Editorial
Austin J Surg. 2014;1(1): 1005.
Portal Venoplasty in the Field of Hepatobiliary Pancreatic Surgery and Liver Transplantation
Tsukasa Nakamura*a,b, Toshimasa Nakaoa, Shumpei Haradaa, Takashi Ito, Hidetaka Ushigome ,Norio Yoshimuraa
aDepartment of Organ Transplantation and General Surgery, Kyoto Prefectural University of Medicine, Japan
bDepartment of Organ Transplantation and General Surgery, Kyoto Prefectural University of Medicine, Japan
*Corresponding author: Tsukasa Nakamura, Department of Organ Transplantation and General Surgery, Kyoto Prefectural University of Medicine, Kajii-cho 465, Kamigyo-ku, Kyoto-prefecture, Japan
Received: February 05, 2014; Accepted: February 10, 2014; Published: February 14, 2014
Recent advances in portalvenoplasty enable us to accomplish complex pancreaticoduodenectomy including liver dissection (PD), and liver transplantation (LT). Technical issues regarding PD and LT surgery is essentially the same. In the field of PD, cases where a malignant tumour directly invades the portal vein, it will require a venous resection combined with reconstruction in order to obtain complete tumour clearance (R0).
In a case where the tumour does not involve the entire circumference, a wedge resection can simply be done with a longitudinal vascular anastomosis. When venoplasty with primary repair is difficult, a size–matched patch is required. The great saphenous vein (GSV) is most widely used as a venous patch graft. However, venoplasty of a wedge resection is often complicated and sometimes leads to venous torsion, stenosis and increased risk of thrombosis. In this case, it is better to perform the complete resection of the tumour–involved PV and primary end–to–end anastomosis rather than undertaking a complicated wedge resection, when semitotal circumference tumour involvement is confirmed. Generally, performing the extended Kocher manoeuvre enables the PV to be close enough to be an astomosed, provided the gap between the portal veins is less than 5 cm in length. However, in cases where the gap is longer than required for a venous graft it is mentioned below.
When portal flow clamping takes more than 30 minutes, the port–caval bypass technique is used, by means of the Anthron antithrombogenic catheter [1], it is recommended to prevent congestion in the intestine. This catheter is most commonly used to bypass the superior mesenteric vein (SMV) to the femoral vein in order to avoid portal congestion, and allows ample time for resection and reconstruction. Generally, these port venoplasty techniques are well established and surgical morbidity is minimized [2].
In the field of LT, there are some differences between a donation after brain death (DBD) LT, donation after cardiac death (DCD) LT and live donor LT in terms of portal venoplasty. Generally, the portal vein of the graft in the case of a live donor is inevitably shorter in length compared to that of a cadaveric donor. Furthermore, anatomical variation and a size mismatch of the PV are sometimes encountered in a live donor LT. Nevertheless, the aim for obtaining enough portal flow is fundamentally the same regardless of LT types. Therefore, although careful attention should be paid to a live donor LT, it can be argued that a wide variety of techniques in portal venoplasty, which also can be applied in PD, are required for surgeons who deal with a LT. In this paper, abnormal cases, where normal portal venoplasty cannot be achieved primarily due to PVthrombosis (PVT), are argued.
The incidence of what we encounter with PVT in cases of a LT is quite varied depending on the status of patients. A variety of methods can be used to obtain normal portal flow. It seems to be a good surgical strategy to follow the order as mentioned below.
- Firstly thromboendovenectomy and a normal end–to–end anastomosis is recommended for anon–organized thrombosis, because a newly transplanted liver is expected to be able to maintain normal coagulability. This can be achieved from the stump of the PV by means of inserting vascular forceps and picking it up, followed by opening the SMV, below the pancreas, in order to remove an extended thrombus as the occasion demands.
