Review Article
Austin J Surg. 2014;1(6): 1028.
Radiotherapy as Primary Treatment of Olfactory Groove Meningiomas is Associated with Preserved Cranial Nerve Function and Excellent Quality of Life
Zaorsky NG1, Andrews DW2, Podrat J1, Gunn V2, Liu H2, Werner-Wasik M1, Farrell C2, Evans JJ2, Moshel YA2, Judy KD2 and Shi W1*
1Department of Radiation Oncology, Thomas Jefferson University, USA
2Department of Neurological Surgery, Thomas Jefferson University, USA
*Corresponding author: Wenyin Shi, Department of Radiation Oncology, Jefferson Medical College & Kimmel Cancer Center, Thomas Jefferson University, 111 S. 11th Street, Bodine Center for Cancer Treatment, Philadelphia, PA 19107, USA
Received: July 28, 2014; Accepted: August 30, 2014; Published: September 04, 2014
Abstract
Objective: Data on radiotherapy (RT), including fractionated stereotactic radiotherapy (FSRT) and stereotactic radiosurgery (SRS), for olfactory groove meningiomas (OGMs) is limited, in part because of the rarity of the disease in this location. We present the first report of efficacy, toxicity, CN function and quality of life (QOL) in patients treated with primary RT for OGMs.
Methods: We retrospectively identified seven patients who were treated with primary FSRT or SRS. Patients were followed clinically, with magnetic resonance imaging (MRI), and were sent Sino-Nasal Outcome Tests (SNOT- 20s).
Results: At a median follow-up time of 64 months (range, 21 to 125), rates of local control, overall survival, and cause specific survival were 100%, 86%, and 100%, respectively. At presentation, four patients had hypo/anosmia; two, symptomatic deficit of visual acuity or fields; two, facial pain; two, tinnitus. After RT, three patients regained olfaction; two had improvement in visual function; two, decreased facial pain; two, resolved tinnitus. Three patients had a decrease and four had no change in lesion size on radiographic imaging. Headaches resolved in the few patients who presented with them. SNOT-20scores correlated with an excellent QOL pre-RT and on follow-up.
Conclusion: Patients with OGMs treated with primary RT maintained excellent rates of local control and cause-specific survival, had preserved or improved CN function (notably, olfactory nerve function improved in three patients), and maintained excellent QOL. Further prospective studies are necessary to determine the role of RT in the multimodal care of OGMs.
Keywords: Cranial nerves; Olfactory groove meningioma; Radiotherapy; Quality of life
Introduction
Olfactory groove meningiomas (OGMs) are rare, slow-growing tumors that account for 10% of intracranial meningiomas. Treatment options for OGMs include observation, surgery, and radiotherapy (RT), which includes both stereotactic radiosurgery (SRS) and fractionated stereotactic radiation therapy (FSRT).Surgery has historically been recommended as the primary therapy for OGMs because of excellent control rates and perioperative mortality of nearly 0% [1].
However, surgical resection of an OGM has limitations. First, total resection is limited if the tumor encroaches on critical neurovascular areas or extends laterally much beyond the vertical plane of the medial orbital wall, which challenges endoscopic approaches [1]. Moreover, all patients develop deficits of cranial nerve (CN) I. Other postoperative complications may include cerebrospinal fluid leaks, post-treatment seizures, frontal lobe edema, and other CN dysfunctions [2,3]. Next, studies suggest that OGM recurrence may be as high as 23% at 7 years [2,4]. Finally, patient comorbidities may preclude intubation and increase the risk of postoperative morbidity and mortality.
Currently, RT is reserved for select meningioma locations (e.g. the optic nerve sheath [ONSMs], cavernous sinus, base of skull, acoustic meatus) that are associated with a high surgical morbidity; for tumors that are unresectable; or for patients unable to tolerate surgery [1]. Recent studies have shown that FSRT [5] and SRS [6] have outcomes similar to surgery with limited morbidity. Moreover, RT may also maintain or improve CN function in ONSMs [7,8] and acoustic meningiomas [9]. To our knowledge, there is no published data on the efficacy of RT for OGMs for tumor control and CN function. We hypothesized that in select patients with OGMs, primary RT (either SRS or FSRT) resulted in tumor control, CN function preservation, and maintenance of excellent patient QOL.
