Case Report
Austin Surg Case Rep. 2024; 9(1): 1063.
Delayed Esophageal Perforation After Anterior Cervical Spine Plating
Taek Yong Ko, MD¹; Jin Hyuk Choi²*
1Department of Thoracic and Cardiovascular Surgery, Kosin University, College of Medicine, Kosin Gospel Hospital, Busan, Korea
2Department of Surgery, Kosin University, College of Medicine, Kosin Gospel Hospital, Busan, Korea
*Corresponding author: Jin Hyuk Choi Department of Surgery, Kosin University, College of Medicine, Kosin Gospel Hospital, 262 Gamcheon-ro, Seo-gu, Busan 49267, Korea. Tel: 82-51-990-6466; Fax: 82-51-990-3066 Email: drchoijinhyuk@gmail.com
Received: June 13, 2024 Accepted: July 01, 2024 Published: July 08, 2024
Abstract
Early diagnosis and active treatment for esophageal perforation are very important because the condition can lead to acute mediastinitis, which has a fatal prognosis. Esophageal perforation is classified as spontaneous such as in Boerhaave syndrome, esophageal cancer, and ulcer; traumatic due to external injury, foreign body, or corrosive injury; and iatrogenic due to endoscopy or balloon dilatation and nasogastric tube. Esophageal perforation is one of the most serious complications of cervical surgery and typically occurs within the first week after surgery. This report describes a case of delayed esophageal perforation caused by a metal bone plate fixed to an anterior cervical spine and a related literature review.
Keywords: Esophageal perforation; Diverticulum; Esophagus; Bone plate
Case Presentation
A 45-year-old man was admitted to a local hospital with chronic odynophagia of two months. Following anterior cervical spine fracture, the patient was mostly bedridden. Endoscopy was performed and a foreign body in the posterior wall of the esophagus was observed. The patient was transferred to our hospital for surgical treatment of esophageal perforation due to foreign body. At the time of admission, his vital signs were stable except for elevated blood pressure. No abnormalities were observed except for mild leukocytosis (11,000/uL), and the electrocardiogram was also normal. Chest X-ray showed a cervical spine metal plate and no abnormal findings such as pneumomediastinum, pneumothorax, or pleural effusion. As seen on neck Computed Tomography (CT), a metal plate was well fixed at C6-7, with mild swelling of the surrounding soft tissue. Esophagogram showed a pseudodiverticulum anterior to the plate without leakage. On endoscopy, about 19 cm from the incisor, a metal plate extending from the lower part of the pyriform sinus to the upper esophageal sphincter was revealed to be penetrating the posterior wall of the esophagus (Figure 1). The esophageal perforation was thought to be caused by persistent pressure and friction between the posterior wall of the esophagus and the plate, resulting in the formation of a pseudodiverticulum. Three months prior to the patient’s admission, right pleural effusion was diagnosed in the patient and was suspected to be tuberculous pleurisy. Thoracoscopic surgical exploration was performed, showing no evidence of tuberculosis. This was interpreted as a complication occurring during the process of esophageal perforation, with improvement predicted following drainage and antibiotic treatment. We planned foreign body removal with esophageal repair, continuing Total Parenteral Nutrition (TPN), and antibiotic therapy. Surgery was performed under general anesthesia, with the patient in a supine position and the head turned toward the left. Oblique cervical incision was applied. Distinction between the esophagus and surrounding structures was not clear due to previous spine surgery and fibrosis. The patient’s platysma and omohyoid muscles were incised, and lateral traction of the Sternocleidomastoid (SCM) muscle and carotid sheath was performed. After medial traction of the larynx and trachea, the esophagus was dissected carefully, with the surgeons touching the L-tube and the bone plate by hand. The esophagus was incised 3 cm superiorly from the bone plate. At this point, the bone plate and screw were removed (Figure 2). Tissue debridement and massive irrigation were performed to prevent cervical and mediastinal infection. The esophagus was further dissected until clean tissue emerged along the front of the vertebra. The mucosa and muscular layers of the esophagus were primarily repaired by interrupted suture with Vicryl 4-0. A superior-based Sternocleidomastoid (SCM) muscle flap was placed between the esophagus and the cervical spine to reinforce the esophagus. After inserting drainage, the operation was finished. At Postoperative Day (POD) 1, the patient was transferred to the general ward because his vital signs were stable and no abnormal findings were observed by chest X-ray and laboratory tests. Gastrografin esophagogram was performed on POD 8, with no contrast leakage shown at the repair site, but with identification of a left-sided esophageal pseudodiverticulum (Figure 3). The patient started to eat the following day. The patient was discharged on POD 12 because he had no complications following oral intake of foods.
