Case Report
Austin Surg Case Rep. 2018; 3(1): 1023.
Successful Thrombin Injection of BA-PSA after Two Weeks of Failed Manual Compression in Patient on Triple Therapy
Yeritsyan NB1, Stolt M1, Halloul Z2, Udelnow A2, Braun-Dullaeus RC1 and Herold J1*
1Division of Cardiology and Angiology, University Hospital Magdeburg, Leipziger, Germany
2Division of Vascular Surgery, University Hospital Magdeburg, Leipziger, Germany
*Corresponding author: Herold J, Division of Cardiology and Angiology, University Hospital Magdeburg, Germany
Received: January 12, 2018; Accepted: January 31, 2018; Published: February 08, 2018
Abstract
An 83-year-old male with a brachial artery pseudoaneurysm (BA-PSA) following transbrachial digital subtraction angiography was admitted to our hospital. After failing both manual and ultrasound-guided compression of the BA-PSA, the percutaneous ultrasound-guided thrombin injection (UGTI) was successfully used as second-line therapy. It was shown that the UGTI is a feasible strategy to treat the brachial artery PSA in older patients on dual antiplatelet and anticoagulant therapy.
Keywords: Brachial Artery; Pseudo Aneurysm; Compression Sonography; Ultrasound-Guided Thrombin Injection
Introduction
Therapeutic angiographies lead to higher incidences of iatrogenic PSA, because these procedures are associated with larger sheath sizes, more aggressive peri- and post-procedural anticoagulation and platelet aggregation inhibitors. The use of catheters with a large diameter (>7 F) [1-3], and platelet inhibitors [2], have been shown to be independent predictive factors of PSA formation in several studies. Parallel to the rapid progression of endovascular technology, upper extremity access has become a preferred interventional strategy during the past decade [4,5]. This resulted in an increase in iatrogenic radial and brachial pseudoaneurysms [5,6]. Until the 1990s, open surgical repair was the preferred method for the treatment of iatrogenic pseudoaneurysms, but this method was replaced by non-invasive methods. However, open surgical repair remains indicated in complicated cases, such as a septic or large PSA causing compression neuropathy. Additional indications include distal ischemia and skin and tissue necrosis [2,7,8]. Surgical repair, which can be performed under either local or general anesthesia, is an effective treatment. However, surgical repair is associated with a high incidence of postoperative complications, ranging from 16% to 71% [9-11]. Conventional approaches to manage PSAs are ultrasound-guided compression (UGC) and the surgical revision of the pseudoaneurysm. Nevertheless, in patients with dual antiplatelet or/and anticoagulation therapy, compressing strategy often fails and may become less efficient, while being painful and time-consuming [12]. The use of pressure bandages (for 12-24h) may cause a reduction of peripheral arterial circulation. Furthermore, it can lead to an increased risk of venous thrombosis due to the compression of the elastic venous walls, sometimes compressed by the pseudoaneurysm itself. Dimensions of the pseudoanerysm and the width and length of the PSA-neck can, in their turn, become factors compromising a successful outcome in UGC.
In the past three decades, there has been increasing popularity in the treatment of iatrogenic PSAs using a minimally-invasive percutaneous US-guided thrombin injection (UGTI) [5,13]. The efficacy of this approach is shown to be higher than that of compression repair alone [14]. Low complication rates, relative ease of performance, and shorter procedure times favor the use of US-guided thrombin injection in treating femoral pseudoaneurysm. However, limited studies have tested the UGTI in the management of BA-PSA, and the existing data are inconclusive on the safety and efficacy of this procedure [6,13,15,16]. The complex anatomical features of the brachial PSA, such as close proximity to the median nerve in the medial brachial fascial compartment and its smaller diameter predisposing to thrombosis, make the choice of treatment strategy highly challenging [16].
In this case report we describe a successful treatment of BA-PSA with UGTI after two weeks of failed manual compression and UGC in an aged patient on anticoagulation and dual antiplatelet therapy. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008.
Case Presentation
An 83-year-old man was initially admitted with a non-healing ulcer on the second toe of the left foot for an emergency digital subtraction angiography. The patient was a smoker with a history of ischemic heart disease, who underwent coronary stent placements using the right-side transfemoral access a month prior. In addition, his medical history included hypertension, pulmonary disease, chronic kidney disease, and subclavian artery occlusive disease with stenting of the right subclavian artery. He was positively tested for Methicillin-resistant Staphylococcus aureus (MRSA). Two years earlier the patient underwent an amputation on the right leg due to progressive peripheral arterial occlusive disease.
