Review Article
Austin J Surg. 2014;1(3): 1011.
Fatty Acid Synthase Inhibitor Cerulenin Suppresses Colorectal Cancer in Combination with Oxaliplatin
Soichiro Murata1*, Risa Shiragami2, Takehito Maruyama1, Keiji Koda2 and Nobuhiro Ohkohchi1
1Department of Surgery, Division of Gastroenterological and Hepatobiliary Surgery and Organ Transplantation, University of Tsukuba, Japan
2Department of Surgery, Teikyo University Chiba Medical Center, Japan
*Corresponding author: Soichiro Murata, Department of Surgery, Division of Gastroenterological, Hepatobiliary Surgery and Organ Transplantation, University of Tsukuba, 1-1-1 Tennodai, Tsukuba, Ibaraki, 305-8575, Japan
Received: February 09, 2014; Accepted: April 09, 2014; Published: April 11, 2014
Abstract
Fatty acid synthase (FASN) is highly expressed in many kinds of human cancers, and the potential use of FASN inhibitors were reported. Colorectal cancer (CRC) is one of the most common cancers with over one million new cases per year. The main cause of death by CRC is distant metastasis, especially liver metastasis. The effective chemotherapy for CRC is required worldwide. Expression of FASN was evaluated in both murine and human CRC cell lines. Cerulenin, a natural inhibitor of FASN, induces apoptosis in these cell lines. The ability of cerulenin to prevent the development of liver metastases of CRC cell lines was investigated. The number and size of metastatic CRC in the liver are significantly reduced by treatment with cerulenin. Oxaliplatin is one of the best chemotherapeutic agents of CRC. Cerulenin has a synergistic effect with oxaliplatin in vitro and in vivo. Cerulenin treatment is associated with reduced levels of phosphorylated Akt in CRC cell lines, suggesting that inhibition of this signal transduction pathway might be involved in the chemopreventive activity of cerulenin. Based on this study, inhibiting FASN would be an effective strategy in treating liver metastases and unresectable CRC, especially in combination with oxaliplatin.
Keywords: Fatty Acid; Inhibitor; Colorectal Cancer; Cerulenin
Introduction
Colorectal cancer (CRC) is one of the most common cancers in the world. Around 90% of CRC deaths are caused by metastasis, not by primary solid tumors [1,2].The liver is the most frequent site of distant metastasis in CRC. Approximately 20% of patients have liver metastases when the primary tumor is diagnosed [3], and half of the patients ultimately develop liver metastases during the course of CRC [4]. In the treatment of liver metastases, hepatic resection is theonly curative treatment. Unfortunately, only 10% to 25% of patients with liver metastases are candidates for liver resection because of tumor number, size or location [5,6]. Also, when curative resection of the metastatic CRC is performed, 30% to 50% of patients have a recurrence in the liver [7,8]. Systemic chemotherapy is the last hope for patients with unresectable liver metastases of CRC. Nowadays, combination regimens of infusion 5–FU with either oxaliplatin (e.g., Folinic acid, 5–Fluorouracil and Oxaliplatin (FOLFOX)) or irinotecan (e.g., Folinic acid, 5–Fluorouracil and Irinotecan (FOLFIRI)) are the popular regimens for metastatic CRC [9]. Recently, three biological agents were indicated for the treatment of colorectal cancer, such as bevacizumab [10], cetuximab [11], and panitumumab [12]. Though recent systemic chemotherapy advances prolong survival in patients with unresectable disease [13], long–term survivors treated with only chemotherapy are rare [14].
The cancer cell is different from normal cells in many points. In contrast to normal cells, the majority of fatty acids in malignant cells are derived from de novo lipogenesis that emphasizes the importance of upregulation of endogenous lipid biosynthesis in malignant transformation [15].