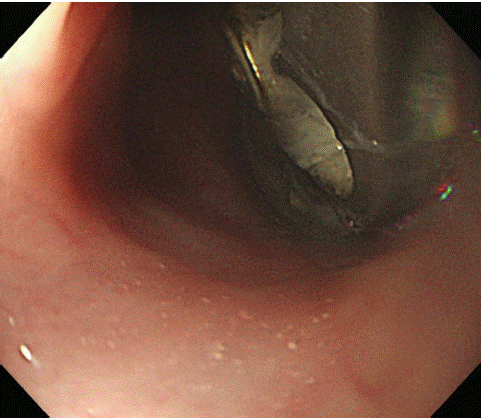
Figure 1: Endoscopy shows a penetrated cervical spine metal plate on the posterior wall of the esophagus.
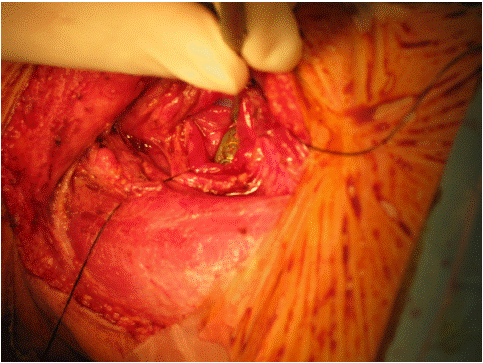
Figure 2: Intra-operative findings show cervical spine metal plate penetrating the posterior wall of the cervical esophagus.
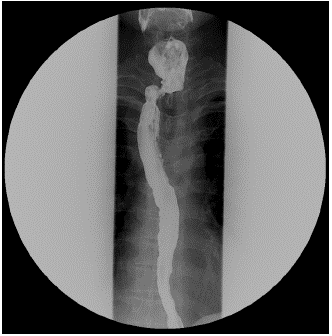
Figure 3: Postoperative esophagogram. There was no observed leakage of contrast agent around the suture site, but a pseudodiverticulum was identified.
Discussion
Esophageal perforation requires early diagnosis and intervention due to its potentially lethal outcomes. The esophagus lacks serosa and is enveloped by loose connective tissue, facilitating the easy dissemination of bacteria, oral secretions, and gastric juices, including digestive enzymes, to the mediastinum upon perforation. Chemical inflammation ensues in the initial phase of esophageal perforation, followed by a bacterial inflammatory response within hours. If the mediastinal pleura ruptures due to pressure from the oral cavity or intra-abdominal pressure, inflammation spreads to the thoracic cavity, potentially progressing to empyema. The etiology of esophageal perforation includes spontaneous perforation such as Boerhaave syndrome, esophageal cancer, and esophageal ulceration, as well as traumatic perforation resulting from trauma, foreign bodies, or corrosive injuries and iatrogenic perforation due to procedures such as endoscopy, balloon dilatation, or nasogastric tube insertion [1]. While spontaneous and traumatic perforations were prevalent in the past, the advent of advanced medical techniques has led to an increase in iatrogenic injuries in most cases of esophageal perforation [2].
Esophageal perforation may arise following surgeries or procedures performed in close proximity to or involving the esophagus, such as cervical spine fracture, sympathectomy, pneumonectomy, hiatal hernia reduction, transesophageal echocardiography, or atrial surgery [3].