The digital subtraction angiography (DSA) was performed using the right-side trans-brachial access. High-grade stenosis along the right and left superficial femoral artery, the left popliteal and the fibular artery were revealed. The antegrade percutaneous transluminal angioplasty of the superficial femoral artery and fibular artery was planned and performed.
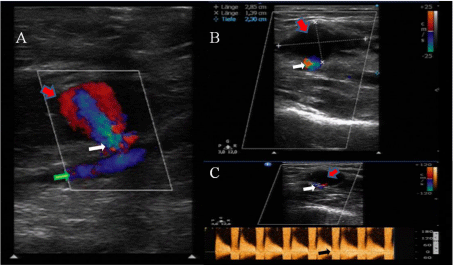
Figure 1: A,B- The pseudo aneurysm (red arrow), originated from the brachial artery (green arrow) and measured 2.85 x 1.39 x 2.3 cm; C- Spectral waveform flow pattern through the aneurysm neck (white arrow) in the pulse wave Doppler (black arrow).
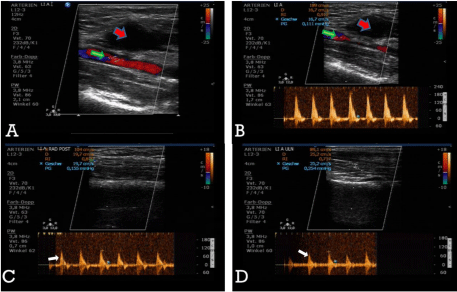
Figure 2: A- The color Doppler image of the brachial pseudo aneurysm (red arrow) immediately after the thrombin injection; B - Restored flow (PW-Doppler) in the brachial artery (green arrow) following effective thrombosis of the pseudo aneurysm; C,D- undisturbed peripheral perfusion of the radial and ulnararteries after the thrombin injection (white arrows).
Four days after the discharge, the patient readmitted to the hospital with pain and a palpable pulsatile mass with a remarkable hematoma in the right upper arm and the antecubital fossa. The radial and ulnar pulses were palpable; there were no signs of motor or sensory deficit of the arm. An ultrasound examination showed a PSA originating from BA (2.85 x 1.39 x 2.3 cm) (Figure 1 A,B) with the typical Yin-Yang flow-pattern through the PSA-neck (Figure 1C). The patient was hospitalized, and a bandage was applied to the arm for the subsequent 24h. Due to increased inflammatory markers (CRP 45.7mg/l (Ref: < 5mg/l), WBC 18.1 Gpt/l, (Ref: 3.7-9.8 Gpt/l) the patient received a calculated antibacterial therapy with a β-lactamase-inhibitor infusion. Ascoronary stenting was performed a month ago, dual antiplatelet therapy with Aspirin (100mg OD) and Clopidogrel (75mg OD) was administered. Additionally, the patient received daily low molecular weight Heparin (Enoxaparin, subcutaneously 0.4ml) for prevention of venous thromboembolism. On the next day, the ultrasound examination revealed a volar medial partially thrombosed but still perfused brachial pseudoaneurysm. A compression pad was applied further together with silicon protection bandage. Considering the high risk of morbidity and mortality associated with an instant surgical treatment in an aged patient with multiple comorbidities, we decided to continue the compression therapy over the next two days. Because of MRSA, massive subcutaneous hematoma and triple therapy surgical repair was declined. Subsequent ultrasound examination showed no further progress in the treatment of pseudoaneurysm, so UGTI was chosen as an alternative approach to the surgical repair. The procedure was performed after detailed clarification of the risks and benefits to the patient, who consented. Prophylactic Enoxaparin was held one day before the procedure. The skin was disinfected, and the cavity and the neck of the aneurysm was imaged by a vascular probe (ultrasound transducer 12-3MHz, Philips, Netherlands) covered with a sterile bag. The sterile gel was applied to the patient’s skin to prevent infections. To avoid intra-arterial injection of thrombin and to prevent embolism, the transducer was placed longitudinally to the PSA for simultaneous imaging of the PSA-cavity and PSA-neck, as well as the blood flow pattern in the brachial artery. We used the human thrombin from the commercial package sold as a fibrin sealant kit (500U/mL, Tissucol Duo 1.0mL, Tissucol set®, Baxter, Germany). The fibrin-containing portion of the set was used and the syringe with the thrombin solution was inserted into a three-way stopcock, with one end connected to an 18-gauge cannula and the other end attached to a saline-filled-syringe. Thrombin was loaded into a small syringe and the PSA-sac was carefully penetrated under ultrasound guidance. Before the thrombin injection, blood was aspirated into the saline-filled-syringe to confirm the exact position of the needle in the cavity [17]. Under color-flow Doppler control, 300μl of thrombin solution (300I.E.) was slowly injected into the cavity, which resulted in an immediate thrombosis of the pseudoaneurysm. The injection was stopped, the needle was withdrawn, and a soft compress was applied for the following six hours. The flow assessment directly after the procedure and 24 hours later using PW-Doppler confirmed the completely thrombosed pseudoaneurysm (Figure 2A). Additionally, preservation of normal peripheral perfusion through the brachial, radial and ulnar arteries was assured by clinical and ultrasound examination (Figure 2 B,C,D).