Fatty acid synthase (FASN)
Fatty acid synthase (FASN), the key enzyme of de novo lipogenesis, is significantly upregulated in many cancers including CRC [15]. In normal adults, FASN is mainly expressed in cells with lipid metabolisms, such as liver and adipose tissues [16].Under usual diet, the de novo fatty acid synthesis in the normal cell is rarely needed and the FASN protein level is low [17].FASN is a 270–kDa cytosolic enzyme containing seven catalytic domains [18].FASN synthesizes palmitate from one acetyl–CoA, seven malonyl–CoA and seven NADPH [16,17]. The expression of FASN has been found to be up–regulated in various human cancer cells including CRC [19–21]. FASN is elevated in aberrant crypt foci (ACF) compared with normal colonic mucosa [22]. Lipogenesis by cancer cells gives proliferative and survival advantages and drug resistance against chemotherapeutic drugs [23]. An increased expression of lipogenic enzymes is associated with a more aggressive metastatic phenotype in CRC [24]. Inhibition of lipogenesis targeting FASN induces apoptosis selectively in human cancer cells both in vitro and in vivo [25–27]. The differential expression of FASN, together with the different responses to FASN inhibition between cancer cells and normal cells, makes FASN a suitable target for cancer treatment. The pharmacological FASN inhibitors are cerulenin, C75, orlistat, luteolin and epigallocatechin–3–gallate (EGCG).
Various kinds of FASN inhibitors
Cerulenin is the first known FASN inhibitor, which is isolated from the culture filtrate of the fungus Cephalosporum caerulens [28– 31]. It was originally used as an antifungal antibiotic and is a potent non–competitive irreversible inhibitor of FASN by binding to the active site of the KS domain [32–34]. Cerulenin treatment significantly decreases fatty acid synthesis and induces selective cytotoxicity in various types of cancer cells [35–37].
C75 is a chemically synthesized analogue of cerulenin [38]. C75 has significant anti–tumor effects against many types of cancer cells, including the human breast [38], prostate [36], and ovary [39], as well as renal carcinoma in xenograft animal models [40].
Orlistat is an anti–obesity drug approved by the U.S. Food and Drug Administration. Orlistat is also reported to inhibit FASN [41]. Several studies have shown that orlistat exhibits anti–tumor effects in many cancer cells in vitro and in vivo by inhibiting FASN activity[41–44]. Orlistathas a very poor solubility and oral bioavailability, and clinical application for cancer treatment is very difficult [45].
Luteolin, 3’,4’,5,7–tetrahydroxyflavone, is found in a variety of vegetables, fruits, and medicinal herbs. Luteolin has been shown to function as an anti–oxidant, anti–inflammatory, and anti–cancer agent [46]. Additionally, luteolin induces cell cycle arrest and apoptosis in the liver and lung cancercell lines [47,48]. Lim do Y et al. indicated that Luteolin inhibited HT–29 cell proliferation by inducing cell cycle arrest and apoptosis [49].
EGCG, which is green tea polyphenol, inhibits the activity of FASN [50,51]. EGCG induces apoptosis in human breast and prostate cancer cells [52–54]. EGCG suppresses FASN expression and downstream phosphatidylinositol–3–kinase (PI3K)⁄Akt pathway [55]. Recently, Maruyama T et al. revealed that EGCG strongly reduces liver metastasis of human CRC in SCID mice [56].
In this review, the effect of cerulenin on cell proliferation and apoptosis in murine CRC cell lines Colon 26 and CMT 93, and in human CRC cell lines HCT116 and RKO is discussed. The effect of cerulenin on the prevention of growth of liver metastatic lesions in mice and the synergistic effect of cerulenin and oxaliplatin are also discussed.
Dose–dependent inhibition of proliferation of CRC cell lines by cerulenin
Murata et al. [57] determined whether cerulenin treatment led to the inhibition of CRC cell proliferation. CRC cells were treated with various doses of cerulenin for 24 h, and cell viability was assayed using WST–8 assay and BrdU assay. Murine CRC cell lines Colon 26 and CMT93 were selected. As the dose of cerulenin increased from 100 to 200 µM, cell growth inhibition increased in a dose–dependent fashion in CRC cell lines. Shiragami et al. [58] evaluated human CRC cell lines HCT116 and RKO. Cerulenin–induced growth inhibition was found to be statistically significant in 12.5–100 µM of cerulenin.
Induction of apoptosis via activation of caspasedependent pathway by cerulenin
The overexpression of FASN has been seen to cooperate with survival pathways, including the phosphatidylinositol–3– kinase (PI3K) / Akt pathway. CRC cell lines expressed FASN and phosphorylated Akt constitutively, and treatment of CRC cells with cerulenin suppressed FASN expression, dephosphorylated constitutive activated Akt, and increased cleaved caspase–3 in murine CRC cell lines Colon 26 and CMT 93, and in human CRC cell line HCT116 cells [57,58]. Inhibiting FASN decreases phosphorylation of Akt in ovarian cancer cells and suppresses human epidermal growth factor receptor 2 (HER2) in breast cancer cells [39,59]. The mechanism by which FASN inhibitor decreases Akt activation is still unclear, but one mechanism that is advocated is that fatty acids synthesized by FASN are incorporated into membrane phospholipids, which are known modulators of Akt activation [60,61]. FASN has a major role in the synthesis of phospholipids including phosphatidylinositol trisphosphate (PIP3) [62]. PIP3 binds to Akt and activates kinase phosphoinositidedependent protein kinase–1(PDK–1) with high affinity, and the phosphorylation of Akt is dependent on PIP3 [62].
