Mini Review
Austin J Surg. 2014;1(7): 1035.
Impact of Extent of Resection for Gliomas
Robert Bailey1 and Timothy H Lucas1,2*
1Department of Neurosurgery, University of Pennsylvania, USA
2Center for Neuroengineering & Therapeutics, University of Pennsylvania, USA
*Corresponding author: Timothy Lucas, Department of Neurosurgery, Translational Neuromodulation Lab, Center for Neuroengineering & Therapeutics, 3400 Spruce Street, 3rd Floor Silverstein, Philadelphia, PA 19104, USA
Received: September 03, 2014; Accepted: September 25, 2014; Published: October 07, 2014
Keywords
Glioma; Extent of resection; Brain mapping; Overall survival
Introduction
Brain tumors exact significant physical, emotional and economic burdens on patients, providers and the nation. In an era of personalized medicine and targeted therapeutics, surgical intervention remains pivotal in glioma management.
The goals of glioma surgery are to obtain a tissue diagnosis, reduce mass effect and achieve cytoreduction. Tissue diagnostics encompass pathological grading and comprehensive genetic profiling to target personalized therapeutics. Within the closed intracranial compartment, the rapid growth of a neoplasm causes mass effect and increased intracranial pressure. Decompression of space-occupying tumors lessens mass effect. Finally, cytoreduction lowers the burden of neoplastic clones that must be targeted with subsequent radiotherapy and chemotherapy.
Surgical success is ultimately defined by patient survival. A number of metrics are independent predictors of survival. Extent of Resection (EOR) is one such metric. In this overview, we review EOR in the context of the two common neoplastic conditions faced by neurosurgeons: Low Grade Glioma (LGG) and High Grade Glioma (HGG). These conditions differ greatly in treatment and prognosis, so we consider each individually. We begin by considering perioperative factors important for both conditions.
Perioperative Considerations
Treatment in the field of surgical oncology usually begins with wide surgical excision of the lesion to ensure clear tumor margins and lymph nodes free of disease. Glioma surgery is distinguished by the unique property of these lesions to infiltrate functional brain regions. Neurological deficits resulting from attempted resection of eloquent areas reduce quality of life, delay adjuvant therapy and hasten demise [1]. Consequently, Gross Total Resection (GTR) is not planned in cases where eloquent brain regions are invaded. Planning glioma surgery must balance maximal safe resection with the risk of inducing neurological deficits in a highly constrained physical environment.
Further, it is generally accepted that neoplastic cells infiltrate beyond the limits of the visible and radiographic boundaries of the lesion. A radiographic ’gross total resection’ more accurately describes a 99% volumetric cytoreduction. The remaining 1% represents diffuse microscopic residual disease that is too small to visualize radiographically with intravenous contrast agents. Surgical planning therefore anticipates the need for adjuvant therapy to the surgical margins and the possibility of local recurrence.
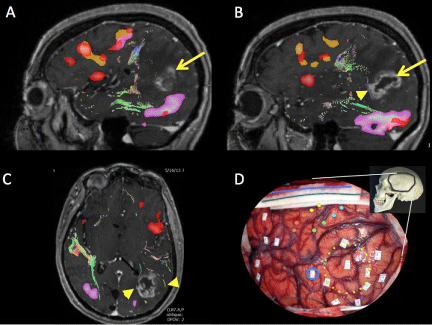
Planning glioma surgery requires specialized radiography and techniques. Specifically, the preoperative workup includes structural Magnetic Resonance Imaging (MRI), Functional MRI (fMRI), magnetic resonance spectroscopy and Diffusion Tensor Imaging (DTI) (Figure 1A, B, C). If the lesion is near speech areas, direct stimulation of the brain during surgery may be necessary. These modalities are used to develop a surgical trajectory clear of eloquent cortical regions and the white matter tracts that link them. The most fundamental requirement is the structural MRI. Isotropic voxels of T1-weighted sequences are acquired in gapless slices to create a 3-dimentional volume that is registered to the patient’s superficial landmarks with optical imaging in the operating room to build a model of the brain during surgery. This affords the surgeon a real-time anatomical navigation tool that can ’see’ through bone, dura and brain to visualize deep lesions. So enabled, the glioma surgeon may strategize an approach to optimize the resection while minimizing iatrogenic deficits. This technique has been transformative in modern neurosurgery and is the backdrop against which we consider the evidence for extent of resection.