Discussion
The UGTI was efficiently used as a minimally-invasive procedure to treat the iatrogenic BA-PSA after ineffective compression therapy [5,18,19]. Limited data on safety and efficacy of UGTI suggest that there is a higher rate of recurrence and complications in the management of upper extremity PSA, as compared to the femoral ones [6,15,16,20]. This corresponds to a greater complication rate for the brachial access as compared to the transfemoral and transradial performed interventions [21,22]. The complexity of the brachial approach is determined by several factors: An absence of certified arterial puncture closure devices (the use of vascular closure devices is officially affirmed only for the femoral access) [23] the difficulty of the compression due to mobility of PSA and the shape of humerus, as well as the increased risk of thrombosis [24,25] the close anatomical location to the median nerve with a risk of upper extremity neuropathy in case of nerve compression either by hematoma or compression bandage. Despite its difficulties, the compression technique is considered the gold standard in obtaining hemostasis of the PSA. Due to anatomical characteristics of the BA-PSA, however, there are certain variations compared to the transfemoral arterial access. The smaller caliber of the BA necessitates far gentler pressure to be applied, in order to obtain hemostasis while maintaining antegrade blood ?ow, rather than to obliterate the in ?ow [25]. In our case it was challenging to succeed with the UGC of BA-PSA in the old patient with restricted mobility and under antiplatelet and anticoagulant therapy. A relatively high recurrence rate after PSA treatment (as high as 25% to 35%) had already been reported in patients under anticoagulant therapy [12]. A strong negative correlation was revealed between anticoagulation status and compression success (70% failure rate in patients using anticoagulants vs. 26% in patients not using anticoagulants) [26,27]. The thrombosis of the PSA observed in our patient 24-48h after the compression was partial, and the perfusion in PSA still persisted, despite the normal INR values. The recent finding from Dzijan-Horn M et al, who failed to detect higher INR levels in patients with repeated manual compression attempts as compared to those successfully treated with manual compression, matches our observation [17]. Further, the resistance of PSA to compression might result from its long persistence due to endothelialization of the false cavity and PSA-neck [28].
In the presence of acutely impaired peripheral perfusion, nerve compression or ‘wide’ PSA neck, surgical revision is recommended [17]. Here, palpable peripheral arterial pulses in the absence of ultrasound evidence of thrombosis confirmed the undisturbed peripheral circulation in our patient. The delayed manifestation of neurological symptoms, especially in patients with peripheral neuropathy, questioned their utility as a criterion enforcing a second-line strategy choice in our case. As the skin maceration was present over the subcutaneous hematoma and could be problematic in a surgical approach to our aged patient with vast co-morbidities, including MRSA-infection, we applied UGTI to manage the BA-PSA in spite of a visualized ‘wide’ PSA neck. The high success rate of this approach is reported elsewhere: It avoids the technical difficulties of compression, is fast, relatively painless, and safe [13,14]. Distal embolization from the newly created thrombus, and arterial thrombosis as a result from thrombin injected directly into artery, are potential complications of this procedure. These are of greater concern in cases with BA-PSA, as the superficial location of the PSA predisposes for a shorter PSA-neck [24]. It makes difficult the single improvements in UGTI technique (e.g. the injection under simultaneous manual PSA-neck compression) [29]. Some reports relate the wider PSA-neck to the larger doses of thrombin (1000U/mL) required [30]. Nevertheless, in our case, sufficient thrombosis was achieved by a relative minimal dose of human thrombin (300U/mL) with the PSA-neck width of 2.55mm.