Methods
After obtaining institutional review board approval, we identified seven consecutive patients with symptomatic and OG Msat XXX between 1994 and 2011who were treated with primary RT. Medical records for these patients were reviewed, including in-patient hospital notes, MRI scans, RT treatment records, and radiation oncology and neurosurgery follow-up encounters. The initial diagnosis was rendered either during surgery or on the basis of the characteristic appearance on a T1-weigthed, contrast-enhanced fat suppression MRI study.
Radiotherapy
Our institutional policy dictates that all patients be evaluated and discussed by a multidisciplinary tumor board, consisting of neurosurgeons, radiation oncologists, and neuroradiologists. Treatment decisions for SRS versus FSRT are based on a thorough discussion evaluating tumor size, symptoms, nerve function, performance status, and patient preference. Patients with intact CN function and larger lesions are generally offered FSRT. However, patient preference of SRS due to the convenience of single treatment may override this recommendation.
Patients treated before June 2004 with FSRT received RT via a [Varian Clinac-600SR linear accelerator] (LINAC, Varian 600SR; Varian Corp., Palo Alto, CA) and were immobilized with Brown- Roberts-Wells and Gill-Thomas-Cosman relocatable frames, using the Radionics planning system. Patients treated after 2004 with FSRT received RT via a Varian/Brain LAB Novalis stereotactic system, including Brain LAB masks for immobilization, ExacTRAC image guided localization, and Brain SCAN treatment planning system. The stereotactic radiation technique involved a conventional fraction paradigm to maximize the chance of CN preservation and minimize the risk of radiation-induced optic neuropathy and/or optic neuritis. A high conformality was established by non-coplanar arcs or beams and differential beam weighting. Patients treated with SRS before May 2006 received treatment via Gamma Knife Model U (Elekta Instruments, Atlanta, GA) and after with Gamma Knife model 4C.
Toxicity evaluation and follow-up
Patients were initially seen in follow-up three months after treatment by the radiation oncology and neurosurgery teams. Patients were then followed yearly with detailed clinical exams and MRIs for a scheduled period of 10 years. Follow-up was measured from the beginning of RT until the last documented encounter by either team. Tumor control was defined as stable or decreased tumor size on MRI. Toxicity was defined as an event starting within 3 months of SRS or within 3 months from the beginning of FSRT.
Detailed CN examinations were performed prior to RT and on follow-up examinations. Olfaction was based on patients’ subjective report; ophthalmological exams were performed in symptomatic patients. Additionally, SNOT-20s [10] were sent to patients around the time of follow-up. The twenty questions (each graded 0 to 5) of the original SNOT-20 were averaged to create the following symptom subscales: (1) rhinological, including needing to blow one’s nose, sneezing, runny nose, cough, post-nasal discharge, and anosmia; (2) ear/facial, including ear fullness, dizziness, ear pain, and facial pain; (3) sleep, including difficulty sleeping and waking at night; and (4) psychological, including fatigue, reduced productivity, reduced concentration, frustration/restlessness/irritability, sadness, and embarrassment [11].
Results
Patient and treatment characteristics
Patient and tumor characteristics are listed in Table 1. There were four women and three men with a median age of 57 years (range, 50 to 73). Patients were treated because of symptoms, which were: hypo/ anosmia (4 patients), headaches (3 patients), and visual symptoms (3 patients) were common. The median tumor size was 3.3 cm (range, 1.7 to 4.5).
Pt.