Figure 1:Multimodal mapping of eloquent brain regions and tracts in patient with dominant hemisphere high grade glioma. Sagittal contrast-enhanced T1- weighted MRI merged with verb generation fMR (orange and red regions) and sentence comprehension fMR (purple) (Panels A and B). Important white matter bundles illustrated as colored lines (arrowhead) (Panel B). Axial MRillustrating important white matter tracts (arrowheads; Panel C). Intraoperative photo of same patient during language mapping of left temporo-parientofrontal regions. Skull insert demonstrates orientation and relative location of craniotomy. Tumor location encircled in yellow. Essential speech site mapped along anterior margin of tumor boundary (blue circle). Motor and sensory areas mapped along central sulcus (filled colored circles).
Surgical planning requires an understanding of the functional organization of the brain. The functional organization of the brain is highly plastic–constantly adapting to changing behavioral conditions [2,3]. The organization of essential language sites, for example, is highly variable across individuals and cannot be predicted on the basis of surface landmarks alone [4,5]. The addition of multiple languages [6] or language deficits [7] further alters the organization of these essential sites. The variable organization of essential speech sites underscores the need for surgery tailored to the patient’s functional anatomy. Functional mapping may be performed with electrical stimulation during a wake craniotomy or functional magnetic resonance imaging performed preoperatively.
Electrical Stimulation Mapping
The standard of care for localizing essential language cortices is Electrical Stimulation Mapping (ESM) performed during surgery. As originally described by Ojemann and Berger [4], ESM consists of the application of low amplitude currents directly to small regions of cortex during neurophysiological testing. These focal currents depolarize local networks of neurons and interneurons–transiently arresting their function. This allows the surgeon to interrogate the brain surface and map regions of interest. Essential eloquent sites are identified and protected during surgery. In similar fashion, the subcortical white matter pathways may be mapped to delineate the deep limits of resection. This technique may be more challenging; however, as the white matter bundles are visually indistinct. Nevertheless, it may be performed successfully. Duffau and colleagues have applied this technique to mapping and preserving the critical white matter bundles in the dominant hemisphere. Authors agree that mapping is necessary to perform maximal safe resection for gliomas within eloquent regions [8-11].
Functional MRI
Functional MRI detects task-related changes in Blood Oxygen Level-Dependent (BOLD) signal in activated brain networks. BOLD signal is thought to represent neural activity such as gamma oscillations of cortical circuits associated with behavior [12-14]. In contrast to electrical stimulation mapping, fMRI simultaneously detects changes throughout the brain across multiple time series. However, BOLD signal is not limited to ’essential’ network activity and may yield both false-positive and false negative information. A meta-analysis by Giussani and colleagues identified 9 reports of cases in which patients underwent language fMRI and the gold standard electrical stimulation mapping [15]. The sensitivities for fMR to predict language localization ranged from 59% to 100% while the specificities ranged from 0% to 97%. This magnitude of variance in sensitivity and specificity prevents language fMR from replacing ESM as a stand-alone modality for language localization.
Sensorimotor fMRI for identifying the location of primary sensory and motor cortices, conversely, is highly sensitive and specific. In large series of glioma surgeries, fMRI has proven as effective as standard intraoperative methods. This enables surgeons to maximize EOR, defined as the volume of tumor remaining after resection divided by the volume prior to surgery expressed as a percentage. Talacchi and colleagues analyzed the rate of achieving GTR in 171 glioma patients undergoing either sensorimotor identification by fMRI or intraoperative monitoring [16]. FMR proved as effective as intraoperative mapping for permitting GTR (71% fMRI group vs 73% IOM group, p=ns). The rate of GTR using either modality was significantly higher than when the surgeon utilized neuronavigation alone (40%, p=0.02). This success directly translated into improved overall survival (p<0.01). Sensorimotor fMRI is a reliable tool for guiding surgery in motor and sensory regions.