Reduction of liver metastasis of CRC by cerulenin
Murata et al. [57] evaluated the potential effectiveness of cerulenin for metastatic liver tumors of the CRC cell line. By treatment with 30 mg⁄kg of cerulenin every 3 days, significant reduction of liver metastasis was observed. Tumor growth was significantly reduced by cerulenin administration. In the cerulenin group, apoptotic tumor cells were observed in the metastatic liver tumor.
Many efforts to treat xenograft cancers with cerulenin or its derivative of C75 were reported in ovary [63], prostate [36], mesothelioma [64], lung cancer [65], CRC [66,67], and murine CRC liver metastasis using cerulenin [57]. To investigate the physiological consequences of in vivo inhibition of FASN, Murata et al. [57] administered cerulenin (30 mg⁄kg body weight every 3 days) to mice by i.p. injection. In this amount there was no significant weight loss in the cerulenin treatment group. In the prior studies, the use of cerulenin or C75 was hampered by transient but severe anorexia and weight loss. Therefore, this compound could also limit use in the clinical setting [68,69]. Loftus et al. [68] reported that cerulenin treatment of 60 mg⁄kg daily for 7 days caused severe weight loss. Pizer et al. [63] reported that cerulenin treatment of 80 mg⁄kg per daily for 7 days caused severe weight loss. These reports suggest that 30 mg⁄kg every 3 days is acceptable for reducing side effects. At this amount, serum triglyceride was significantly decreased in the cerulenin group. That means that FASN was actually inhibited in our animal model. Newly developed FASN inhibitor C93, which inhibits FASN but has no significant effect on fatty acid oxidation, is effective for treatment of human lung cancer xenografts. As a result, C93 does not cause anorexia or weight loss [70,71]. Although there is no report discussing CRC, C93 might be an effective agent for CRC treatment with minimal side effects.
Induction of apoptosis via activation of caspase–dependent pathway by cerulenin combined with oxaliplatin
Shiragami et al. [58] determined whether oxaliplatin treatment led to the inhibition of human CRC cell proliferation. CRC cells were treated with various doses of oxaliplatin for 24 h and as the dose of oxaliplatin increased from 0.5 to 2.5 µM, cell growth inhibition increased in a dose–dependent manner in CRC cell lines HCT116 and RKO.They also revealed that cerulenin and oxaliplatin have synergic antitumor effects. In a xenotransplant mouse model, the combination therapy, which consists of 2.5 mg⁄kg of oxaliplatin and 15 mg⁄kg of cerulenin, had the same tumor shrinkage effect compared to the oxaliplatin 5 mg⁄kg group; which means that by adding cerulenin, oxaliplatin dose could be reduced.
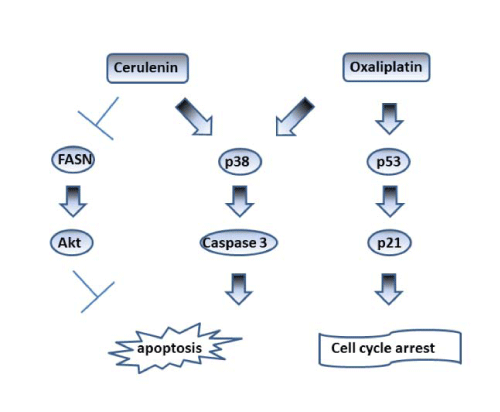
Shiragami et al. [58] determined the mechanism of the observed synergistic effect of cerulenin and oxaliplatin. Dephosphorylated constitutive activated Akt, activation of p38 and increased cleaved caspase–3 were observed in CRC cells with cerulenin treatment. Oxaliplatin induced p53–p21 pathway and p38, but did not increase cleaved caspase–3. In combination with cerulenin and oxaliplatin, p53–p21 pathway and p38 activation occurred and induced caspase–3 cleavage, finally causing apoptosis. Figure 1 shows the scheme of the combination therapy consisting of cerulenin and oxaliplatin.
