Review Article
Austin Surg Case Rep. 2021; 6(1): 1041.
Efficacy and Safety of Ibutilide for the Cardioversion of Atrial Fibrillation: A Systematic Review and Meta-Analysis
Gong CC¹, Tang Y², Huang Y² and Liu X²*
¹Zhejiang University School of Medicine Children’s Hospital, China
²Department of Critical Care Medicine, the Affiliated Hospital of Guizhou Medical University, China
*Corresponding author: Liu X, Department of Critical Care Medicine, the Affiliated Hospital of Guizhou Medical University, China
Received: February 16, 2021; Accepted: March 17, 2021; Published: March 24, 2021
Abstract
Background: Ibutilide has been approved for cardioversion of Atrial Fibrillation (AF), but its side-effects include a high risk of torsade de pointes, besides, one recent meta-analysis showed ibutilide was inferior to vernakalant for conversion (AF<7 days). Hence, the aim of this study is to evaluate the efficacy and safety of ibutilide for the cardioversion of AF within 90 days.
Methods: The Embase, PubMed, Web of Science, Cochrane Central databases and clinical trials.gov were comprehensively searched for relevant studies from January 1991 to May 2020 using the keywords “ibutilide” and “atrial fibrillation”. Only Randomized Controlled Trials (RCTs) comparing ibutilide with placebo or other Anti-Arrhythmic Drugs (AADs) for the termination of AF (duration of AF ≤90 days) were included. The primary outcome was successful cardioversion in response to ibutilide versus placebo or other AADs within 4h. Related adverse events were defined as secondary outcomes.
Results: A total of 1712 patients in 13 RCTs met the eligibility criteria. Four trials compared ibutilide to placebo; nine trials compared ibutilide to other active drugs. The results revealed that ibutilide had a higher success rate for the termination of recent-onset atrial fibrillation compared to placebo within 4h [Risk Ratio (RR), 4.64; 95% Confidence Interval (CI), 1.30-16.56, P=0.006]; and ibutilide also showed superiority to DL-sotalol, Propafenone, Procainamide for successful termination of recent-onset AF within 4h. As compared to other active drugs, Ibutilide was associated with a lower risk of hypotension (RR 0.23, 95% CI 0.09-0.57, P=0.002); but significantly increased the incidence of Polymorphic ventricular tachycardia (RR 3.78, 95% CI 1.08-13.23, P=0.04).
Conclusion: Intravenous ibutilide could be an accessible choice for the cardioversion of recent-onset AF patients without contraindications, but under strict monitored condition is needed for at least 6 hours.
Keywords: Atrial fibrillation; Cardioversion; Ibutilide; Meta-analysis
Abbreviations
AF: Atrial Fibrillation; AADS: Anti-Arrhythmic Drugs; RR: Risk Ratio; CI: Confidence Interval; RCTs: Randomized Controlled Trials
Introduction
Atrial Fibrillation (AF) is the most prevalent cardiac arrhythmia, occurring in 1-2 % of the general population. Patients with AF have an increased risk of death, hospitalizations, and a lower quality of life [1]. Sinus restoration can relieve rhythm-related symptoms and attenuate functional impairment [2-4]. As such, early chemical or electrical conversion is the preferred choice for the treatment of AF, but electro cardioversion requires sedation or anesthesia [5], which limits its use. Pharmacological treatment is therefore more appealing for the conversion of recent-onset AF.
Ibutilide is a class III intravenous anti-arrhythmic agent that blocks the rapid component of the cardiac delayed rectifier potassium current and activates a late inward sodium current [6]. Ibutilide has been approved for the termination of recent-onset atrial fibrillation and atrial flutter [7]. However, given the limited sample size in previous trials [8-10], the superiority of ibutilide over other Anti- Arrhythmic Drugs (AADs) remains undefined. The aim of this review was to investigate the effectiveness and safety of ibutilide for the conversion of recent-onset AF compared with placebo and other AADs.
Methods
This meta-analysis was performed according to the Cochrane Handbook for Systematic Reviews of Interventions [11] and presented in accordance with Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines (PRISMA Guidelines) [12].
Search strategy
Two researchers (Gong C, and Tang Y) independently performed a comprehensive literature search. Trials comparing ibutilide with placebo or other AADs for the termination of recent-onset AF (duration of AF ≤90 days) were retrieved from PubMed, Embase, Web of Science, the Cochrane Library and Clinical Trials.gov until May, 2020. A basic search was carried out using the key words as follows: (“atrial fibrilation” or “atrial arrhythmia”) and (“ibutilide” or “ibutilide fumarate”). No language limitation was applied for the selection of articles.