Age at RT (y)
Sex
Presenting symptoms
Diameter on initial image (cm)
Laterality
Initial surgery plan
Surgical complications
Reason for RT
Time from surgery to RT (m)
1
57
f
anosmia
3.5
midline
No
N/A
symptoms
2
60
m
hyposmia
3.7
midline
No
N/A
symptoms
3
64
m
decreased visual acuity; mild fatigue
2.3
left
STR
none
symptomatic (visual changes) from RD
30
4
51
f
sensed flashing lights; decreased visual acuity, fields; L facial pain; HA; fatigue
3.2
midline
No
N/A
symptoms
5
47
f
fatigue, malaise; but likely due to comorbidities
1.3
left
STR
CSF leak; sunken skull
symptomatic from RD
123
6
69
m
anosmia; generalized seizure; syncope; change in taste (subjective); fatigue
1
left
STR
decreased sense of taste; L eye blind spots; 20/40 B vision
symptomatic from RD
52
7
56
f
decreased visual acuity, depth perception, fields; hyposmia; L orbital pain; mild HA; fatigue; memory loss
1.7
left
No
N/A
symptoms
8
37
f
tinnitus; mild HA
1.5
midline
No
N/A
symptoms
9
68
f
hyposmia; seizure
5.5
midline
GTR
bilateral MCA strokes, likely thromboembolic
RDnoted on post-op MRI
10
10
73
f
absent vision in L eye; slowly developing R hemianopsia
4.5
midline
GTR
lost vision on R post-op, but resolved
RD on post-op MRI
4
11
61
f
anosmia; mental status changes; personality changes; memory loss; apathy
5.5
midline
GTR
LR in 1 year on MRI, then memory loss
32
12
46
f
anosmia; decreased taste sensation (subjective); HA
2.2
midline
GTR
none
LR 2 years after on MRI, then HAs
56
13
72
m
asymptomatic
4.5
midline
No
N/A
OGM size
14
65
f
tinnitus; migraines; anosmia
3.3
right
No
N/A
mean
59
3.1
43
med
61
3.2
32
Table 1: Patient and tumor characteristics.
Dose and treatment characteristics are listed in Table 2. The median tumor volume was 3.56mL (range, 1.13 to 7.10). Six patients were treated with FSRT to a median total prescribed dose of 54.0 Gy (range, 50.0 to 56.0) in 1.8 to 2.0 Gy fractions; and two patients were treated with SRS to dose of 16 Gy in one fraction. The median ratio of maximum dose to prescribed dose (MD: PD) for FSRT patients was 1.13. The median maximum dose to the chiasm for FSRT patients was 28 Gy; the SRS patients received 1.9 and 2.0 Gy.
Pt.
Clinical tumor volume (mL)
RT type
Dose (Gy)
Dose/
frax (Gy)
# frax
Prescribed to % isodose line
MD/PD ratio
# of isocenters
Max dose to tumor
Mean dose tumor
Max to R optic nerve
Max to L optic nerve
Max to optic chiasm
1
2.27
FSRT on LINAC
54.0
1.8
30
90%
1.21
1
65.1
59.4
48.0
51.0
28.5
2
4.69
FSRT on LINAC
50.0
2.0
25
88%
1.13
2
63.5
56.0
49.2
43.7
50.5
3
3.57
SRS on LINAC
16.0
16.0
1
89%
1.12
1
19.1
17.6
3.8
10.0
3.9
4
7.10
FSRT on LINAC
54.0
1.8
30
94%
1.11
1
59.7
57.3
29.1
23.4
10.8
5
1.30
SRS on GK
15.0
15.0
1
89%
1.15
1
17.2
15.5
1.9
2.7
1.3
6
1.50
FSRT on LINAC
50.4
1.8
28
89%
1.14
1
57.7
56.0
46.5
56.6
54.9
7
3.40
SRS on GK
16.0
16.0
1
50%
2.00
1
32.0
30.0
4.5
7.2
2.0
8
6.20
SRS on GK
16.0
16.0
1
50%
2.37
10
38.0
23.0
5.3
5.2
1.9
9
2.60
SRS on GK
18.0
18.0
1
50%
2.00
3
36.0
23.8
3.9
6.3
2.1
10
1.90
FSRT on LINAC
52.2
1.8
29
70%
1.09
5
56.8
44.0
41.4
42.6
46.9
11
1.70
SRS on GK
15.0
15.0
1
55%
1.70
2
25.5
18.6
2.3
1.9
1.5
12
1.50
SRS on GK
17.0
17.0
1
50%
2.04
9
34.7
21.4
1.5
1.9
0.9
13
2.60
FSRT on LINAC
56.0
2.0
28
85%
1.18
3
65.9
63.4
5.0
36.8
1.0
14
1.13
FSRT on LINAC
54.0
1.8
30
92%
1.04
1
56.0
55.0
52.0
47.0
54.5
FSRT median
54.0
1.8
29
89%
1.13
1
58.7
56.7
46.5
43.8
46.9
SRS median
16.0
16.0
1
50%
2.00
2
32.0
21.4
3.8
5.2
1.9
Table 2: Dose and treatment characteristics.