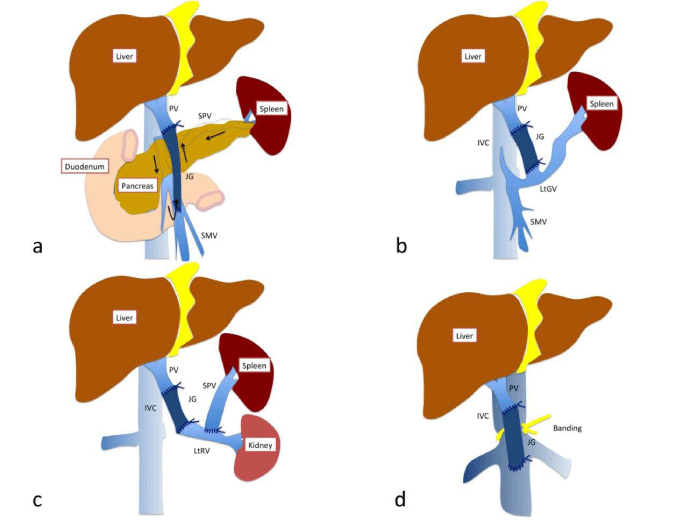
- Secondly, in the case where a thrombus is firmly adhered to the PV behind the pancreatic head, it is good to utilise a SMV–portal anastomosis by means of an interpositional graft (Figure 1a). This method requires a relatively long venous graft and a careful design for the anastomosis, because the long gap is connected by a SMV–venous graft side–to–end anastomosis and a graft–portal end–to–end anastomosis which can be potentially twisted.
- We sometimes encounter cases where there are diffuse thrombi of the PV. In these cases, a collateral vessel–portal anastomosis can be done (Figure 1b), provided that the remaining collateral vessels are ligated properly. Through this, enough portal flow for the liver can be obtained. The left gastric vein, middle colic vein etc. can be used.
- A renoportal anastomosis with the presence of spontaneous or artificial splenorenal shunts is an alternative approach to portalvenoplasty (Figure 1c). This method may be preferred, because the left renal blood flow relatively matches the required portal flow, and the retro hepatic caval flow is almost intact compared to the following cavoportal anastomosis.
- Nevertheless, sometimes it is difficult to perform a renoportal anastomosis, due to the existence of previous severe pancreatitis etc. The next alternative is a cavoportal anastomosis with the portal systemic shunt which maintains the intestine–liver circulation (Figure 1d). This can be divided in two different ways: a cavoportal anastomosis (end–to–end) with the complete caval dissection; a caval side–to–portal end anastomosis with caval banding in order to make a pressure gradient. From the aspect of importance of physiological retro hepatic blood flow, the latter way may be preferred. It is noteworthy that a favourable survival rate can be obtained by these portal venoplasties [3].
Figure 1 :An illustration of representative port venoplasty in the field of liver transplantation. a. SMV-portal anastomosis, b. LtGV-portal anastomosis, c. Renoportal anastomosis with an artificial splenorenal shunt, d. cavoportal anastomosis with IVC banding. PV: portal vein, JG: jumping graft, SMV: superior mesenteric vein, IVC: inferior vena cava, SPV: splenic vein, LtRV: left renal vein, LtGV: left gastric vein.
While performing a portalvenoplasty, a venous graft is sometimes required to completely accomplish what is mentioned above. A wide range of veins can be used as an interpositional venous graft, depending on each situation. In the case of PD, it is true that the left renal venous graft is useful, because it can be grafted without an extra incision. Commonly, the Kocher manoeuvre is performed when the left renal vein has already been visualised during a pancreaticoduodenectomy. Then, it can be harvested from the same field of vision or through the mesenteric window. In an adult to adult LT or PD with a relative long gap, the external iliac venous graft may be a good option, because a longer and size matched venous graft is obtained. An extra peritoneal approach with a Gibson incision is considered to be one of the best ways to graft the external iliac vein. This approach is essentially the same as that of a renal transplantation. It is better to ligate the lymph vessels, surrounding the external iliac vein, in order to prevent a lymphocele. Other complications, including leg pain or oedema, are usually sub–clinical.
In summary, with a wide variety of portalvenoplasty options that surgeons can utilise during surgery, surgical indications can be extended and patients will benefit from these advanced methods.
References
- Tashiro S, Uchino R, Hiraoka T, Tsuji T, Kawamoto S, et al. Surgical indication and significance of portal vein resection in biliary and pancreatic cancer. Surgery. 1991; 109: 481-487.
- Menon V G, Puri V C, Annamalai A A, Tuli R,Nissen N N. Outcomes of vascular resection in pancreaticoduodenectomy: single-surgeon experience. Am Surg. 2013; 79: 1064-1067.
- Ravaioli M, Zanello M, Grazi G L, Ercolani G, Cescon M, et al. Portal vein thrombosis and liver transplantation: evolution during 10 years of experience at the University of Bologna. Ann Surg. 2011; 253: 378-384.