Eligibility criteria
Trials were selected based on the following inclusion criteria: (1) Adult patients; (2) Ibutilide versus placebo or other AADs for treatment of recent-onset AF (duration of AF =90 days); (3) Randomized controlled trials.
Quality assessment
The methodological quality for the included studies was evaluated separately by two researchers(Gong C and Tang Y) using the Cochrane risk of bias criteria [11] and each quality item was graded as low risk, high risk, or unclear risk. We defined other bias as trials in which baseline characteristics were significant variation between different treatment groups. The included trials were graded as low quality, high quality or moderate quality based on the criteria in the following: (1) trials were evaluated as low quality if either randomization or allocation concealment was assessed as a high risk of bias, without taking the risk of other items into consideration; (2) trials were considered as high quality when both randomization and allocation concealment were assessed as a low risk, along with all other items assessed as low or unclear risk of bias in a trial; (3) trials were considered as moderate quality if they did not meet criteria for high or low quality [13].
Data extraction
The following information from each trial was extracted independently by two researchers (Fang H and Huang Y): first author, year of publication, country of origin, sample size, treatment strategies, the duration of AF, the time point for evaluating conversion efficacy, the length of follow-up and patient population. Disagreements on data extraction and quality assessment between the 2 reviewers were resolved by consensus (Liu X).
Outcomes
The primary outcome was the success conversion rate of AF within 4h, secondary outcomes were as follows: the incidence of Polymorphic ventricular tachycardia and hypotension.
Statistical analysis
Risk Ratio (RR) with 95% Confidence Interval (CI) were calculated for dichotomous data. Analyses were performed using Review Manager 5.3 (Revman: The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark). We assessed the heterogeneity among studies using Cochran’s Q-test (P<0.05 for statistical significance) and the I2 index (I2>50% for substantial heterogeneity). The random-effect model was chosen to pool the data.
Results
Selection of studies
Following the database search, a total of 1386 studies were identified. After the removal of duplications, 961 studies were screened according to titles and abstracts. Then, full texts of 26 potentially eligible trials were assessed based on predefined eligibility criteria. A total of 13 eligible trials were finally included in the metaanalysis (Figure1).
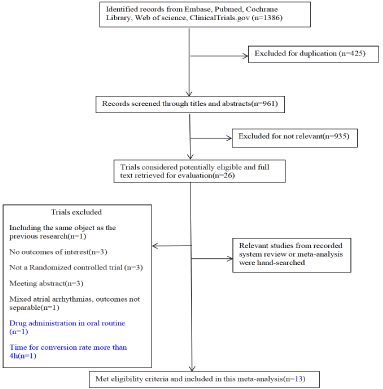
Figure 1: The flow diagram of study selection.
Description of the included RCTs
Four RCTs [14-17] depicted the efficacy and safety of ibutilide vs. Placebo, the cardioversion efficacy in all these trials was evaluated within 90min. A further nine studies [8-10,18-23] compared ibutilide with other AADs (including DL-sotalol, Amiodarone, Propafenone, Flecainide, Vernakalant, Procainamide), of which, the cardioversion efficacy in seven trials [8-10,18-21] was observed within 90min, while in other trials [22,23] was observed at more than 90 min and even up to 4 h. In addition, three studies [15,17,20] were designed to clarify that the treatment effects of ibutilide were dose-dependent. Detailed information of the patient characteristics. The outcomes of quality assessments were that two trials were of high quality, ten trials were of moderate quality, and one trials were of low quality.
Outcomes of the pooled studies
ibutilide versus placebo: A meta-analysis of 4 RCTs (total of 614 patients, Figure 2) demonstrated that ibutilide was superior to placebo for termination of recent-onset atrial fibrillation within 4h (RR 4.64, 95% CI 1.30-16.56, P=0.02). In addition, there were no significant difference between ibutilide and placebo for the incidence of Polymorphic ventricular tachycardia and hypotension events (Figure 4,5).
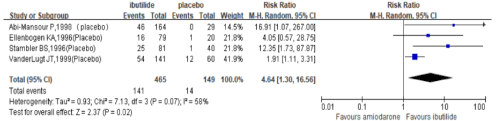
Figure 2: Relative risks of the conversion rate in trials comparing ibutilide to placebo within 4h.