Efficacy, toxicity, quality of life
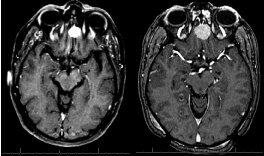
Treatment efficacy is shown in Table 3. After a median follow-up time of 64 months (range, 21 to 125), local control (LC) was 100%. Patient #6 died secondary to comorbidities (overall survival [OS], 86%); none has died secondary to his/her OGM (cause specific survival [CSS], 100%). Three patients had a decrease and four had no change in lesion size on radiographic imaging. Figure 1 shows a sample response on CT from patient #4.
Title: MRI of an OGM before and after treatment.
Legend: Pre- and post-RT MRI scan of patient #4 demonstrating stable size of the OGM after RT. Notably, most patients do not have a change in the OGM size after treatment.
Figure 1:Title: MRI of an OGM before and after treatment.
Legend: Pre- and post-RT MRI scan of patient #4 demonstrating stable size of the OGM after RT. Notably, most patients do not have a change in the OGM size after treatment.
Pt.
Clinical tumor volume (mL)
Follow-up (m)
Size on MRI
Changes in presenting symptoms
RTOG toxicity / grade
Time from RT until death (m)
1
2.27
37
decreased
improved olfaction
none
N/A
2
4.69
64
unchanged
unchanged
erythema
1
N/A
3
3.57
48
unchanged
unchanged
none
60
4
7.10
71
unchanged
improved HAs, fatigue; worsened visual acuity, unclear if it is secondary to RT injury vs. RD; improved orbitofacial pain
alopecia
1
N/A
5
1.30
69
unchanged
unchanged, but likely due to comorbidities
none
N/A
6
1.50
88
unchanged
improved vision, fatigue; seizure resolution; unchanged anosmia; worsened rhinological symptoms
none
N/A
7
3.40
102
decreased
improved visual symptoms, rhinological symptoms, olfaction, facial pain, sleep disturbances, HAs, memory loss
peritumoral brain edema lasting for months, but likely due to comorbidities
2
N/A
8
6.20
125
unchanged
improved tinnitus, HAs
peritumoral brain edema, lasting for 3 weeks
3
N/A
9
2.60
N/A
N/A
resolution of seizures; died of comorbidities shortly after
none
7
10
1.90
99
unchanged
worsened visual acuity and fields, new HAs secondary to another metastatic cancer
none
106
11
1.70
53
unchanged
improved rhinological, ear/facial, sleep, psychological, visual symptoms; improved frontal lobe symptoms
none
N/A
12
1.50
184
unchanged
improved HAs; otherwise unchanged
none
N/A
13
2.60
31
decreased
unchanged
none
66
14
1.13
21
unchanged
improved HAs, anosmia, tinnitus
none
N/A
mean
3.02
55
60
median
2.27
49
Table 3: Efficacy of RT on tumor and patient outcome.
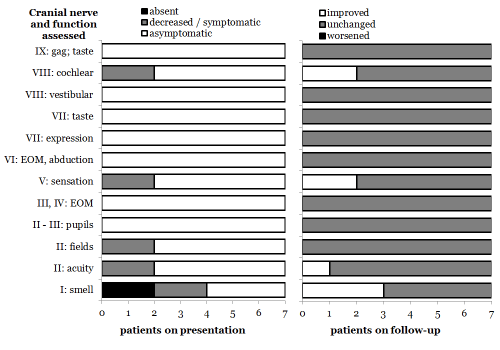
At presentation, four patients had hypo/anosmia; two had decrease in visual acuity or fields; two had facial pain; two had tinnitus. Three patients (#1, #4, and #7) had subjective improvement in their sense of smell. Two patients (#5 and #7) had an improvement in orbitofacial pain and tinnitus. No patients experienced worsening of other CN functions. Figure 2 compares symptoms associated with tumor mass effect on cranial nerves before RT to those at follow-up. For all patients, CNs X through XII was intact both before and after therapy. Headaches resolved in the three patients who presented with them. Patients #4 and #5 experienced acute peritumoral brain edema (RTOG grade 2 and 3), resolving after weeks. No patients had chronic toxicities.
Title: Cranial nerve function assessed at presentation and at follow-up for patients with OGMs treated with primary RT.
Legend: Detailed cranial nerve examinations were performed prior to RT and on follow-up examinations. Patients had a preservation of most cranial nerve functions after RT. No patients had deterioration of cranial nerve function; many had improved function.
Figure 2:Title: Cranial nerve function assessed at presentation and at follow-up for patients with OGMs treated with primary RT.
