Case Report
Austin Surg Case Rep. 2019; 4(1): 1029.
Pneumatosis Intestinalis after Correction of Postinfarction VSD
Yong Ko T and Ho Cho S*
Department of Thoracic and Cardiovascular Surgery, Kosin Gospel Hospital, Busan, Korea
*Corresponding author: Seong Ho Cho, Department of Thoracic and Cardiovascular Surgery, Kosin Gospel Hospital, Busan, Korea
Received: August 17, 2019; Accepted: September 17, 2019; Published: September 24, 2019
Abstract
Pneumatosis Intestinalis (PI) refers to the phenomenon in which air is present in the bowel wall. It can be mistaken for ileus, which frequently occurs after general anesthesia due to abdominal distention. Delays in proper diagnosis and treatment can cause fatal results. Depending on a patient’s underlying disease, PI can occur at any age, and its incidence in the general population has been reported as 3 per 10,000 persons. The bowel wall air can affect a various amount of intestine, and it can be accompanied by pneumoperitoneum, pneumoretroperitoneum and portal venous gas inclusion. In this report, we present a case of fatal PI after correction of a postinfarction VSD.
Introduction
Pneumatosis Intestinalis (PI) is defined as the accumulation of gas in the bowel wall. This unique image finding was first discovered by Du Vernoi in 1730 during cadaver dissection [1]. The incidence of pneumatosis intestinalis is increasing because of the improvements in and the use of radiographic abdominal studies, such as abdominal CT. In addition, many medical procedures including surgery, medications, and diagnostic procedures have contributed to the increase in PI. Based on autopsy studies, the incidence of PI in the general population has been estimated as 3 per 10,000 individuals [2]. PI can be a fatal disease, especially when combined with ischemic colitis which necessitates surgical intervention. When PI occurs immediately after surgery, it is sometimes difficult to distinguish from postoperative ileus. If a patient shows symptoms of ileus and infection after surgery, physicians must consider PI in the differential diagnosis.
Case Presentation
A 82-year-old woman visited a local medical center for chest pain and dyspnea which began 5 days prior to presentation. Coronary arteriography revealed a total occlusive lesion from the mid LAD. PCI was immediately attempted for revascularization but failed. Transesophageal echocardiography demonstrated a ventricular septal defect in the mid portion of interventricular septum, and the patient was transferred to our hospital for surgical intervention. At the time of admission, the chest pain, described as squeezing, persisted. The patient’s past medical history was notable only for hypertension. On physical examination, a cardiac murmur was prominent, and ST elevation was observed in the V2 ~ 5 lead on initial ECG. Laboratory examinations revealed increased cardiac enzymes: troponin-I level was 660 ng/L, and pro-BNP level was › 35,000 pg/ml. A chest x-ray demonstrated cardiomegaly and pulmonary edema. The patient received oxygen, aspirin, heparin, inotropics, and diuretics to treat IHD and HF. After two-lumen right subclavian catheterization for hemodialysis, the patient underwent surgery for VSD. Ventriculotomy was performed at the left lateral 2 cm of the mLAD, and the interventricular septal defect was observed. The VSD was first closed with bovine pericardium, sutured to normal tissue using pledget buttressed 3-0 Prolene. Another bovine pericardium was then sutured to the repaired VSD using 3-0 Prolene with running sutures for reinforcement, thus concluding the operation.
After surgery, the patient was noted to have persistent thrombocytopenia and low cardiac output. Insertion of an IABP was attempted through both femoral arteries but failed. As the treatments for IHD and CHF continued, the patient improved and was transferred to the general ward. However, on POD 4, she complained of dyspnea with cold sweats and was readmitted to the ICU for unstable V/S and low oxygen saturation. In the ICU, her HS-CRP, D-dimer, and BUN/Cr continuously increased, and her abdominal distension was severe. On postoperative day six, an enhanced abdominal CT scan revealed severe jejunal distension and an air bubble in the jejunum wall, resulting in the diagnosis of pneumatosis intestinalis (Figure 1 and 2). A small bowel infarction was suspected as the cause of the patient’s pneumatosis intestinalis and sepsis, so we recommended diagnostic and therapeutic laparotomy. However, the patient’s family refused surgery due to her age, and she expired on POD 7.
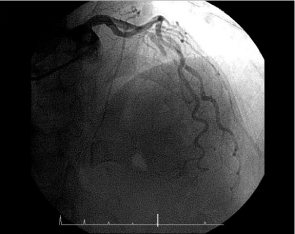
Figure 1: The preoperative coronary arteriogram showed total occlusion of
the mLAD.
