Case Report
Austin J Surg. 2014;1(5): 1025.
Denver Peritoneovenous Shunt Performed Under Local Anaesthesia for Intractable Ascites Following Esophagogastrectomy
Yoshito Nako1, Atsushi Shiozaki1*, Hitoshi Fujiwara1, Hirotaka Konishi1, Ryo Morimura1, Yasutoshi Murayama1, Shuhei Komatsu1, Hisashi Ikoma1, Yoshiaki Kuriu1, Takeshi Kubota1, Masayoshi Nakanishi1, Daisuke Ichikawa1, Kazuma Okamoto1, Toshiya Ochiai2, Chouhei Sakakura1 and Eigo Otsuji1
1Department of Surgery, Division of Digestive Surgery, Kyoto Prefectural University of Medicine, Japan
2Department of Surgery, Division of Digestive Surgery, North Medical Center, Kyoto Prefectural University of Medicine, Japan
*Corresponding author: Atsushi Shiozaki, Department of Surgery, Division of Digestive Surgery, Kyoto Prefectural University of Medicine, 465 Kajii-cho, Kamigyo-ku, Kyoto 602-8566, Japan
Received: May 20, 2014; Accepted: August 18, 2014; Published: August 21, 2014
Abstract
We herein described a 70-year-old male patient with lower thoracic esophageal cancer (T1aN2M0, Stage II) and gastric cancer in the upper body (T1bN0M0, Stage IA), who underwent subtotal esophagectomy and proximal gastrectomy with reconstruction using colon interposition in January, 2011. He exhibited abdominal distension in April, 2011. Ultrasonography revealed massive ascites, the cytology of which was class II, and mediastinal lymph node recurrence was detected using PET/CT. Although the patient received DCF chemotherapy (2 courses of 5-FU 600 mg/m2 days 1-5, docetaxel 60 mg/ m2 day1, and cisplatin 50 mg/m2 day 4) and achieved a complete response in the mediastinal lymph nodes, no improvements were noted in massive ascites. Lymphorrhea was not detected by lymphangiography. Although a definite diagnosis had not been obtained, a Denver peritoneovenous shunt (DPVS) was placed in August, 2011. A marked amelioration in ascites was observed following surgery, and the patient was subsequently discharged without complications. DPVS is a simple procedure that can be successfully applied to the treatment of intractable ascites.
Keywords: Denver peritoneovenous shunt; Intractable ascites; Esophagectomy
Introduction
Ascites following surgery for digestive cancer is frequently attributed to postoperative lymphorrhea, peritoneal metastasis, cirrhosis, and under nutrition. However, difficulties are associated with the treatment and management of ascites after surgery because it may be intractable and the cause may be unclear. Conventional medical treatments, such as the restriction of dietary sodium and administration of diuretics, are typically used as the first line of therapy for ascites. Repeated Paracentesis is also frequently performed to ameliorate abdominal distention because of its simplicity, but has been linked to complications such as cardiac failure by massive fluids shift, infection, intestinal damage, and, most importantly, the loss of nutrients, which eventually result in cachexia. Concentrated ascites reinfusion therapy (CART) or a peritoneovenous shunt (PVS) may be chosen for cases that have not shown improvements by either medication or repeated paracentesis [1].
We here in described a case of intractable ascites after surgery for esophageal and gastric cancer that was successfully treated by the placement of a Denver peritoneovenous shunt (DPVS).
Case Presentation
The patient was a 70-year-old male with lower thoracic esophageal cancer (T1aN2M0, Stage II) [2,3] and gastric cancer in the upper body (T1bN0M0, Stage IA) [4,5], who underwent subtotal esophagectomy and proximal gastrectomy with reconstruction using colon interposition in January, 2011. His postoperative recovery was uneventful, and he was discharged 48 days after surgery. However, he exhibited abdominal distension in April, 2011, and was admitted to the hospital for examinations and treatments.