Legend: Detailed cranial nerve examinations were performed prior to RT and on follow-up examinations. Patients had a preservation of most cranial nerve functions after RT. No patients had deterioration of cranial nerve function; many had improved function.
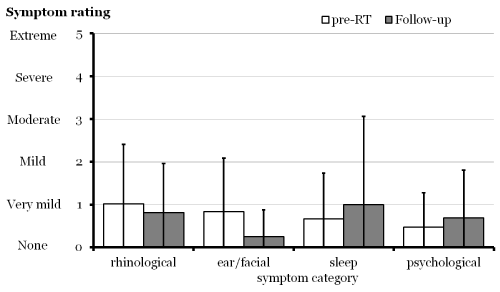
Figure 3 compares the pre- and post-RT results of the SNOT-20 survey sent to patients after RT. Patients had very mild rhinological, ear/facial, sleep, psychological, visual, and frontal lobe subscale scores on presentation; they maintained excellent quality of life on follow-up. Headaches resolved in the three patients who presented with them.
Assessment of quality of life using Sino-Nasal Outcome Tests (SNOT-20s) for patients with OGMs treated with RT.
Legend: Quality of life was measured with SNOT-20 surveys, which were sent to patients around the time of follow-up. The twenty questions (each graded 0 to 5) were averaged to create the following symptom subscales: (1) rhinological, including needing to blow one’s nose, sneezing, runny nose, cough, post-nasal discharge, and anosmia; (2) ear/facial, including ear fullness, dizziness, ear pain, and facial pain; (3) sleep, including difficulty sleeping and waking at night; and (4) psychological, including fatigue, reduced productivity, reduced concentration, frustration/restlessness/irritability, sadness, and embarrassment. Patients maintained excellent quality of life before and after RT.
Figure 3: Title:Assessment of quality of life using Sino-Nasal Outcome Tests (SNOT-20s) for patients with OGMs treated with RT.
Legend: Quality of life was measured with SNOT-20 surveys, which were sent to patients around the time of follow-up. The twenty questions (each graded 0 to 5) were averaged to create the following symptom subscales: (1) rhinological, including needing to blow one’s nose, sneezing, runny nose, cough, post-nasal discharge, and anosmia; (2) ear/facial, including ear fullness, dizziness, ear pain, and facial pain; (3) sleep, including difficulty sleeping and waking at night; and (4) psychological, including fatigue, reduced productivity, reduced concentration, frustration/restlessness/irritability, sadness, and embarrassment. Patients maintained excellent quality of life before and after RT.
Discussion
We investigated the efficacy and safety of primary RT on patients OGMs. We showed that in select patients, primary RT is an effective treatment modality: at a median follow-up time of 64 months, LC, OS, and CSS were 100%, 86%, and 100%, respectively. CN function was unchanged or improved in all patients. Notably, the function of CN I was subjectively improved in the three patients who presented with hypo/anosmia. If these patients were treated with primary surgery, they likely would have lost this function. Patient QOL was excellent both before and after RT. The results suggest that in select patients with OGMs, primary RT alone may be a safe and effective treatment option with less morbidity than would be expected from surgery.
Historically, surgery has been considered to be the primary treatment of all meningiomas, unless tumor- or patient-related risks precluded the procedure. Specifically, for OGMs, the lateral supraorbital approach has been a preferred [1]. For all meningiomas, primary SRS and FSRT are reserved for select locations (e.g. the optic nerve sheath, cavernous sinus, base of skull, acoustic meatus), where surgical risk is expected to be higher [12,13]. Recent reports have shown that primary RT for anatomically selected meningiomas is associated with excellent tumor control and CN function preservation or improvement [7-9]. For OGMs, CN dysfunction (especially anosmia) is common after surgery.
Regarding SRS vs. FSRT for patients with OGMs (Table 1), only one study [14] has compared the two modalities for all types of meningiomas, and the authors reported no clear difference in local control, toxicity, or effect on neurological function. Similarly, we did not find a clear difference in efficacy between these modalities. Benign meningiomas have been shown to be equally well controlled with SRS of 15 Gy in 1 fraction and FSRT of 54 Gy in 30 fractions [15].