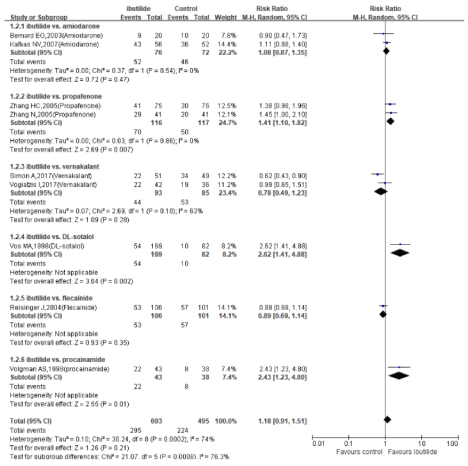
Figure 3: Relative risks of the conversion rate in trials comparing ibutilide to other active drugs within 4h: subgrouped based on different active drugs in control
group.
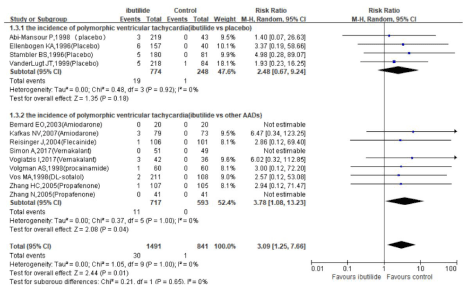
Figure 4: Relative risks of the polymorphic ventricular tachycardia events in trials comparing ibutilide to comparators: subgrouped based on whether the control
group received placebo or active drugs.
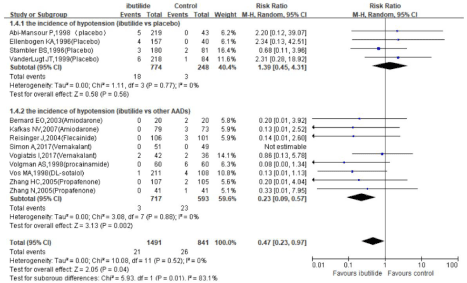
Figure 5: Relative risks of the hypotension events in trials comparing ibutilide to comparators: subgrouped based on whether the control group received placebo
or active drugs.
ibutilide versus other anti-arrhythmic drugs: A pool of 9 studies showed that no significant difference in the conversion rate was observed between the ibutilide and the active drugs (Figure 3, RR 1.18, 95% CI 0.91-1.51, P=0.21), also there existed substantial heterogeneity (I2=74%), which might caused by different comparator drugs and drug dosing. Then we did a subgroup analysis based on different control drugs, and we found ibutilide was more effective than DL-sotalol, Propafenone and Procainamide.
Pooled data from the 9 trials showed that ibutilide led to a higher risk of Polymorphic ventricular tachycardia (Figure 4 RR 3.78, 95% CI 1.08-13.23, P=0.04). But upon comparison with AADs, ibutilide retained a lower risk of hypotension (Figure 5, RR 0.23, 95% CI 0.09- 0.57, P=0.002).
Discussion
This analysis suggested that ibutilide could be an accessible choice for the cardioversion of recent-onset AF patients, which had minimal effects on blood pressure. However, ibutilide had a relatively much higher risk of ventricular tachycardia than other AADs.
Ibutilide is a class III antiarrhythmic agent that exerts its function by markedly prolonging the effective refractory period and monophasic action potential duration in the atrium [24-26]. Our meta-analysis showed that ibutilide was superior to DL-sotalol, Propafenone, Procainamide for rapidly successful termination of recent-onset AF, which was in coincidence with ibutilide function. However, the included trials showed that vernakalant and flecainide exhibited higher conversion rate than ibutilide, further to dig out the data, it seemed that these two active drugs had higher efficacy for conversion with the same shorter AF duration (<48h). This needs to be verified through relevant clinical trials. Besides, although Vernakalant is promoted as much less proarrhythmic along with a higher conversion rate, it is more expensive and not available in the US. Moreover, Intravenous flecainide are mainly available in many European countries, meanwhile, drug contraindications are also vital, which needs taking into consideration when drug selection.
What’s more, significant heterogeneity of the data on cardioversion rate in the trials were observed, this substantial heterogeneity may be due to the changes in the dose of ibutilide, the duration of atrial fibrillation. A random-effect model was therefore employed for the meta-analysis to reduce the false positive outcomes.
Moreover, we found that ibutilide was associated with a lower incidence of hypotension compared with other AADs. Previous studies also indicated that ibutilide was available for conversion of patients with hemodynamically unstable arrhythmia [27,28]. These results were in accordance with our data. For example, in the Varriale, et al. Trial [27], 27 of 34 ibutilide treated patients with symptomatic and/or hemodynamically unstable disorders reversed into sinus rhythm, with few cases of serious adverse events. Similarly, a recent study [28] showed that ibutilide was effective for refractory ventricular tachycardia in patients with hemodynamic instability.