Diffusion Tensor Imaging
Since its introduction in 1994 [17], Diffusion Tensor Imaging (DTI) has grown in adoption as a surgical planning tool. DTI tractography enables visualization of white matter tracts by providing information about the molecular displacement of protons within anisotropic tissues such as axons. DTI behaves like in vivo quantitative histology–permitting the surgeon to reconstruct critical pathways noninvasively. DTI has improved our understanding of the brain’s connectivity [18] and informs glioma resection [19-22]. Historically surgeons would stop short of maximal resection when a tumor boundary encroached upon suspected white matter bundles so as to avoid the possibility of inducing iatrogenic neurological deficits. The ability to visualize these bundles during tumor resection ensures that the surgical boundaries are based on white matter anatomy. The rate of achieving successful GTR is higher when the surgeon has access to this information. A randomized controlled trial confirmed this hypothesis by examining the impact of DTI in patients with gliomas near the corticospinal pathways [23]. The rate of GTR, neurological outcomes and survival were all improved when surgery is guided by tractography.
This utility has permitted surgeons to estimate EOR prior to surgery and counsel patients. Invasion of important white matter bundles such as the corticospinal tract is a strong predictor of sub-total resection, even in small tumors [24]. Patients and surgeons may now engage in informed discussions about the probability of neurological deficit.
Extent of resection of low grade gliomas
Low grade gliomas represent the minority of intrinsic brain tumors in the adult population. The most common adult Low Grade Gliomas (LGG) area strocytomas, oligodendrogliomas and oligoastrocytomas. The median survival ranges from 5-7 years. While these lesions are generally slow-growing, their diffuse, infiltrative nature prevents them from being surgically curable [25]. They are commonly characterized by a long indolent period followed by a terminal phase of malignant transformation. Chemotherapy and radiotherapy have minimal effect [26]. Two notable exceptions are ’co-deleted’ oligodendroglioma and MGMT methylated astrocytoms. Oligodendroglioma with 1p and 19q chromosomal deletions have higher response rates to procarbazine-lomustine-vincrinstine chemotherapy. Astrocytomas with promoter methylation of the DNA repair gene O6-Methylguanine-DNA-methyltransferase (MGMT) demonstrate less resistance to alkylating chemotherapeutics. For these reasons, many surgeons employ a conservative strategy of tissue biopsy followed by expectant management until progression is observed [27-29]. This strategy is not without controversy, however. Proponents of aggressive attempts at surgical resection cite improvements in overall survival associated with GTR. Considerable debate remains in the neuro-oncology literature concerning optimal LGG management.
Management of LGG varies as a function of presentation and tumor location. Tumors found incidentally are more likely to be smaller and occur in non-eloquent locations than symptomatic lesions. These factors are associated with increased EOR for incidental lesions (95.7%) compared with symptomatic lesions (77.1%) [30]. Not surprisingly, predictors of poor prognosis include tumors presenting with neurological deficits, tumors originating in non-frontal locations and large tumors [31].
Supporting the role for early aggressive surgical intervention areimproved chances at GTR before symptom progression and improved overall survival [32]. The survival advantage of GTR compared to subtotal resection has been estimated to be as high as 30 months [33]. Extent of resection is an independent predictor of overall survival [34], even when GTR is not possible. Ius and colleagues recently examined overall survival as a function of EOR for LGG near eloquent cortex [35]. The 5-year survival rate was 93% for resections greater than 90% of the original tumor volume, 84% for resections greater than 70% of the original tumor volume and 41% for those less than 70% of the original volume. This nonlinear response curve suggests that the observed survival benefit may have an inflection point at, or around, 70% EOR. This phenomenon is thought to represent a reduction in the probability of malignant transformation due to cytoreduction. This observation waits empirical testing.
The observation that survival is related to EOR led to the introduction of supra-maximal resections in brain surgery [36]. Having long been a tradition in general surgery practices, the rationale for supra-maximal resections is based upon the fact that neoplastic cells extend beyond the area of abnormality visualized on MRI. Indeed, glioma cells are found up to 20 mm outside the boundaries of ’well-defined’ gliomas [37]. This explains natural history of the disease, which is characterized by local tumor recurrence and progression [38-43].
In supra-maximal resections, tissue removal proceeds until eloquent cortex is encountered, often well beyond the radiographic limits of the tumor [36]. Such resections are volumetrically larger (36.8 cm3vs26.6 cm3, p <0.05) and are associated with a trend towards lower rates of tumor recurrence (26% vs 41%) and lower rate of transformation. Aggressive resections also result in high rates of neurological deficit (60%) which temper the enthusiasm for universal adoption of this technique.