Clinical symptoms and signs of esophageal perforation correlate with the site and timing of perforation. Cervical esophageal perforations frequently occur just inferior to the cricopharyngeus muscle, with the surrounding tissues enveloping the esophagus, mitigating the lethality of the condition. Symptoms such as neck pain and dysphagia may manifest. Thoracic and abdominal esophageal perforations easily contaminate the mediastinum or abdominal cavity, swiftly progressing to mediastinitis and intraperitoneal infection. Inflammation-induced rupture of the mediastinal pleura results in thoracic cavity contamination and pleural effusion. Negative pressure within the thoracic cavity facilitates the ingress of gastric contents, exacerbating contamination. This broad infection precipitates systemic symptoms such as fever, tachycardia, tachypnea, sepsis, and shock [4].
Our patient presented with dysphagia accompanied by pain for two months. The right pleural effusion noted three months prior had prompted thoracoscopic biopsy, under suspicion of tuberculous pleurisy, but which yielded negative mycobacterium findings. Subsequently, escalating dysphagia and pain led the patient to endoscopy, revealing the presence of esophageal foreign body with esophageal perforation.
Early diagnosis of esophageal perforation significantly mitigates mortality and complications. Chest X-ray findings such as cervical or thoracic subcutaneous emphysema, pneumothorax, pneumomediastinum, pleural effusion, or abdominal free air warrant suspicion of perforation. Esophagogram is the gold standard of diagnosis. Examination is performed using the water-soluble contrast agent Gastrografin, and if the results are unclear or if there is a possibility of respiratory aspiration, then diluted barium examination is conducted [5]. Endoscopy enables precise localization of the perforation, while CT aids in diagnosis and follow-up.
Surgical and non-surgical approaches are available based on the severity and location of perforation. Non-surgical treatment entails broad-spectrum intravenous antibiotics, nutritional support, and containment of intra-thoracic and abdominal infections. Surgical treatments include primary closure, esophagectomy with reconstruction, and irrigation and drainage. Selection of treatment should be made in consideration of the time from disease onset and the site of occurrence [6]. Abbas G. et al. proposed an esophageal perforation severity score, reporting that in patient groups with lower scores, non-surgical treatments such as esophageal stent insertion yielded better outcomes in comparison to surgical treatments [7].
The author reports a case of delayed esophageal perforation due to a metal plate employed in cervical spine fixation, necessitating surgical intervention.
Author Statements
Conflict of Interest
The author has no conflicts of interest to declare.
Funding
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea(NRF), funded by the Ministry of Education(NRF-2021R1G1A1011865).
References
- Cho SW, Hong KW, Kim S, Lee HS, Kim HS, Lee JW, et al. Clinical Results and Risk Factor Analysis of Surgical Treatment for Esophageal Perforation. Korean J Thorac Cardiovasc Surg. 2008; 41: 347-353.
- Cho SJ. Shin JS, Hwang JJ, Choi YH, Kim HJ. Surgical treatment of esophageal perforation. Korean J Thorac Cardiovasc Surg. 1994; 27: 598-602.
- Venuta F, Rendiana EA, De Giacomo T, Ciccone AM, Mercadante E, Coloni GF. Esophageal perforation after sequencial double-lung transplantation. Chest. 2000; 117: 285-287.
- Barrett N, Allison PR, Johnstone AS, Bonham-Carter RE. Discussion on unusual aspects of esophageal disease. Proc R Soc Med. 1956; 49: 529.
- Sarr MG, Pemberton JH, Payne WS. Management of instrumental perforation of the esophagus. J Thorac Cardiovasc Surg. 1982; 84: 211-8.
- Fischer A, Thomusch O, Benz S, Dobschuetz EV, Baier P, Hopt UT. Nonoperative treatment of 15 benign esophageal perforations with self-expandable covered metal stents. Ann Thorac Surg. 2006; 81: 467-72.
- Abbas G, Schuchert MJ, Pettiford BL, Pennathur A, Landreneau J, Landreneau J, et al. Contemporaneous management of esophageal perforation. Surgery. 2009; 146: 749–755; discussion 755–756.