In addition, pooled data from clinical trials showed that ibutilide was associated with a relatively much higher risk of polymorphic ventricular tachycardia compared with other active drugs, which occurred in 2.0% (30/1491) of participants. But in general, rapidly resolved following discontinuation [8-10,18-23]. Furthermore, one unscripted real-world clinical practice [29] reported that ibutilideinduced ventricular tachycardia was uncommon [0.6% (2/361)] and only one case of polymorphic ventricular tachycardia occurred for mild hypokalemia. Ibutilide is therefore a safe antiarrhythmic agent when patients are monitored through close electrocardiograph assessments and are administered magnesium or potassium to maintain electrolyte balance as well as avoidance combination with drugs of prolongation QT intervals [20,23].
There were several limitations in this study. Firstly, the sample size of many of the included trials were relatively small, affecting the quality of the review. Secondly, some RCTs were of low quality, including randomization or allocation concealment that was of high risk. Thirdly, the dose of ibutilide was variable and was difficult to stratify with relevant efficacy and safety data; meantime,patients in our included trials with different atrial fibrillation duration. Finally, few trials did cost effectiveness comparation, one of important factors affecting doctor to make drug selection.
Conclusion
In this meta-analysis of 13 RCTs, ibutilide was an accessible choice for the cardioversion of recent-onset atrial fibrillation when patients were closely monitored.
Acknowledgment
This work was supported by the National Science Foundation of China (No.81701958,No.81960357), the Key Special Project “Active Health and Scientific and Technological Response to Ageing” of National Key R&D Program (2018YFC2001904), the Special Fund of Wu Jie ping Medical Foundation for Clinical Scientific Research (320.6750.18001).
References
- Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, Ernst S, et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J. 2010; 31: 2369-429.
- Steg PG, Alam S, Chiang CE, Gamra H, Goethals M, Inoue H, et al. Symptoms, functional status and quality of life in patients with controlled and uncontrolled atrial fibrillation: data from the RealiseAF cross-sectional international registry. Heart. 2012; 98: 195-201.
- Singh SN, Tang XC, Singh BN, Dorian P, Reda DJ, Harris CL, et al. Quality of life and exercise performance in patients in sinus rhythm versus persistent atrial fibrillation: a Veterans Affairs Cooperative Studies Program Substudy. J Am Coll Cardiol. 2006; 48: 721-730.
- Sandhu RK, Smigorowsky M, Lockwood E, Savu A, Kaul P, McAlister FA. Impact of Electrical Cardioversion on Quality of Life for the Treatment of Atrial Fibrillation. Can J Cardiol. 2017; 33: 450-455.
- Barnett AS, Kim S, Fonarow GC, Thomas LE, Reiffel JA, Allen LA, et al. Treatment of Atrial Fibrillation and Concordance With the American Heart Association/American College of Cardiology/Heart Rhythm Society Guidelines: Findings From ORBIT-AF (Outcomes Registry for Better Informed Treatment of Atrial Fibrillation). Circ Arrhythm Electrophysiol. 2017; 10.
- Foster RH, Wilde MI, Markham A. Ibutilide. A review of its pharmacological properties and clinical potential in the acute management of atrial flutter and fibrillation. Drugs. 1997; 54: 312-330.
- January Craig T, Wann L Samuel, Alpert Joseph S, Calkins Hugh, Cigarroa Joaquin E, Cleveland Joseph C, et al. ACC/AHA Task Force Members. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014; 130: e199-267.
- Reisinger J, Gatterer E, Lang W, Vanicek T, Eisserer G, Bachleitner T, et al. Flecainide versus ibutilide for immediate cardioversion of atrial fibrillation of recent onset. Eur Heart J. 2004; 25: 1318-1324.
- Simon A, Niederdoeckl J, Skyllouriotis E, Schuetz N, Herkner H, Weiser C, et al. Vernakalant is superior to ibutilide for achieving sinus rhythm in patients with recent-onset atrial fibrillation: a randomized controlled trial at the emergency department. Europace. 2017; 19: 233-240.
- Vogiatzis I, Papavasiliou E, Dapcevitch I, Pittas S, Koulouris E. Vernakalant versus ibutilide for immediate conversion of recent-onset atrial fibrillation. Hippokratia. 2017; 21: 67-73.
- Higgins JPT. Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions, version 5.1.0. 2011.
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and metaanalyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009; 339: b2700.
- Zhao JG, Zeng XT, Wang J, Liu L. Association Between Calcium or Vitamin D Supplementation and Fracture Incidence in Community-Dwelling Older Adults: A Systematic Review and Meta-analysis. JAMA. 2017; 318: 2466- 2482.