Another complication of the UGTI is an allergic reaction to thrombin, which can be avoided by using its human analogue [17,30]. In our patients we favor the commonly used injection of human lyophilized Thrombin from Tissucol set® (Baxter, Germany) [30], and there was no incidence of allergic manifestations among 250 patients treated with UGTI in our clinic.
Although the treatment of the BA-PSA by UGTI in our patient was efficient, further experience is required to confirm the efficacy of this approach as a second-line therapy after initially failing compression. In the majority of similar cases, patients receive surgical repair with a high risk of complication. The course and outcome of any approach treating pseudoaneurysms may become compromised by age and concomitant medication. The value of this casein management of BA-PSA comprises the use of UGTI as a feasible strategy to treat complicated brachial artery PSA in older patients on antiplatelet and anticoagulant therapy. We believe that the present case report adds to the collective knowledge of the individualized treatment of the BA-PSA, which is valuable for clinicians facing upper extremity pseudoaneurysm.
Acknowledgments
Funding
Sponsorship for this study and article processing charges was funded by University Hospital Magdeburg, Division of Cardiology and Angiology.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published.
Disclosures
All authors declare that they have no conflict of interest.
Compliance with ethics guidelines
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. Informed consent was obtained from the patient for inclusion in the study as well as for any identifying information in this article.
Data availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- Ates M, Sahin S, Konuralp C, Gullu U, Cimen S, Kizilay M, et al. Evaluation of risk factors associated with femoral pseudoaneurysms after cardiac catheterization. J Vasc Surg. 2006; 43: 520-524.
- Popovic B, Freysz L, Chometon F, Lemoine J, Elfarra M, Angioi M, et al. Femoral pseudoaneurysms and current cardiac catheterization: Evaluation of risk factors and treatment. Int J Cardiol. 2010; 141: 75-80.
- Smilowitz NR, Kirtane AJ, Guiry M, Gray WA, Dolcimascolo P, Querijero M, et al. Practices and complications of vascular closure devices and manual compression in patients undergoing elective transfemoral coronary procedures. The American journal of cardiology. 2012; 110: 177-182.
- Bhat T, Teli S, Bhat H, Akhtar M, Meghani M, Lafferty J, et al. Access-site complications and their management during transradial cardiac catheterization. Expert Rev Cardiovasc Ther. 2012; 10: 627-634.
- Herold J, Brucks S, Boenigk H, Said SM, Braun-Dullaeus RC. Ultrasound guided thrombin injection of pseudoaneurysm of the radial artery after percutaneous coronary intervention. Vasa. 2011; 40: 78-81.
- Krueger K, Zaehringer M, Strohe D, Stuetzer H, Boecker J, Lackner K. Postcatheterization pseudoaneurysm: Results of US-guided percutaneous thrombin injection in 240 patients. Radiology. 2005; 236: 1104-1110.
- O'Sullivan GJ, Ray SA, Lewis JS, Lopez AJ, Powell BW, Moss AH, et al. A review of alternative approaches in the management of iatrogenic femoral pseudoaneurysms. Annals of the Royal College of Surgeons of England. 1999; 81: 226-234.
- Webber GW, Jang J, Gustavson S, Olin JW. Contemporary management of postcatheterization pseudoaneurysms. Circulation. 2007; 115: 2666-2674.
- Piffaretti G, Mariscalco G, Tozzi M, Rivolta N, Castelli P, Sala A. Predictive factors of complications after surgical repair of iatrogenic femoral pseudoaneurysms. World journal of surgery. 2011; 35: 911-916.
- San Norberto Garcia EM, Gonzalez-Fajardo JA, Gutierrez V, Carrera S, Vaquero C. Femoral pseudoaneurysms post-cardiac catheterization surgically treated: Evolution and prognosis. Interactive cardiovascular and thoracic surgery. 2009; 8: 353-357.
- Ricci MA, Trevisani GT, Pilcher DB. Vascular complications of cardiac catheterization. American journal of surgery. 1994; 167: 375-378.
- Dean SM, Olin JW, Piedmonte M, Grubb M, Young JR. Ultrasound-guided compression closure of postcatheterization pseudoaneurysms during concurrent anticoagulation: A review of seventy-seven patients. Journal of vascular surgery. 1996; 23: 28-35.
- Kang SS, Labropoulos N, Mansour MA, Michelini M, Filliung D, Baubly MP, et al. Expanded indications for ultrasound-guided thrombin injection of pseudoaneurysms. Journal of Vascular Surgery. 2000; 31: 289-298.