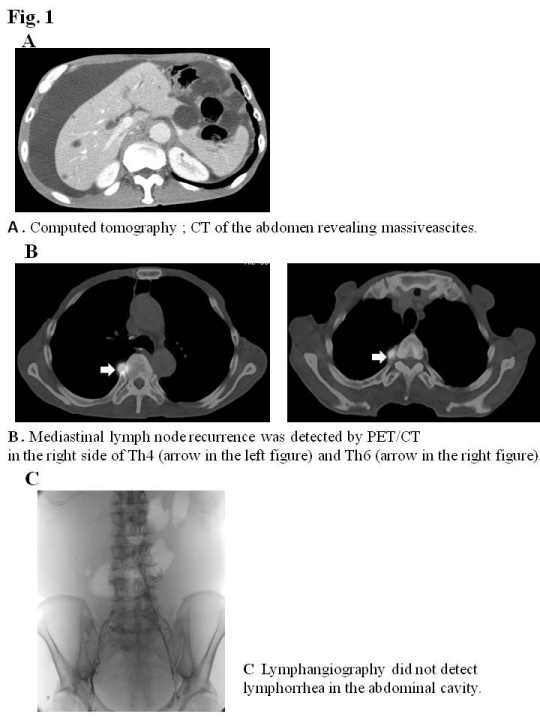
He had an operation scar on his upper abdomen, and marked abdominal distension. A laboratory examination on admission revealed that total protein and albumin were slightly decreased at 5.4 g/dl and3.5 g/dl, respectively. Carcinoembryonic antigen; CEA, squamous cell carcinoma antigen; SCC-Ag, and cytokeratin 19 fragment; CYFRA, which are tumor markers were all within normal limits. A serological study for hepatitis B and C was negative. A cytological examination showed that ascites was class II. Computed tomography; CT of the abdomen revealed massive ascites (Figure 1A). No recurrence was detected in the remnant stomach or ileocolic reconstruction by gastrointestinal endoscopy. Loop diuretics and paracentesis were administered and improved ascites, therefore, the patient was discharged. Two weeks later, he presented with abdominal distention. PET/CT revealed massive ascites and mediastinal lymph node recurrence on the right side of Th4 (SUV4.7) and Th6 (SUV9.2) (Figure 1B). The patient received two courses of DCF chemotherapy (5-FU 600mg/m2: days 1-5, docetaxel 60mg/m2: day1, and cisplatin 50mg/m2: day 4) to treat mediastinal lymph node recurrence, and resulted in a complete response. However, no improvements were noted in massive ascites. Therefore, the patient was re-examined to establish the cause of massive ascites.
Figure 1:
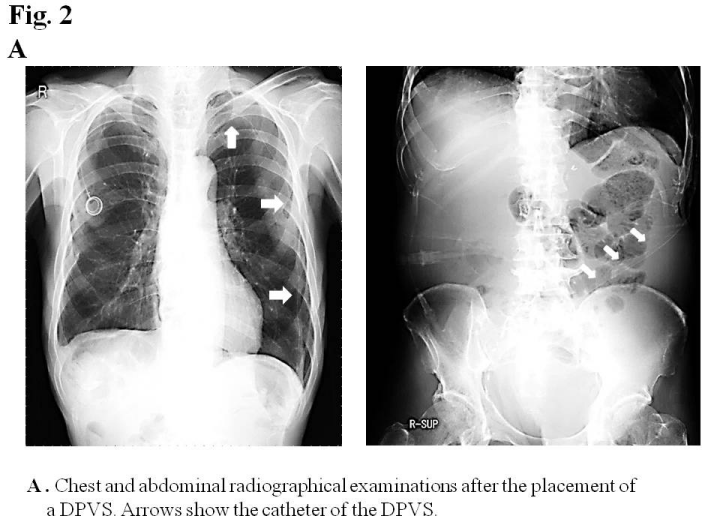
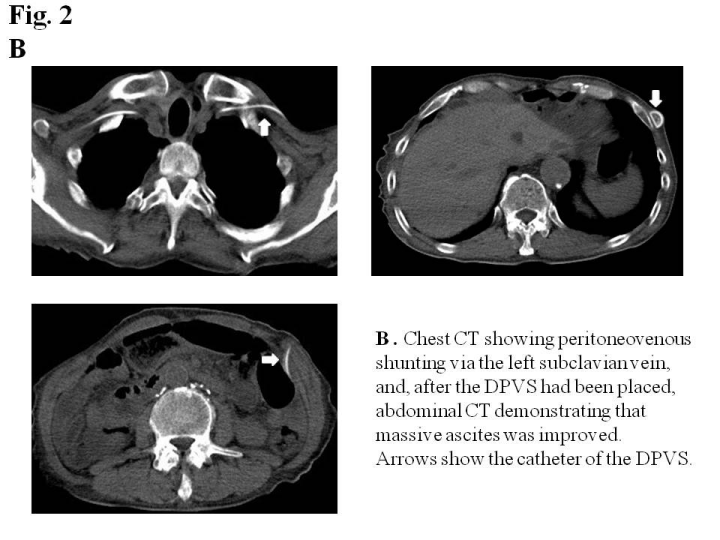
An examination of ascites revealed that clear appearance, low specific gravity, low protein concentration, and low LDH ratio indicated transudate ascites (e.g. due to cirrhosis), whereas the Rivalta reaction was positive, and the cell ingredient was neutrophil indicated exudative ascites (e.g. due to inflammation or malignancy). A cytological examination revealed that ascites was negative for malignant cells (class II). Ultrasonography and abdominal contrast CT demonstrated massive ascites, not liver cirrhosis, liver metastasis, or peritoneal metastasis. Lymphangiography followed by abdominal CT did not detect lymph leak caused by surgical injury of lymphatic vessel in the abdominal cavity (Figure 1C). Although the diagnosis of intractable ascites in the re-examination was not definitive, we placed a DPVS in August, 2011to relieve abdominal distention and improve his quality of life. Peritoneovenous shunting was performed under local anesthesia. At first, the left subclavian vein was approached for venous access. Next, the pump chamber pocket was created at the lower left rib cage to facilitate manual compression of the pump. The venous tube was passed subcutaneously, and the pump camber was placed in the pocket. Finally, the peritoneal catheter was inserted, and the abdominal tube was passed subcutaneously to the pump camber (Figure 2A). The all procedures were performed under local anesthesia, and the operative time was within 1 hour. A marked improvement in ascites was observed after surgery. The patient was discharged 23 days after surgery without any complications. Although he died due to the mediastinal and intra abdominal lymph node recurrence of esophageal cancer in July, 2012, ascites did not recur (Figure 2B) and his quality of life had been improved by DPVS.
Figure 2a:
Figure 2b :
Discussion
