Jpn J Surg. 1983; 13: 173-176.
- Basheer, Daniel J, Padhi S. Compressive myeloradiculopathy from bony metastasis as the initial presentation of poorly differentiated adenocarcinoma stomach- a case report. Australasian Medical Journal. 2013; 6: 515–519.
- Ahn JB, Ha TK, and Kwon SJ. Bone metastasis in gastric cancer patients. Journal of Gastric Cancer. 2011; 11: 38-45.
- Chung YS, Choi TY, Ha CY, Kim HM, Lee KJ, Park CH, et al. An unusual case of osteoblastic metastasis from gastric carcinoma. 2002; 43: 377-380.
- Nishidoi H, Koga S. Clinic pathological study of gastric cancer with bone metastasis. 1987; 14: 1717-1722.
- Kim HS, Yi SY, Jun HJ. Clinical outcome of gastric cancer patients with bone marrow metastases. Oncology. 2007; 73: 192-197.
- Turkoz FP, Solak M, Kilickap S, Ulas A, Esbah O, Oksuzoglu B, et al. Bone metastasis from gastric cancer: the incidence, clinicopathological features, and influence on survival. Journal of Gastric Cancer. 2014; 14: 164-172.
- Kusumoto H, Haraguchi M, Nozuka Y, Oda Y, Tsuneyoshi M, Iguchi H. Characteristic features of disseminated carcinomatosis of the bone marrow due to gastric cancer: the pathogenesis of bone destruction. Oncol Rep. 2006; 16: 735-740.
- Blanchette P, Lipton JH, Barth D, Mackay H. Case report of very late gastric cancer recurrence. 2013; 20: 161-164.
- Nishidoi H, Koga S. Clinic pathological study of gastric cancer with bone metastasis. 1987; 14: 1717-1722.
- Nishidoi H, Koga S. Clinic pathological study of gastric cancer with bone metastasis. Gan To Kagaku Ryoho. 1987; 14: 1717–1722.
- Carstens SA, Resnick D. Diffuse sclerotic skeletal metastasis as an initial feature of gastric carcinoma. Arch Intern Med. 1980; 140: 1666-1668.
- Blanchette P, Lipton JH, Barth D, Mackay H. Case report of very late gastric cancer recurrence. 2013; 20: 161-164.
- Noda N, Sano T, Shirao K, Ono H, Katai H, Sasako M, et al. A case of bone marrow recurrence from gastric carcinoma after a nine-year disease-free interval. 1996; 26: 472-475.
- Hayes DF. Prognostic and predictive factors revisited. Breast. 2005; 14: 493- 499.
- Kobayashi M, Okabayashi T, Sano T, Araki K. Metastatic bone cancer as a recurrence of early gastric cancer: characteristics and possible mechanisms. World J Gastroenterol. 2005; 11: 5587-5591.
- Wiedswang G, Borgen E, Kåresen R, Kvalheim G, Nesland JM, Qvist H, et al. Detection of isolated tumor cells in bone marrow is an independent prognostic factor in breast cancer. J Clin Oncol. 2003; 21: 3469-3478.
- Kim NH, Kim JH, Chin HM, Jun KH. Leptomeningeal carcinomatosis from gastric cancer: single institute retrospective analysis of 9 cases. Annals of Surgical Treatment and Research. 2014; 86: 16-21.
- Hussain S, Chui S. Gastric carcinoma presenting with extensive bone metastases and marrow infiltration causing extradural spinal haemorrhage. British Journal of Radiology. 2006; 79: 261-263.
- Ajani JA, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, et al. Clinical benefit with docetaxel plus fluorouracil and cisplatin compared with cisplatin and fluorouracil in a phase III trial of advanced gastric or gastroesophageal cancer adenocarcinoma: the V-325 Study Group. J Clin Oncol. 2007; 25: 3205-3209.
- Park HS, Rha SY, Kim HS, Hyung WJ, Park JS, Chung HC, et al. A prognostic model to predict clinical outcome in gastric cancer patients with bone metastasis. Oncology. 2011; 80: 142-150.
- Kobayashi T, Sasaki T, Ibuka T, Imai K, Monma K, Sasaki N, et al. Sequential MTX and 5-FU therapy of gastric, cancer with systemic bone metastasis and disseminated intravascular coagulation. Gan To Kagaku Ryoho. 1992; 19: 69-74.
- Noda N, Sano T, Shirao K, Ono H, Katai H, Sasako M, et al. A case of bone marrow recurrence from gastric carcinoma after a nine-year disease-free interval. Jpn J Clin Oncol. 1996; 26: 472-475.