Figure 1 :The scheme of the combination therapy consists of cerulenin and oxaliplatin. Cerulenin inhibits FASN and deactivates Akt. Cerulenin also activates p38. These reactions finally activate caspase-3 and induce apoptosis.Oxaliplatin induces the p53-p21 pathway which causes cell cycle arrest. In combination with cerulenin and oxaliplatin, p53-p21 pathway and p38 are activated, which causes cell cycle arrest and apoptosis. This figure was modified from [58].
p38 MAP kinase is a member of the MAP kinase family and is activated by a variety of cellular stresses, including osmotic shock, inflammatory cytokines, UV light and growth factors [72–76]. Activated p38 MAP kinase appears to have multiple targets in the apoptotic pathway. p38 MAP kinase activates caspase and induces apoptosis [77]. Oxaliplatin activates p38 MAP kinase phosphorylation in human CRC [78]. The p53 protein plays a pivotal role in the maintenance of genomic stability [79,80]. Activation of p53 can lead to either cell cycle arrest and DNA repair or apoptosis [81]. DNA damage induces p53 activation and activated p53 up–regulates p21 transcription [82].
Oxaliplatin is a most promising chemotherapeutic agent, which consists of FOLFOX for unresectable CRC [9,83]. Neurotoxicity is a severe and treatment–limiting side–effect of several chemotherapeutic agents [84]. Sensory neurotoxicity is a potentially limiting factor in many patients who might otherwise achieve good results with oxaliplatin therapy [85]. In patients with severe neurotoxicity, reduction or discontinuation of oxaliplatin is often required. This study revealed that cerulenin can potentiate oxaliplatin in in vitro and in vivo. Cerulenin is one of the better combinations with oxaliplatin, which achieves reduction of oxaliplatin and long–term tolerated chemotherapy for unresectable CRC.
Conclusion
In conclusion, cerulenin has a cytotoxic effect on murine and human CRC cell lines. Moreover, cerulenin potentiated cytotoxicity of oxaliplatin. Cerulenin would be effective in treatment of unresectable CRC, especially liver metastasis. Also, cerulenin is synergistically effective in combination with oxaliplatin, which reduces the dose of oxaliplatin and would make it possible for the patient to endure the chemotherapy over a longer period.
References
- Gupta GP, Massagué J. Cancer metastasis: building a framework. Cell. 2006; 127: 679-695.
- Kune GA, Kune S, Field B, White R, Brough W, Schellenberger R, et al. Survival in patients with large-bowel cancer. A population-based investigation from the Melbourne Colorectal Cancer Study. Diseases of the colon and rectum. 1990; 33: 938-946.
- Manfredi S, Lepage C, Hatem C, Coatmeur O, Faivre J, Bouvier AM. Epidemiology and management of liver metastases from colorectal cancer. Ann Surg. 2006; 244: 254-259.
- Geoghegan JG, Scheele J. Treatment of colorectal liver metastases. Br J Surg. 1999; 86: 158-169.
- Spolverato G, Ejaz A, Azad N, Pawlik TM. Surgery for colorectal liver metastases: The evolution of determining prognosis. World J Gastrointest Oncol. 2013; 5: 207-221.
- Leporrier J, Maurel J, Chiche L, Bara S, Segol P, Launoy G. A population-based study of the incidence, managementand prognosis of hepatic metastases from colorectal cancer.Br J Surg. 2006; 93: 465-474.
- de Jong MC, Pulitano C, Ribero D, Strub J, Mentha G, Schulick RD, et al. Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: an international multi-institutional analysis of 1669 patients. Ann Surg. 2009; 250:440-448.
- Neeff HP, Drognitz O, Holzner P, Klock A, Bronsert P, Hopt UT, et al. Outcome after repeat resection of liver metastases from colorectal cancer. Int J Colorectal Dis. 2013; 28: 1135-1141.
- Scheer MG, Sloots CE, van der Wilt GJ, Ruers TJ. Management of patients with asymptomatic colorectal cancer and synchronous irresectable metastases. Ann Oncol. 2008; 19: 1829-1835.
- Kabbinavar F, Hurwitz HI, Fehrenbacher L, Meropol NJ, Novotny WF, Lieberman G, et al. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol. 2003; 21: 60-65.
- Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. The New England Journal of Medicine2004; 351: 337-345.
