Case Presentation
Austin Surg Case Rep. 2017; 2(1): 1017.
Aortic Root Dilatation and Loss of the Aortomitral Curtain due to Paravalvular Leakage after Double Valve Replacement: A Case Report
Cho SH¹ and Sung Kim W²*
¹Department of Thoracic and Cardiovascular Surgery, Kosin University Gospel Hospital, South Korea
²Department of Thoracic and Cardiovascular Surgery, Samsung Medical Center, Sunkyunkwan University School of Medicine, South Korea
*Corresponding author: Sung Kim W, Department of Thoracic and Cardiovascular Surgery, Samsung Medical Center, Sunkyunkwan University School of Medicine, South Korea
Received: June 09, 2017; Accepted: June 27, 2017; Published: July 04, 2017
Abstract
A man who had underwent double valve replacement surgery was admitted with paravalvular leakage. Aortic root dilated and only a small amount of tissue was found around the aortomitral curtain. These anatomical change was thought to be the result of longstanding PVL. We performed bent all operation with aortomitral reconstruction and mitral valve replacement. The patient was managed successfully and we report it.
Keywords: Heart valves; Heart valve prosthesis implantation
Case Presentation
A 50-year-old man was admitted to a local medical center with symptoms of progressive dyspnea for three weeks. About 24 years previously he underwent Double Valve Replacement (DVR) surgery for infective endocarditis of the aortic and mitral valves. A transthoracic echocardiogram revealed severe aortic regurgitation with a dilated aortic root and moderate left ventricular dysfunction. The patient was transferred to our hospital for further evaluation and management, and additional echocardiography and thoracic computed tomographic angiography were performed. The Transthoracic Echocardiogram (TTE) showed moderate-to-severe aortic regurgitation due to paravalvular leakage, a well-functioning prosthetic mitral valve and mild-to-moderate tricuspid regurgitation (Figure 1). CT angiography revealed a60-mm ascending thoracic aortic aneurysm and 66mm dilated aortic root (Figure 2).
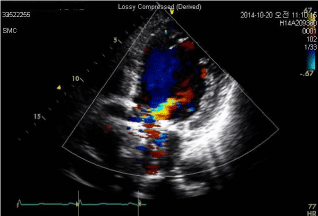
Figure 1: Preoperative transthoracic echocardiogram.
A preoperative Transthoracic Echocardiogram (TTE) showed moderate-tosevere
aortic regurgitation from paravalvular leakage.
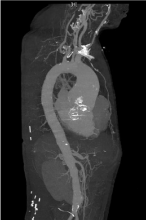
Figure 2: Preoperative CT angiography.
CT angiography revealed a 60mm ascending thoracic aortic aneurysm and
66mm dilated aortic root.
Our preoperative plan was to perform the bent all procedure, which comprises ascending aorta and hemi arch replacement and tricuspid annuloplasty. A median sternotomy and conventional cardiopulmonary bypass were performed with moderate hypothermia and ante grade and retrograde blood cardioplegia. The aorta was transected. The leakage point was located between the annulus of the noncoronary sinus and the prosthesis. The sewing ring of the mechanical mitral valve was exposed and found to be adhered to the mechanical aortic valve for about 2cm (Figure 3). A partial, continuous annular suture had been used to secure the pledgeted annular mattress suture during the previous mitral valve replacement and connected the sewing rings of the mechanical aortic valve to the sewing ring of the mechanical mitral valve. We replaced the mitral valve with a reconstruction of the aorto-mitral continuity using bovine pericardium and a two-patch technique. The superior vena cava was divided just distal to the venous cannula to expose the left atrial roof. A vertical incision in the aorta was extended into the left atrial roof. The previously inserted prosthetic valves were removed and a bovine pericardial patch was tailored to replace the anterior mitral annulus and the aorto-mitral curtain. After insertion of the prosthetic mitral valve, a second pericardial patch was sutured to the anterior portion of the prosthetic mitral valve simultaneously with the first patch. After replacement of the mitral valve, everted pledget buttressed mattress sutures were added along the aortic annulus and pericardial patch. Coronary buttons of the coronary ostia and margin of aortic tissue were mobilized and the aorta was trimmed. Residual tissue from the first pericardial patch was excised and a St. Jude 25mm composite graft (Valsalva graft) was implanted. Coronary buttons were implanted into the newly constructed composite graft using running 5-0 polypropylene sutures. A distal anastomosis was constructed to the aorta, which had been trimmed to the lesser curvature of aortic arch. The second patch was folded over and used to close the left atrial roof. Tricuspid annuloplasty was performed using a 29mm Duran ring.
