Case Report
Austin J Surg. 2014;1(4): 1016.
Acute Postoperative Cardiorespiratory Arrest after En Bloc Thymectomy: a Multidisciplinary Diagnostic and Therapeutic Dilemma
El-Eshmawi A*, Castillo JC, Pawale A and Reddy RC
Department of Cardiothoracic Surgery, Mount Sinai School of Medicine, USA
*Corresponding author: El-Eshmawi A, Department of Cardiothoracic Surgery, The Mount Sinai Medical Center, 1190 5th Avenue, Box 1028, New York, NY 10029-6574, USA
Received: May 19, 2014; Accepted: July 09, 2014; Published: July 14, 2014
Introduction
Postoperative cardiorespiratory arrest may be related to several reasons including the extent of surgery, hemodynamic status of the patient, availability and feasibility of bedside monitoring, and preexisting comorbidities. Regarding the latter, peripheral deep venous thrombosis is a major cause of postoperative morbidity and mortality, particularly in the setting of acute pulmonary embolism (PE). Acute pulmonary embolism often presents abruptly with no prodromal symptomatology and can be potentially fatal. Since prompt embolectomy can be lifesaving, immediate suspicion and diagnosis becomes crucial. However, the consequent patient’s instability often makes rapid diagnosis very difficult due to the impossibility of proceeding with the appropriate imaging techniques. In this manuscript, we report the case of a patient with sudden cardiovascular collapse in whom a strong suspicion multidisciplinary approach leading to an expeditious clinical diagnosis of acute PE. Subsequently, emergent assessment of the pulmonary artery and surgical embolectomy with concomitant inferior vena cava filter (IVC) insertion were successfully performed. In addition, this case illustrates and emphasizes the need for a thoughtful decision making process when confirmatory imaging tests are not feasible.
Case Presentation
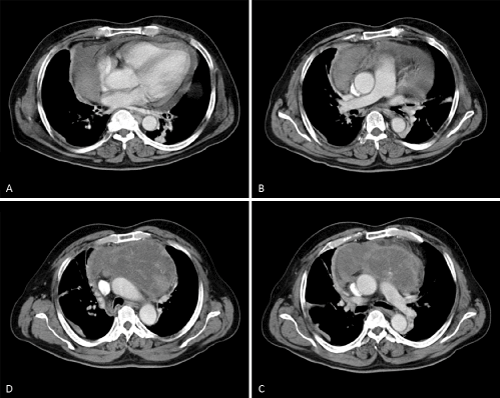
A 55-year-old man with no known past medical history presented to the emergency room complaining of acute chest pain, shortness of breath, intermittent productive cough and hemoptysis, as well as hypertension. Chest X-Ray showed no abnormalities other than a widened mediastinum. Acute aortic dissection was suspected and a computed tomography angiogram (CTA) of the chest was obtained. The CTA showed the presence of a large anterior mediastinal mass (15 x 8.5 x 9.3 cm) with mass effect on the great vessels and compression of the innominate vein and associated moderate pericardial effusion (Figure1). Analysis with radiocontrast agent did not show any vascular filling defect along the great vessels. Left and right ventricular size and function were normal. A computed tomography guided biopsy was then performed and the diagnosis of thymic carcinoid tumor was made. Preoperative workup excluded metastatic disease.
The patient was taken to the operating room electively and an en bloc tumor resection was performed through a median sternotomy. The patient was extubated on postoperative day one. However, two hours later, the patient developed severe respiratory distress, oxygen desaturation, and ultimately acute cardiogenic shock. After a short period of cardiopulmonary resuscitation, the patient was intubated and full monitoring was reestablished. Central venous pressure was noted to be fairly high and very minimal chest tubes drainage was observed. Transesophageal echocardiogram revealed severe right ventricular dysfunction without signs of pericardial tamponade or aortic dissection. Based on these findings, a potential acute pulmonary embolism was already suspected. The patient continued to be hemodynamically labile with increasing pressors requirements despite the initiation of intravenous heparin. Hemodynamic instability precluded further imaging studies.
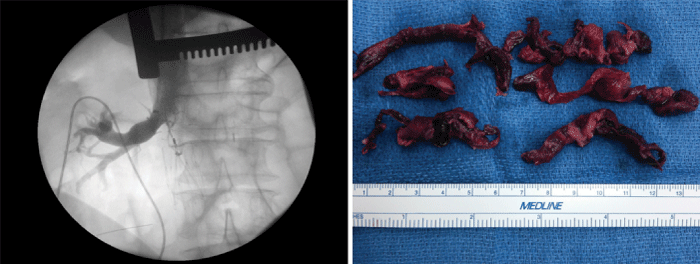
A multidisciplinary approach with intensive care, hematology, cardiology, Pulmonology as well as cardiothoracic surgery was convened. After quorum, the patient was emergently taken to the operating room. The median sternotomy incision was re-explored. The patient was systemically heparinized, cannulated and placed on cardiopulmonary bypass (CPB) and a pulmonary arteriotomy was performed under moderate systemic hypothermia. Complete removal of all visible thrombi was performed at a temperature of 30°C (Figure 2, right). In addition, the right atrium and ventricle were explored during rewarming and no additional clots were found. An inferior vena cava filter was implanted via the right atrium under further fluoroscopic guidance (Figure 2, left). An intra-aortic balloon pump was placed for post bypass biventricular dysfunction and the patient was smoothly weaned off CPB. The intra-aortic balloon pump was removed on postoperative day one and the patient was systemically heparinized as soon as the chest tube drainage was acceptable. Pre discharge echocardiography demonstrated good biventricular function. The patient remains currently asymptomatic and no new episodes of thrombosis have been reported.