- Van CutsemE, Peeters M, Siena S, Humblet Y, Hendlisz A, Neyns B, et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. Journal of Clinical Oncology 2007; 25: 1658-1664.
- Aprile G, Lutrino SE, Ferrari L, Casagrande M, Bonotto M, Ongaro E. Evidence-based appraisal of the upfront treatment for unresectable metastatic colorectal cancer patients. World J Gastroenterol. 2013; 19: 8474-8488.
- DyGK, Hobday TJ, Nelson G, Windschitl HE, O'Connell MJ, Alberts SR, et al. Long-term survivors of metastatic colorectal cancer treated with systemic chemotherapy alone: a North Central Cancer Treatment Group review of 3811 patients, N0144. Clinical colorectal cancer. 2009; 8: 88-93.
- Furuta E, Okuda H, Kobayashi A, Watabe K. Metabolic genes in cancer: their roles in tumor progression and clinical implications. Biochim Biophys Acta. 2010; 1805: 141-152.
- Kusakabe T, Maeda M, Hoshi N, Sugino T, Watanabe K, Fukuda T, et al. Fatty acid synthase is expressed mainly in adult hormone-sensitive cells or cells with high lipid metabolism and in proliferating fetal cells. J Histochem Cytochem. 2000; 48: 613-622.
- WeissL, Hoffmann GE, Schreiber R, Andres H, Fuchs E, Körber E, et al. Fatty-acid biosynthesis in man, a pathway of minor importance. Purification, optimal assay conditions, and organ distribution of fatty-acid synthase.Biological chemistry Hoppe-Seyler. 1986; 367: 905-912.
- Smith S, Witkowski A, Joshi AK. Structural and functional organization of the animal fatty acid synthase. Prog Lipid Res. 2003; 42: 289-317.
- Nguyen PL, Ma J, Chavarro JE, Freedman ML, Lis R, Fedele G, et al. Fatty acid synthase polymorphisms, tumor expression, body mass index, prostate cancer risk, and survival. J Clin Oncol. 2010; 28: 3958-3964.
- Zhou Y, Niu C, Li Y, Gao B, Zheng J, Guo X, et al. Fatty acid synthase expression and esophageal cancer. Mol Biol Rep. 2012; 39: 9733-9739.
- Long QQ, Yi YX, Qiu J, Xu CJ, Huang PL. Fatty acid synthase (FASN) levels in serum of colorectal cancer patients: correlation with clinical outcomes. Tumour Biol. 2014; 35: 3855-3859.
- Kearney KE, Pretlow TG, Pretlow TP. Increased expression of fatty acid synthase in human aberrant crypt foci: possible target for colorectal cancer prevention. Int J Cancer. 2009; 125: 249-252.
- Ong ES, Zou L, Li S, Cheah PY, Eu KW, Ong CN. Metabolic profiling in colorectal cancer reveals signature metabolic shifts during tumorigenesis. Mol Cell Proteomics. 2010;.
- Luque-García JL, Martínez-Torrecuadrada JL, Epifano C, Ca&nTilde;amero M, Babel I, Casal JI. Differential protein expression on the cell surface of colorectal cancer cells associated to tumor metastasis. Proteomics. 2010; 10: 940-952.
- Kuhajda FP. Fatty-acid synthase and human cancer: new perspectives on its role in tumor biology. Nutrition. 2000; 16: 202-208.
- Kuhajda FP. Fatty acid synthase and cancer: new application of an old pathway. Cancer Res. 2006; 66: 5977-5980.
- Yoshii Y, Furukawa T, Oyama N, Hasegawa Y, Kiyono Y, Nishii R, et al. Fatty acid synthase is a key target in multiple essential tumor functions of prostate cancer: uptake of radiolabeled acetate as a predictor of the targeted therapy outcome. PLoS One. 2013; 8: e64570.
- Nomura S, Horiuchi T, Hata T, Omura S. Inhibition of sterol and fatty acid biosyntheses by cerulenin in cell-free systems of yeast. J Antibiot (Tokyo). 1972; 25: 365-368.
- Nomura S, Horiuchi T, Omura S, Hata T. The action mechanism of cerulenin. I. Effect of cerulenin on sterol and fatty acid biosynthesis in yeast. J Biochem. 1972; 71: 783-796.
- Vance D, Goldberg I, Mitsuhashi O, Bloch K. Inhibition of fatty acid synthetases by the antibiotic cerulenin. Biochem Biophys Res Commun. 1972; 48: 649-656.