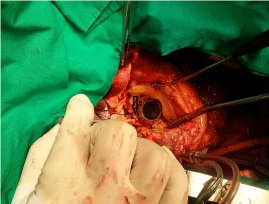
Figure 3: Intraoperative findings.
A 1mm probe was inserted into the left ventricle through the hole between the
sewing ring and aortic annulus (black arrow).
The patient was discharged without complications on the thirteenth postoperative day. One year after the operation, transthoracic echocardiography was performed. There was no paravalvular leakage and his ejection fraction had normalized.
Discussion
Paravalvular Leak (PVL) is an uncommon complication associated with prosthetic valve replacement, and 3% of patients with PVL require reoperation because of heart failure and/or hemolysis [1-4]. Although the factors causing PVL are still controversial, several factors increase PVL risk. Using operative findings, Kuwabara et al. classified and defined the following causes of PVL: cutting of the annulus, suture laxity, infection, and valve-on-valve structure [5]. Some studies suggest different factors are associated with PVL depending on time of onset. These studies found that early onset PVLs are usually caused by technical aspects of the surgical implant and late PVLs are associated with consequences of suture dehiscence caused by endocarditis or the gradual resorption of incompletely debrided annular calcifications [6]. Several reports describe the most frequent PVL sites. De Cicco et al. Reported that aortic PVL is more frequent at the nadir of the right coronary and noncoronarycusp, and that mitral PVL, seen by viewing the mitral annulus in a clock format from the surgeon’s view, is more frequent between hour 5 and hour 6 and between hour 10 and hour 11 [7]. However, no reports describe the frequency of PVL sites in DVRs. In our case, the PVL was at the nadir of the non coronary sinus. The patient underwent DVR for infective endocarditis about 24 years ago. No medical records were available to determine if the PVL occurred in the early postoperative period or recently. However, it was thought that the PVL occurred in the early postoperative period because the preoperative and operative findings of ascending aortic aneurysm and root dilatation suggested longstanding volume overload caused by PVL. Furthermore, only a small amount of tissue was found around the aortomitral curtain. It was not certain if the loss of aortomitral continuity occurred as a result of longstanding PVL; however, it was thought that the dilated aortic root might have contributed to the aortomitral curtain loss. The sewing rings of the previously replaced mechanical aortic and mitral valves were connected by continuous running sutures. These operative findings suggest that the previous endocarditis extended to the aortomitral curtain and the tissue of the aortomitral curtain was too fragile to secure the sewing ring with pledget buttressed annular sutures. We suspect this abnormal aortomitral connection induced friction between the sewing ring and aortic annulus that caused the PVL. The patient underwent aortomitral reconstruction with the bent all procedure and was successfully managed. We feel that if the initial operation was an aortomitral reconstruction rather than a simple DVR, this unusual case of PVL might not have occurred.
References
- Jindani A, Neville EM, Venn G, Williams BT. Paraprosthetic leak: a complication of cardiac valve replacement. J Cardiovasc Surg . 1991; 32: 503-508.
- Misawa Y, Saito T, Konishi H, Oki S, Kaminishi Y, Takahashi H, et al. When and how does nonstructural mechanical prosthetic heart valve dysfunction occur? Jpn J Thorac Cardiovasc Surg. 2003; 51: 355-360.
- Nishida T, Sonoda H, Oishi Y, Tanoue Y, Nakashima A, Shiokawa Y, et al. Single-institution, 22-year follow-up of 786 CarboMedics mechanical valves used for both primary surgery and reoperation. J Thorac Cardiovasc Surg. 2014; 147: 1493-1498.
- Antunes MJ. Reoperations on cardiac valves. J Heart Valve Dis. 1992; 1: 15-28.
- Fumiaki K, Akihiko U, Yoshimori A, Yuji N, Shinichi M, Hideki O, et al. Pathogenesis of paravalvular leakage as a complication occurring in the late phase after surgery. J Artif Organs. 2011; 14: 201-208.
- Kliger C, Eiros R, Isasti G, Einhorn B, Jelnin V, Cohen H, et al. Review of surgical prosthetic paravalvular leaks: diagnosis and catheter-based closure. European Heart Journal. 2013; 34: 638-648.
- Cicco G, Lorusso R, Colli A, Nicolini F, Fragnito C, Grimaldi T, et al. Aortic valve periprosthetic leakage: anatomic observations and surgical results. Ann Thorac Surg. 2005; 79: 1480–1485.