- Sheiman RG, Brophy DP. Treatment of Iatrogenic Femoral Pseudoaneurysms with Percutaneous Thrombin Injection: Experience in 54 Patients. Radiology. 2001; 219: 123-127.
- Garvin RP, Ryer EJ, Yoon HR, Kendrick JB, Neidrick TJ, Elmore JR, et al. Ultrasound-guided percutaneous thrombin injection of iatrogenic upper extremity pseudoaneurysms. Journal of Vascular Surgery. 2014; 59: 1664-1669.
- Kuma S, Morisaki K, Kodama A, Guntani A, Fukunaga R, Soga Y, et al. Ultrasound-guided percutaneous thrombin injection for post-catheterization pseudoaneurysm. Circ J. 2015; 79: 1277-1281.
- Dzijan-Horn M, Langwieser N, Groha P, Bradaric C, Linhardt M, Böttiger C, et al. Safety and Efficacy of a Potential Treatment Algorithm by Using Manual Compression Repair and Ultrasound-Guided Thrombin Injection for the Management of Iatrogenic Femoral Artery Pseudoaneurysm in a Large Patient Cohort. Circulation: Cardiovascular Interventions. 2014; 7: 207-215.
- Lorenz E, Herold J, Udelnow A, Pech M, Pech M, Halloul Z. Percutaneous thrombin embolisation of an iatrogenic pseudoaneurysm of the subclavian artery with neuroprotection in a critically ill patient. Vasa. 2015; 44: 391-394.
- Peters S, Braun-Dullaeus R, Herold J. Pseudoaneurysm. Incidence, Therapy and Complications. Hamostaseologie. 2017; 38.
- Kang SS, Labropoulos N, Mansour MA, Baker WH. Percutaneous ultrasound guided thrombin injection: A new method for treating postcatheterization femoral pseudoaneurysms. J Vasc Surg. 1998; 27: 1032-1038.
- Armstrong PJ, Han DC, Baxter JA, Elmore JR, Franklin DP. Complication Rates of Percutaneous Brachial Artery Access in Peripheral Vascular Angiography. Annals of Vascular Surgery. 2003; 17: 107-110.
- Jolly SS, Amlani S, Hamon M, Yusuf S, Mehta SR. Radial versus femoral access for coronary angiography or intervention and the impact on major bleeding and ischemic events: A systematic review and meta-analysis of randomized trials. American Heart Journal. 2009; 157: 132-140.
- Sheth RA, Ganguli S. Closure of Alternative Vascular Sites, Including Axillary, Brachial, Popliteal, and Surgical Grafts. Techniques in Vascular & Interventional Radiology. 2015; 18: 113-121.
- Skibo L, Polak JF. Compression repair of a postcatheterization pseudoaneurysm of the brachial artery under sonographic guidance. American Journal of Roentgenology. 1993; 160: 383-384.
- Sugawara Y, Koyama H, Miyata T, Sato O. Delayed closure of a postcatheterization pseudoaneurysm of the brachial artery by sonographically guided digital compression. American Journal of Roentgenology. 1996; 166: 220.
- Eisenberg L, Paulson EK, Kliewer MA, Hudson MP, DeLong DM, Carroll BA. Sonographically guided compression repair of pseudoaneurysms: Further experience from a single institution. American Journal of Roentgenology. 1999; 173: 1567-1573.
- Schaub F, Theiss W, Busch R, Heinz M, Paschalidis M, Schömig A. Management of 219 Consecutive Cases of Postcatheterization Pseudoaneurysm. Journal of the American College of Cardiology. 1997; 30: 670–675.
- Heis HA, Bani-Hani KE, Elheis MA, Yaghan RJ, Bani-Hani BK. Postcatheterization femoral artery pseudoaneurysms: Therapeutic options. A case-controlled study. International Journal of Surgery. 2008; 6: 214-219.
- McNeil NL, Clark TWI. Sonographically Guided Percutaneous Thrombin Injection Versus Sonographically Guided Compression for Femoral Artery Pseudoaneurysms. American Journal of Roentgenology. 2001; 176: 459-462.
- Vázquez V, Reus M, Piñero A, Abellán D, Canteras M, de Rueda ME, et al. Human Thrombin for Treatment of Pseudoaneurysms: Comparison of Bovine and Human Thrombin Sonogram-Guided Injection. American Journal of Roentgenology. 2005; 184: 1665-1671.