- D'Agnolo G, Rosenfeld IS, Awaya J, Omura S, Vagelos PR. Inhibition of fatty acid synthesis by the antibiotic cerulenin. Specific inactivation of beta-ketoacyl-acyl carrier protein synthetase. Biochim Biophys Acta. 1973; 326: 155-156.
- Goldberg I, Walker JR, Bloch K. Inhibition of lipid synthesis in Escherichia coli cells by the antibiotic cerulenin. Antimicrob Agents Chemother. 1973; 3: 549-554.
- Omura S. The antibiotic cerulenin, a novel tool for biochemistry as an inhibitor of fatty acid synthesis. Bacteriol Rev. 1976; 40: 681-697.
- Omura S. Cerulenin. Methods Enzymol. 1981; 72: 520-532.
- Jeong NY, Lee JS, Yoo KS, Oh S, Choe E, Lee HJ, et al. Fatty acid synthase inhibitor cerulenin inhibits topoisomerase I catalytic activity and augments SN-38-induced apoptosis. Apoptosis. 2013; 18: 226-237.
- Chen HW, Chang YF, Chuang HY, Tai WT, Hwang JJ. Targeted therapy with fatty acid synthase inhibitors in a human prostate carcinoma LNCaP/tk-luc-bearing animal model. Prostate Cancer Prostatic Dis. 2012; 15: 260-264.
- Zhao W, Kridel S, Thorburn A, Kooshki M, Little J, Hebbar S, et al. Fatty acid synthase: a novel target for antiglioma therapy. Br J Cancer. 2006; 95: 869-878.
- Kuhajda FP, Pizer ES, Li JN, Mani NS, Frehywot GL, Townsend CA. Synthesis and antitumor activity of an inhibitor of fatty acid synthase. Proc Natl Acad Sci U S A. 2000; 97: 3450-3454.
- Rahman MT, Nakayama K, Rahman M, Katagiri H, Katagiri A, Ishibashi T, et al. Fatty acid synthase expression associated with NAC1 is a potential therapeutic target in ovarian clear cell carcinomas. Br J Cancer. 2012; 107: 300-307.
- Horiguchi A, Asano T, Asano T, Ito K, Sumitomo M, Hayakawa M. Pharmacological inhibitor of fatty acid synthase suppresses growth and invasiveness of renal cancer cells. J Urol. 2008; 180: 729-736.
- Kridel SJ, Axelrod F, Rozenkrantz N, Smith JW. Orlistat is a novel inhibitor of fatty acid synthase with antitumor activity. Cancer Res. 2004; 64: 2070-2075.
- MenendezJA, Vellon L, Lupu R. Anti-tumoral actions of the anti-obesity drug orlistat (XenicalTM) in breast cancer cells: blockade of cell cycle progression, promotion of apoptotic cell death and PEA3-mediated transcriptional repression of Her2/neu (erbB-2) oncogene. Annals of Oncology2005; 16: 1253-1267.
- Little JL, Wheeler FB, Fels DR, Koumenis C, Kridel SJ. Inhibition of fatty acid synthase induces endoplasmic reticulum stress in tumor cells. Cancer Res. 2007; 67: 1262-1269.
- CarvalhoMA, Zecchin KG, Seguin F, Bastos DC, Agostini M, Rangel AL, et al. Fatty acid synthase inhibition with Orlistat promotes apoptosis and reduces cell growth and lymph node metastasis in a mouse melanoma model. International Journal of Cancer. 2008; 123: 2557-2565.
- LiuH, Liu JY, Wu X, Zhang JT. Biochemistry, molecular biology, and pharmacology of fatty acid synthase, an emerging therapeutic target and diagnosis/prognosis marker. International Journal of Biochemistry and Molecular biology. 2010; 1: 69-89.
- Lin Y, Shi R, Wang X, Shen HM. Luteolin, a flavonoid with potential for cancer prevention and therapy. Curr Cancer Drug Targets. 2008; 8: 634-646.
- Chang J, Hsu Y, Kuo P, Kuo Y, Chiang L, Lin C. Increase of Bax/ Bcl-XL ratio and arrest of cell cycle by luteolin in immortalized human hepatoma cell line. Life Sci. 2005; 76: 1883-1893.
- Leung HW, Wu CH, Lin CH, Lee HZ. Luteolin induced DNA damage leading to human lung squamous carcinoma CH27 cell apoptosis. Eur J Pharmacol. 2005; 508: 77-83.