- Stambler BS, Wood MA, Ellenbogen KA, Perry KT, Wakefield LK, VanderLugt JT. Efficacy and safety of repeated intravenous doses of ibutilide for rapid conversion of atrial flutter or fibrillation. Ibutilide Repeat Dose Study Investigators. Circulation. 1996; 94: 1613-1621.
- Ellenbogen KA, Stambler BS, Wood MA, Sager PT, Wesley RC, Meissner MC, et al. Efficacy of intravenous ibutilide for rapid termination of atrial fibrillation and atrial flutter: a dose-response study. J Am Coll Cardiol. 1996; 28: 130-136.
- Abi-Mansour P, Carberry PA, McCowan RJ, Henthorn RW, Dunn GH, Perry KT. Conversion efficacy and safety of repeated doses of ibutilide in patients with atrial flutter and atrial fibrillation. Study Investigators. Am Heart J. 1998; 136: 632-642.
- VanderLugt JT, Mattioni T, Denker S, Torchiana D, Ahern T, Wakefield LK, et al. Efficacy and safety of ibutilide fumarate for the conversion of atrial arrhythmias after cardiac surgery. Circulation. 1999; 100: 369-375.
- Zhang HC, Guo JH, Fang Q, Zheng YA, Sun YM, Zhu WQ, et al. Immediate cardioversion of atrial fibrillation and atrial flutter lasting less than 90 days by ibutilide versus propafenone: a multicenter study. Zhonghua Yi Xue Za Zhi. 2005; 85: 798-801.
- Zhang N, Guo JH, Zhang H, Li XB, Zhang P, Xn Y. Comparison of intravenous ibutilide vs. propafenone for rapid termination of recent onset atrial fibrillation. Int J Clin Pract. 2005; 59: 1395-1400.
- Vos MA, Golitsyn SR, Stangl K, Ruda MY, Van Wijk LV, Harry JD, et al. Superiority of ibutilide (a new class III agent) over DL-sotalol in converting atrial flutter and atrial fibrillation. The Ibutilide/Sotalol Comparator Study Group. Heart. 1998; 79: 568-575.
- Volgman AS, Carberry PA, Stambler B, Lewis WR, Dunn GH, Perry KT, et al. Conversion efficacy and safety of intravenous ibutilide compared with intravenous procainamide in patients with atrial flutter or fibrillation. J Am Coll Cardiol. 1998; 31: 1414-1419.
- Kafkas NV, Patsilinakos SP, Mertzanos GA, Papageorgiou KI, Chaveles JI, Dagadaki OK, et al. Conversion efficacy of intravenous ibutilide compared with intravenous amiodarone in patients with recent-onset atrial fibrillation and atrial flutter. Int J Cardiol. 2007; 118: 321-325.
- Bernard EO, Schmid ER, Schmidlin D, Scharf C, Candinas R, Germann R. Ibutilide versus amiodarone in atrial fibrillation: a double-blinded, randomized study. Crit Care Med. 2003; 31: 1031-1034.
- Buchanan LV, Turcotte UM, Kabell GG, Gibson JK. Antiarrhythmic and electrophysiologic effects of ibutilide in a chronic canine model of atrial flutter. J Cardiovasc Pharmacol. 1993; 22: 10-14.
- Buchanan LV, Kabell G, Brunden MN, Gibson JK. Comparative assessment of ibutilide, D-sotalol, clofilium, E-4031, and UK-68,798 in a rabbit model of proarrhythmia. J Cardiovasc Pharmacol. 1993; 22: 540-549.
- Nabih MA, Prcevski P, Fromm BS, Lavine SJ, Elnabtity M, Munir A, et al. Effect of ibutilide, a new class III agent, on sustained atrial fibrillation in a canine model of acute ischemia and myocardial dysfunction induced by microembolization. Pacing Clin Electrophysiol. 1993; 16: 1975-1983.
- Varriale P, Sedighi A. Acute management of atrial fibrillation and atrial flutter in the critical care unit: should it be ibutilide? Clin Cardiol. 2000; 23: 265-268.
- Sendra-Ferrer M, Gonzalez MD. Ibutilide for the control of refractory ventricular tachycardia and ventricular fibrillation in patients with myocardial ischemia and hemodynamic instability. J Cardiovasc Electrophysiol. 2019; 30: 503-510.
- Vinson David R, Nelya L, Margaret WE, Aaron MR, Matthew DS, Mary ER, et al. Pharm CAFE Investigators of the CREST Network. Ibutilide Effectiveness and Safety in the Cardioversion of Atrial Fibrillation and Flutter in the Community Emergency Department. Ann Emerg Med. 2018; 71: 96-108.