Figure 1 :preoperative computed tomographic angiography showing a large anterior mediastinal mass. The patency of the central pulmonary vasculature can be easily appreciated.
Figure 2 :Intraoperative angiogram of the inferior vena cava. Note the position of the IVC filter below the right renal vein (left). Intraoperative image of pulmonary emboli after surgical removal (right).
Discussion
Acute pulmonary embolism occurs in about 5% of patients undergoing thoracostomy [1]. Historically, large autopsy series have found PE to be a major cause of postoperative mortality [2]. In this context, early diagnosis and aggressive management play a key role in patients’ survival. Available bedside tests include biochemical screening with a D-dimer assay, echocardiography and Duplex scan of the lower extremities. V-Q scanning, PA angiography and CTA are very sensitive confirmatory tests that require patient manipulation and transportation. A D-dimer assay is of little perioperative value, especially in clinical scenarios with intraoperative hemorrhage. Duplex scanning is able to detect the venous thrombosis as a source of PE but does not help with the diagnosis of PE. Echocardiography may demonstrate right heart dysfunction (indirect evidence) but can only visualize clots in the proximal PA.
Postoperative V-Q scan in patients who underwent cardiothoracic surgery has been progressively replaced by CTA in our clinical practice. Nevertheless, both tests require patient transportation hence are contraindicated in the hemodynamically unstable patient. At his point, a multidisciplinary approach with different clinical perspectives might be the only reasonable course of action available since no other options are feasible. When medical management is attempted, thrombolytic therapy is considered the only available tool in the armamentarium. Although anticoagulation is routinely contraindicated in the early postoperative period [3], recent publications have reported successful outcomes [4,5]. From our point of view, in such clinical scenarios, anticoagulation should be individualized. In our particular case, considerations to take into account included the vast nature of the tumor and vascularization and the early occurrence of hemodynamic instability (within 18 hours of surgery), all of which would argue against any decision in favor of thrombolysis.
In patients whom anticoagulation is contraindicated or in those patients in frank cardiogenic shock, interventional or surgical embolectomy is considered as last option [6]. Interventional therapies offer an immediate and minimally invasive way of relieving some of the hemodynamic burden of PE without exposure to thrombolysis or CPB. However, their application requires special expertise and also involves patient transportation to the interventional radiology suit. The most recent recommendations, derived from the ACCP guidelines on antithrombotic and fibrinolytic therapy, recommend surgical embolectomy for patients with high burden of PE who cannot receive fibrinolytic therapy due to bleeding risk or whose critical status precludes sufficient time for fibrinolysis to become effective (Grade 2C) [7]. This guideline was applicable to our case and directed our decision making.
Surgical pulmonary embolectomy can be done without CPB. However, this is limited to saddle emboli that can be extracted blindly through an incision in the main pulmonary artery. Cardiopulmonary bypass allows for careful visualization of primary and secondary arteries, permits a complete embolus extraction, and accelerates the correction of metabolic derangements frequently seen in the post arrest scenarios. Furthermore, CPB allows decompression and reperfusion of the dysfunctional right ventricle while supporting the systemic circulation and minimizing end-organ damage. Systemic hypothermia may also be protective for the brain and other end-organs. It is indeed our institutional policy to always perform pulmonary embolectomy with CPB and mild systemic hypothermia.
In patients who are receiving cardiopulmonary resuscitation or highly unstable in the non-operating room set up or in hospitals without CPB facilities, peripheral venoarterial extracorporeal membrane oxygenator (ECMO) could be very useful till the patient is stabilized and transported to the operating room for surgical pulmonary embolectomy as described above.
References
- Ziomek S, Read RC, Tobler HG, Harrell JE Jr, Gocio JC, Fink LM, et al. Thromboembolism in patients undergoing thoracotomy. Ann Thorac Surg. 1993; 56: 223-226.
- Kalweit G, Huwer H, Volkmer I, Petzold T, Gams E. Pulmonary embolism: a frequent cause of acute fatality after lung resection. Eur J Cardiothorac Surg. 1996; 10: 242-246.
- Jaff MR, McMurtry MS, Archer SL, Cushman M, Goldenberg N, Goldhaber SZ, et al . Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation. 2011; 123: 1788-1830.
- Sakuragi T, Sakao Y, Furukawa K, Rikitake K, Ohtsubo S, Okazaki Y, et al. Successful management of acute pulmonary embolism after surgery for lung cancer. Eur J Cardiothorac Surg. 2003; 24: 580-587.
- Sano Y. [Prophylaxis and management of postoperative pulmonary embolism in lung surgery]. Kyobu Geka. 2008; 61: 721-725.
- Lee L, Kavinsky CJ, Spies C. Massive pulmonary embolism: review of management strategies with a focus on catheter-based techniques. Expert Rev Cardiovasc Ther. 2010; 8: 863-873.
- Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ; American College of Chest Physicians. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008; 133: 454S-545S.