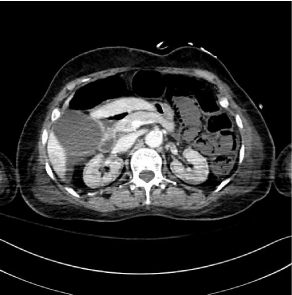
Figure 2: A postoperative abdominal CT revealed jejunal distention and
intramural air bubbles in the jejunal wall.
Discussion
The mechanism of PI has been controversial for many years. St. Peter and co-workers have focused their study on the origin of the gas and have suggested three major pathophysiological mechanisms of pneumatosis [3].
(i) Intraluminal gas: Increased intraluminal pressure, which may occur due to blunt trauma, can cause pneumatosis intestinalis, even in patients with a normal mucosal barrier. However, at least some degree of mucosal injury is associated with pneumatosis intestinalis in many cases. Defects in the mucosa may induce enhanced gut permeability to gas. (ii) Bacterial gas production: Gas-producing bacteria can form intramural gas through direct invasion into the bowel wall or by alteration of the intraluminal gas content. Gas-producing bacteria can reach the intramural layer through increased intraluminal pressure, mucosal injury, or both factors. The latter mechanism can be explained by the theory of counter perfusion super saturation. The intraluminal hydrogen pressure, which is produced by bacteria in the bowel, exceeds the nitrogen pressure in the blood, causing a hydrogen diffusion gradient towards the submucosal vessels [4]. (iii) Pulmonary gas: Alveolar rupture can cause air migration along vascular channels in the mediastinum, and the air can move caudally via the retroperitoneum to the mesentery of the bowel. Pulmonary gas migration to the subserosal layer of the intestine has been observed in patients with pulmonary disease. Gas migration is more persuasive etiology of PI than a transmural origin of gas in these patients.
PI can present with a wide variety of clinical manifestations ranging from benign to fatal. Patients with benign manifestations are usually asymptomatic, and only mild abdominal discomfort may occur in some [5].
Fatal PI can present with fever, abdominal and rebound tenderness, and abdominal distention, especially if there is intestinal ischemia or bowel perforation. Serum lactate level of › 2 mmol/L, serum bicarbonate level of ‹ 20 mmol/L, hyperamylasemia of › 200 IU/L and an acidosis with a blood pH of ‹ 7.3 [6]. Hawn MT et al. proposed that an increased serum lactate level of › 2 mmol/L in PI was associated with a poor outcome, rating over 80% in mortality [7].
A.J. Greenstein et al. reviewed patients with PI and presented the surgical indications for PI [8]. Surgical intervention was favoured in patients with a WBC › 12 c/mm3 and/or emesis in patients greater than 60 years old.
In the present case, we considered intestinal ischemia caused by myocardial infarction as the causative factor of PI. The ischemia might have caused injury to the intestinal mucosa, allowing gasproducing bacteria and intraluminal gases to invade the intestinal wall and form intramural gas. In patients who are intubated, sedated, or under analgesic drugs, it is difficult to ascertain abnormal findings, such as abdominal tenderness and rebound tenderness, on the physical examination. If PI is suspected, enhanced abdominal CT should be considered and immediate surgical intervention should be undertaken.
References
- Du Vernoi JG. Anatomische Beobachtungen der unter der außeren und inneren Haut der Gederme eingeschlossenen Luft. Phys Med Abbhand Acad Wissensch in Petersb 1783:1823.
- Ho LM, Paulson EK, Thompson WM. Pneumatosis intestinalis in the adult: benign to life threatening causes. Am J Roentgenol 2007; 188: 1604-1613.
- St. Peter SD, Abbas MA, Kelly KA. The spectrum of pneumatosis intestinalis. Arch Surg 2003;138: 68-75.
- Florin TH, Hills BA. Does counterperfusion supersaturation cause gas cysts in pneumatosis cystoides coli, and can breathing heliox reduce them? Lancet. 1995; 345: 1220-1222.
- Galandiuk S, Fazio VW. Pneumatosis cystoides intestinalis:a review of the literature. Dis Colon Rectum. 1986; 29: 358-363.
- Knechtle SJ, Davidoff AM, Rice RP. Pneumatosis intestinalis: surgical management and clinical outcome. Ann Surg. 1990; 212: 160-165.
- Hawn MT, Canon CL, Lockhart ME, Gonzalez QH, Shore G, Bondora A, Vickers SM. Serumlactic acid determines the outcomes of CT diagnosis of pneumatosis of the gastrointestinal tract. Am Surg 2004; 70: 19-24.
- Greenstein AJ, Nguyen SQ, Berlin A, Corona J, Lee J, Wong E, Factor SH, Divino CM. Pneumatosis intestinalis in adults: management, surgical indications, and risk factors for mortality.J Gastrointest Surg 2007; 11: 1268- 1274.