- Lim do Y, Jeong Y, Tyner AL, Park JH. Induction of cell cycle arrest and apoptosis in HT-29 human colon cancer cells by the dietary compound luteolin. Am J Physiol Gastrointest Liver Physiol. 2007; 292: G66-75.
- Wang X, Tian W. Green tea epigallocatechin gallate: a natural inhibitor of fatty-acid synthase. Biochem Biophys Res Commun. 2001; 288: 1200-1206.
- Wang X, Song KS, Guo QX, Tian WX. The galloyl moiety of green tea catechins is the critical structural feature to inhibit fatty-acid synthase. Biochem Pharmacol. 2003; 66: 2039-2047.
- Vergote D, Cren-Olivé C, Chopin V, Toillon RA, Rolando C, Hondermarck H, et al. (-)-Epigallocatechin (EGC) of green tea induces apoptosis of human breast cancer cells but not of their normal counterparts. Breast Cancer Res Treat. 2002; 76: 195-201.
- Khan N, Bharali DJ, Adhami VM, Siddiqui IA, Cui H, Shabana SM, et al. Oral administration of naturally occurring chitosan-based nanoformulated green tea polyphenol EGCG effectively inhibits prostate cancer cell growth in a xenograft model.Carcinogenesis. 2014; 35: 415-423.
- Yeh CW, Chen WJ, Chiang CT, Lin-Shiau SY, Lin JK. Suppression of fatty acid synthase in MCF-7 breast cancer cells by tea and tea polyphenols: a possible mechanism for their hypolipidemic effects. Pharmacogenomics J. 2003; 3: 267-276.
- PanMH, Lin CC, Lin JK, Chen WJ. Tea polyphenol (-)-epigallocatechin 3-gallate suppresses heregulin-beta1-induced fatty acid synthase expression in human breast cancer cells by inhibiting phosphatidylinositol 3-kinase/Akt and mitogen-activated protein kinase cascade signaling. Journal of Agricultural and Food Chemistry. 2007; 55: 5030-5037.
- Maruyama T, Murata S, Nakayama K, Sano N, Ogawa K, Nowatari T, et al. (-)-Epigallocatechin-3-gallate suppresses liver metastasis of human colorectal cancer. Oncol Rep. 2014; 31: 625-633.
- Murata S, Yanagisawa K, Fukunaga K, Oda T, Kobayashi A, Sasaki R, et al. Fatty acid synthase inhibitor cerulenin suppresses liver metastasis of colon cancer in mice. Cancer Sci. 2010; 101: 1861-1865.
- Shiragami R, Murata S, Kosugi C, Tezuka T, Yamazaki M, Hirano A, et al. Enhanced antitumor activity of cerulenin combined with oxaliplatin in human colon cancer cells. Int J Oncol. 2013; 43: 431-438.
- Menendez JA, Vellon L, Mehmi I, Oza BP, Ropero S, Colomer R, et al. Inhibition of fatty acid synthase (FAS) suppresses HER2/neu (erbB-2) oncogene overexpression in cancer cells. Proc Natl Acad Sci U S A. 2004; 101: 10715-10720.
- Swinnen JV, Van Veldhoven PP, Timmermans L, De Schrijver E, Brusselmans K, Vanderhoydonc F, et al. Fatty acid synthase drives the synthesis of phospholipids partitioning into detergent-resistant membrane microdomains. Biochem Biophys Res Commun. 2003; 302: 898-903.
- Uddin S, Siraj AK, Al-Rasheed M, Ahmed M, Bu R, Myers JN, et al. Fatty acid synthase and AKT pathway signaling in a subset of papillary thyroid cancers. J Clin Endocrinol Metab. 2008; 93: 4088-4097.
- Denley A, Gymnopoulos M, Kang S, Mitchell C, Vogt PK. Requirement of phosphatidylinositol(3,4,5) trisphosphate in phosphatidylinositol 3-kinase-induced oncogenic transformation. Mol Cancer Res. 2009; 7: 1132-1138.
- Pizer ES, Wood FD, Heine HS, Romantsev FE, Pasternack GR, Kuhajda FP. Inhibition of fatty acid synthesis delays disease progression in a xenograft model of ovarian cancer. Cancer Res. 1996; 56: 1189-1193.
- Gabrielson EW, Pinn ML, Testa JR, Kuhajda FP. Increased fatty acid synthase is a therapeutic target in mesothelioma. Clin Cancer Res. 2001; 7: 153-157.