Intractable ascites is defined as ascites that does not recede after medical therapy or reoccurs shortly after fluid has been removed from the abdominal cavity via paracentesis [6]. Because of its resistance to treatment, intractable ascites has been shown to adversely affect the quality of life of patients due to abdominal distension, abdominal pain, and loss of appetite [7]. Zervos et al. reported that the most common underlying causes for intractable ascites were malignancy and cirrhosis, while refractory ascites could be attributed to various causes [1]. Although previous studies demonstrated that lymphorrhea was the cause of postoperative ascites [8,9], this was not the case in our patient. The treatment options for intractable ascites include large volume paracentesis with the intravenous supplementation of albumin, CART, PVS, a transjugular intrahepatic portosystemic shunt, living donor liver transplantation for liver failure [10], and continuous ambulatory peritoneal dialysis for renal failure [11]. However, these interventions carry some risks and undesirable side effects.
The placement of a PVS is a relatively simple procedure that can be performed with little or no trauma or blood loss [1,12]. APVS has been used to effectively treat some intractable ascites diseases including malignant ascites [13,14], cirrhotic ascites [15], and chylous ascites [16]. The systemic expansion of malignant cells after the placement of a PVS represents a major concern in patients with malignant ascites. It currently remains unclear whether the placement of a PVS affects patient survival because each patient has presented with a different background for the initial disease and clinical stage. However, the efficacy of a PVS for the treatment of malignant ascites has been reported because quality of life after surgery was significantly improved [7]. A PVS was first applied for ascites in 1962 and has become increasingly used with improvements and modifications to the device. The success of the Leveen peritoneovenous shunt; LPVS was introduced in 1974, and DPVS in 1979. Although the efficacy of the LPVS in the treatment of intractable ascites has been reported, the DPVS now more frequently used because of the simple surgical technique performed to place it and the ease at which is can be managed [17,18]. Complications associated with the PVS included infections, congestive heart failure, shunt occlusion, and disseminated intravascular coagulation; DIC. DIC was reported immediately after shunt placement in approximately 35% of patients. This complication was attributed to the rapid introduction of fibrin-rich procoagulants into the central venous circulation after shunt placement. Previous studies indicated that this complication could be prevented by withdrawing and disposing of the majority of ascites prior to placement of the shunt [1,19,20]. The postoperative course of our patient was good and no complications were noted. The DPVS is a relatively simple procedure that can be performed under local anesthesia with a short operative time, and is very effective for the treatment of intractable ascites; however, adequate care is essential for preventing complications. Although radical therapies for ascites are indispensable, difficulties have been associated with treating the primary disease in many cases. Therefore, symptomatic treatments that improve the quality of life of patients are important. ADPVS was placed as a symptomatic therapy in the present case and was very effective, even though the diagnosis of intractable ascites was not definitive.