For SRS, the single-dose tolerance of the optic nerves (8-10 Gy) has been shown to be a cutoff for risk of rate of optic neuropathy. Neither of our SRS patients exceeded 10 Gy to the optic nerve. For FSRT, doses > 2.5 Gy per fraction have been shown to significantly increase the risk of optic neuropathy; doses < 1.9 Gy have been shown to have an increased risk of neuropathy when the total dose exceeds 65 Gy. All of our FSRT patients received between 1.8 and 2.0 Gy per fraction, and the maximum total doses to the chiasm using the schedules have been <55 Gy. Although the dose guidelines and common schedules have changed over time, we report no differences in efficacy or toxicity among the SRS and FSRT schedules when they met the established toxicity constraints.
Among studies reporting outcomes of surgery for OGMs, follow-up times average around 4 years (range, 0 to 22), and the combined local control rate is between 77 and 100% [1]. Comparatively, our cohort has a relatively long follow-up of 5.3 years and a local control rate of 100%. While two patients experienced peritumoral brain edema (grade 2 and 3), one of these patients had underlying comorbidities and an autoimmune disorder which likely precipitated the flare, and the other patient’s symptoms subsided in 3 weeks.
To our knowledge, this is the first report specifically examining CN function after RT in OGMs. Several studies have shown that for ONSMs [7,8] and acoustic tumors [9] surgery may be unnecessarily aggressive, and patients may retain special nerve function with RT alone. Among our patients, none had a worsening olfaction after RT and three patients had recovery in their sense of smell (Figure 2). Additionally, both patients who presented with visual symptoms had symptom improvement, which is also encouraging as CN II dysfunction occurs in up to 8% of patients after surgery [1].
Finally, we measured QOL with the SNOT-20, a detailed and validated subjective questionnaire [10], used in patients with endonasal and sinus surgical procedures [16,17]. The SNOT-20 is also validated measure of QOL symptoms, including sleep, energy, cognition, and emotional problems. We present the SNOT-20 with subscales that improve clinical meaningfulness [11]. Our patients maintained a relatively good QOL, with regard to rhinological, ear/ facial, sleep, and psychological subscores.
This study has limitations. First, it is a retrospective cohort, so one may draw association but not causation between RT and the outcomes. Second, there is a limited sample size. However, OGMs are relatively rare, and the largest reported series of minimally invasive endoscopic resection reports on12 patients [18], and no studies have yet assessed CN function after RT. Finally, surveys were mailed to patients around the time of the last follow-up, so there is a risk for recall bias and response bias among respondents.
Future studies will help to personalize therapy for patients with OGMs by stratifying patients based on risk of progression, identifying unique subpopulations of patients who would benefit from a targeted molecular therapy, and predicting treatment response. Stratification of patients may involve the use of an algorithm. For example, perhaps patients with small and medium sized tumors and no signs or symptoms of mass effect could be treated with RT. For larger tumors or those with symptoms related to mass effect, surgery with adjuvant or salvage RT would be indicated.
Additionally, targeted molecular therapy will have a niche in treating meningiomas [1]. Losses on chromosome 22 (e.g. mutations of the NF2 gene, merlin protein) have been most commonly implicated in the formation for most meningiomas. Inactivation of the tumor suppressor genes DAL-1 and matrix metalloproteinase’s, upregulation of the oncogene STAT-3, and signaling dysregulation of the Wnt pathway have also shown to contribute to their formation. The predominance of females with meningiomas suggests that the hormonal axis is associated in their development in progression; although, estrogen and progesterone inhibitors have yet to show efficacy [1]. These biomolecular pathways may also predict response to surgery or RT; for example, the presence of VEGFRs [19] or micro RNA [20] on pathological specimen may predict the incidence of peritumoral edema aid in the recommendation of adjuvant vs. salvage RT.
Conclusion
In this study, patients with OGMs treated with primary RT maintained excellent rates of local control and cause-specific survival, had preserved or improved CN function (notably, olfactory nerve function improved in three patients), and maintained excellent QOL. The results suggest that RT could be offered as a primary treatment option select OGMs. Larger prospective studies are necessary to determine the role of RT in the multimodal care of these patients.
References
- Adappa ND, Lee JY, Chiu AG, Palmer JN. Olfactory groove meningioma. Otolaryngol Clin North Am. 2011; 44: 965-980, ix.
- Nakamura M, Struck M, Roser F, Vorkapic P, Samii M. Olfactory groove meningiomas: clinical outcome and recurrence rates after tumor removal through the frontolateral and bifrontal approach. Neurosurgery. 2007; 60: 844-852.