- Relat J, Blancafort A, Oliveras G, Cufí S, Haro D, Marrero PF. Different fatty acid metabolism effects of (-)-epigallocatechin-3-gallate and C75 in adenocarcinoma lung cancer. BMC Cancer. 2012; 12: 280.
- Huang P, Zhu S, Lu S, Li L, Dai Z, Jin Y. [Cerulenin inhibits growth of human colonic carcinoma in nude mice]. Zhonghua Bing Li Xue Za Zhi. 2000; 29: 435-438.
- Huang PL, Zhu SN, Lu SL, Dai ZS, Jin YL. Inhibitor of fatty acid synthase induced apoptosis in human colonic cancer cells. World J Gastroenterol. 2000; 6: 295-297.
- Loftus TM, Jaworsky DE, Frehywot GL, Townsend CA, Ronnett GV, Lane MD, et al. Reduced food intake and body weight in mice treated with fatty acid synthase inhibitors. Science. 2000; 288: 2379-2381.
- Thupari JN, Landree LE, Ronnett GV, Kuhajda FP. C75 increases peripheral energy utilization and fatty acid oxidation in diet-induced obesity. Proc Natl Acad Sci USA. 2002; 99: 9498-9502.
- Orita H, Coulter J, Lemmon C, Tully E, Vadlamudi A, Medghalchi SM, et al. Selective inhibition of fatty acid synthase for lung cancer treatment. Clin Cancer Res. 2007; 13: 7139-7145.
- Lemmon CR, Woo JH, Tully E, Wilsbach K, Gabrielson E. Nuclear factor-kappaB (NF-kappaB) mediates a protective response in cancer cells treated with inhibitors of fatty acid synthase. J Biol Chem. 2011; 286: 31457-31465.
- Rouse J, Cohen P, Trigon S, Morange M, Alonso-Llamazares A, Zamanillo D, et al. A novel kinase cascade triggered by stress and heat shock that stimulates MAPKAP kinase-2 and phosphorylation of the small heat shock proteins. Cell. 1994; 78: 1027-1037.
- Han J, Lee JD, Bibbs L, Ulevitch RJ. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science. 1994; 265: 808-811.
- Lee JC, Laydon JT, McDonnell PC, Gallagher TF, Kumar S, Green D, et al. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 1994; 372: 739-746.
- Freshney NW, Rawlinson L, Guesdon F, Jones E, Cowley S, Hsuan J, et al. Interleukin-1 activates a novel protein kinase cascade that results in the phosphorylation of Hsp27. Cell. 1994; 78: 1039-1049.
- Raingeaud J, Gupta S, Rogers JS, Dickens M, Han J, Ulevitch RJ, et al. Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J Biol Chem. 1995; 270: 7420-7426.
- Ghatan S, Larner S, Kinoshita Y, Hetman M, Patel L, Xia Z, et al. p38 MAP kinase mediates bax translocation in nitric oxide-induced apoptosis in neurons. J Cell Biol. 2000; 150: 335-347.
- Liu HF, Hu HC, Chao JI. Oxaliplatin down-regulates survivin by p38 MAP kinase and proteasome in human colon cancer cells. Chem Biol Interact. 2010; 188: 535-545.
- Schwartz D, Rotter V. p53-dependent cell cycle control: response to genotoxic stress. Semin Cancer Biol. 1998; 8: 325-336.
- Taylor WR, Stark GR. Regulation of the G2/M transition by p53. Oncogene. 2001; 20: 1803-1815.
- Levine AJ. p53, the cellular gatekeeper for growth and division. Cell. 1997; 88: 323-331.
- Wang Y and Prives C. Increased and altered DNA binding of human p53 by S and G2/M but not G1 cyclin-dependent kinases. Nature. 1995; 376: 88-91.
- Wils J. Adjuvant treatment of colon cancer: past, present and future. J Chemother. 2007; 19: 115-122.
- Bennett BK, Park SB, Lin CS, Friedlander ML, Kiernan MC, Goldstein D. Impact of oxaliplatin-induced neuropathy: a patient perspective. Support Care Cancer. 2012; 20: 2959-2967.
- Zedan AH, Hansen TF, Svenningsen AF, Vilholm OJ. Oxaliplatin-Induced Neuropathy in Colorectal Cancer: Many Questions With Few Answers. Clin Colorectal Cancer. 2013.