In conclusion, the placement of a DPVS is a simple procedure that can be successfully applied for the treatment of intractable ascites.
References
- Zervos EE, Rosemurgy AS. Management of medically refractory ascites. Am J Surg. 2001; 181: 256-264.
- Japan Esophageal Society. Japanese Classification of Esophageal Cancer, tenth edition: part I. Esophagus. 2009; 6: 1–25.
- Japan Esophageal Society. Japanese Classification of Esophageal Cancer, tenth edition: part II and III. Esophagus. 2009; 6: 71–94.
- Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011; 14: 101-112.
- Sano T, Aiko T. New Japanese classifications and treatment guidelines for gastric cancer: revision concepts and major revised points. Gastric Cancer. 2011; 14: 97-100.
- Artemiou O, Marta GM, Klepetko W, Wolner E, Müller MR. Pleurovenous shunting in the treatment of nonmalignant pleural effusion. Ann Thorac Surg. 2003; 76: 231-233.
- Tomiyama K, Takahashi M, Fujii T, Kunisue H, Kanaya Y, Maruyama S, et al. Improved quality of life for malignant ascites patients by Denver peritoneovenous shunts. Anticancer Res. 2006; 26: 2393-2395.
- Yamada T, Jin Y, Hasuo K, Maezawa Y, Kumazu Y, Rino Y, et al. Chylorrhea following laparoscopy assisted distal gastrectomy with D1+ dissection for early gastric cancer: A case report. Int J Surg Case Rep. 2013; 4: 1173-1175.
- Mine S, Udagawa H, Kinoshita Y, Makuuchi R. Post-esophagectomy chylous leakage from a duplicated left-sided thoracic duct ligated successfully with left-sided video-assisted thoracoscopic surgery. Interact Cardiovasc Thorac Surg. 2008; 7: 1186–1188.
- Yamashiki N, Sugawara Y, Tamura S, Nakayama N, Oketani M, Umeshita K, et al. Outcomes after living donor liver transplantation for acute liver failure in Japan: results of a nationwide survey. Liver Transpl. 2012; 18: 1069-1077.
- Nobe K, Inoue T, Nodaira Y, Kimura Y, Okayama M, Gen S. Continuous ambulatory Peritoneal dialysis beyond a decade: cases from a single center. Adv Perit Dial. 2012; 28: 74-78.
- Moore KP, Wong F, Gines P, Bernardi M, Ochs A, Salerno F, et al. The management of ascites in cirrhosis: report on the consensus conference of the International Ascites Club. Hepatology. 2003; 38: 258-266.
- Tueche SG, Pector JC. Peritoneovenous shunt in malignant ascites. The Bordet Institute experience from 1975-1998. Hepatogastroenterology. 2000; 47: 1322-1324.
- Smith DA, Weaver DW, Bouwman DL. Peritoneovenous shunt (PVS) for malignant ascites. An analysis of outcome. Am Surg. 1989; 55: 445-449.
- Söderlund C. Denver peritoneovenous shunting for malignant or cirrhotic ascites. A prospective consecutive series. Scand J Gastroenterol. 1986; 21: 1161-1172.
- Gupta D, Ross K, Piacentino V 3rd, Stepnowski D, McClurken JB, Furukawa S, et al. Use of LeVeen pleuroperitoneal shunt for refractory high-volume chylothorax. Ann Thorac Surg. 2004; 78: e9-12.
- Akimaru K, Ueda Y, Shoji T. Peritoneovenous shunting for intractable cirrhotic and cancerous ascites using different types of shunting tubes. Jpn J Surg. 1988; 18: 502-508.
- Fulenwider JT, Galambos JD, Smith RB 3rd, Henderson JM, Warren WD. LeVeen vs Denver peritoneovenous shunts for intractable ascites of cirrhosis. A randomized, prospective trial. Arch Surg. 1986; 121: 351-355.
- Perera E, Bhatt S, Dogra VS. Complications of denver shunt. J Clin Imaging Sci. 2011; 1: 6.
- Lund RH, Moritz MW. Complications of Denver peritoneovenous shunting. Arch Surg. 1982; 117: 924-928.