- Aguiar PH, Tahara A, Almeida AN, Simm R, Silva AN, Maldaun MV, et al. Olfactory groove meningiomas: approaches and complications. J Clin Neurosci. 2009; 16: 1168-1173.
- Obeid F, Al-Mefty O. Recurrence of olfactory groove meningiomas. Neurosurgery. 2003; 53: 534-542.
- Milker-Zabel S, Zabel A, Schulz-Ertner D, Schlegel W, Wannenmacher M, Debus J, et al. Fractionated stereotactic radiotherapy in patients with benign or atypical intracranial meningioma: long-term experience and prognostic factors. Int J Radiat Oncol Biol Phys. 2005; 61: 809-816.
- Pollock BE, Stafford SL, Utter A, Giannini C, Schreiner SA. Stereotactic radiosurgery provides equivalent tumor control to Simpson Grade 1 resection for patients with small- to medium-size meningiomas. Int J Radiat Oncol Biol Phys. 2003; 55: 1000-1005.
- Andrews DW, Faroozan R, Yang BP, Hudes RS, Werner-Wasik M, Kim SM, et al. Fractionated stereotactic radiotherapy for the treatment of optic nerve sheath meningiomas: preliminary observations of 33 optic nerves in 30 patients with historical comparison to observation with or without prior surgery. Neurosurgery. 2002; 51: 890-902.
- Vagefi MR, Larson DA, Horton JC. Optic nerve sheath meningioma: visual improvement during radiation treatment. Am J Ophthalmol. 2006; 142: 343-344.
- Andrews DW, Suarez O, Goldman HW, Downes MB, Bednarz G, Corn BW, et al. Stereotactic radiosurgery and fractionated stereotactic radiotherapy for the treatment of acoustic schwannomas: comparative observations of 125 patients treated at one institution. Int J Radiat Oncol Biol Phys. 2001; 50: 1265-1278.
- Davis GE, Yueh B, Walker E, Katon W, Koepsell TD, Weymuller EA, et al. Psychiatric distress amplifies symptoms after surgery for chronic rhinosinusitis. Otolaryngol Head Neck Surg. 2005; 132: 189-196.
- Browne JP, Hopkins C, Slack R, Cano SJ. The Sino-Nasal Outcome Test (SNOT): can we make it more clinically meaningful? Otolaryngol Head Neck Surg. 2007; 136: 736-741.
- Pechlivanis I, Wawrzyniak S, Engelhardt M, Schmieder K. Evidence level in the treatment of meningioma with focus on the comparison between surgery versus radiotherapy. A review. J Neurosurg Sci. 2011; 55: 319-328.
- Starke RM, Williams BJ, Hiles C, Nguyen JH, Elsharkawy MY, Sheehan JP, et al. Gamma knife surgery for skull base meningiomas. J Neurosurg. 2012; 116: 588-597.
- Torres RC, Frighetto L, De Salles AA, Goss B, Medin P, Solberg T, et al. Radiosurgery and stereotactic radiotherapy for intracranial meningiomas. Neurosurg Focus. 2003; 14: e5.
- Flickinger JC, Kondziolka D, Maitz AH, Lunsford LD. Gamma knife radiosurgery of imaging-diagnosed intracranial meningioma. Int J Radiat Oncol Biol Phys. 2003; 56: 801-806.
- Piccirillo JF, Merritt MG Jr, Richards ML. Psychometric and clinimetric validity of the 20-Item Sino-Nasal Outcome Test (SNOT-20). Otolaryngol Head Neck Surg. 2002; 126: 41-47.
- Georgalas C, Badloe R, van Furth W, Reinartz S, Fokkens WJ. Quality of life in extended endonasal approaches for skull base tumours. Rhinology. 2012; 50: 255-261.
- Gardner PA, Kassam AB, Thomas A, Snyderman CH, Carrau RL, Mintz AH, et al. Endoscopic endonasal resection of anterior cranial base meningiomas. Neurosurgery. 2008; 63: 36-52.
- Kan P, Liu JK, Wendland MM, Shrieve D, Jensen RL. Peritumoral edema after stereotactic radiosurgery for intracranial meningiomas and molecular factors that predict its development. J Neurooncol. 2007; 83: 33-38.
- Zhi F, Zhou G, Wang S, Shi Y, Peng Y, Shao N, et al. A microRNA expression signature predicts meningioma recurrence. Int J Cancer. 2013; 132: 128-136.