Few definitive conclusions can be reached with the available data on the management of LGG. For obvious ethical and logistical reasons, class I data are difficult to obtain. For the foreseeable future a balance must be reached between maximizing the oncological goals of surgery with the risk of inducing neurological deficits on a case-by-case basis [44].
Extent of resection of high grade gliomas
High-grade gliomas (HGGs) account for 60–75% of all gliomas, and include WHO grade III anaplastic astrocytoma, anaplastic oligodendroglioma, mixed anaplastic oligoastrocytoma and grade IV glioblastomamultiforme (GBM). GBM is the most malignant and prevalent astrocytic tumor. Histological hallmarks include polymorphism, nuclear atypia, mitotic activity, venous thrombosis, neovascularity and necrosis. The median survival for HGG is approximately 15 months [45]. With such a dismal prognosis, there is little controversy concerning the surgical management of HGG– maximal safe resection, followed by aggressive adjuvant therapy.
HGGs are heterogeneous tumors in both genotype and phenotype. This fact contributes to the broad range of individual survival [46,47]. A single glioma may contain several different types of pathological cells. The WHO convention for grading tumors requires that the tumor be classified based on the most malignant feature observed. Therefore the diagnosis of HGG may be rendered even if the predominant cell type is less malignant [48]. HGGs are derived from transformed neural stem cells or de-differentiated mature neural cells, so called ’glioma stem cells’ [49]. De novo HGG is believed to be a result of an aggregation of multiple mutations leading to dysregulation of signaling pathways [50]. This complex interaction of altered signaling pathways confounds treatment strategies.
Noclass I evidence exist concerning extent of resection and overall survival for HGG. A single prospective, randomized study comparing biopsy verses resection demonstrated a modest survival benefit in favor of EOR (2.8 months vs 5.7 months) [51]. Unfortunately, the interpretation of this study is limited by the small sample size and the absence of quantitative tumor volumetrics. In the absence of class I evidence, treatment recommendations are based upon a number of high quality case-control trials. Lacroix and colleagues performed retrospective multivariate analysis on 416 patients with GBM to identify independent predictors of survival [52]. They documented a significant survival advantage when the EOR was ≥ 98% of the enhancing tumor volume, especially when other predictive variables such as age, KPS and the presence of necrosis on MRI, were favorable. This finding is taken as supporting evidence by those who practice an all-or-none surgical strategy for HGG.
An alternative to the all-or-none strategy is one of maximizing safe EOR when GTR is not possible. This strategy is supported by class II and III evidence, predominantly from single institutions and is more widely adopted in the US. Sanai and colleagues identified EOR as an independent predictor of survival using a Cox proportional hazards analysis of 500 consecutive GBM cases [53]. A significant survival advantaged was seen above 78% EOR, with stepwise improvement in survival with increasing EOR. The largest impact on overall survival was realized by the group of patients with EOR ≥ 95%. Unfortunately, even in this group, the median survival was only 14.5 months.
Additional support for a relationship between extent of resection on survival was found in two multi-center studies investigating the role of 5-aminolevulinic acid (ALA) as a surgical aid [54,55]. ALA accumulates in fluorescent porphyrins within malignant glioma cells. When tagged with fluorescent markers, it may be visualized during surgery under proper filters. Improved visualization of the tumor results in greater EOR (65% for ALA vs 36% for white light) [56]. Patients in the ALA group also had higher 6-month progression free survival compared to a surgical control group (41% vs. 21%, respectively). Further, when stratifying patients by Radiation Therapy Oncology Group recursive partitioning analysis (RTOG-RPA), EOR predicted overall survival for prognostically-unfavorable Class IV and V patients. Overall survival for patients with complete resections was 5 months longer that those without (16.7 monthsvs11.8, p<0.0001). These data mirror those of larger studies. McGirt reviewed 1215 cases of pathologically proven Grade III or IV tumors [56]. After adjusting for age, KPS and adjuvant chemotherapy, the authors observed that the median survival for GBM was predicted by stratified EOR. Similarly, maximizing EOR of recurrent disease is associated with modest survival advantage [57], but even in the presence of maximal surgical and medical therapies, prognosis remains dismal.
Conclusion
Surgery remains a central tenant in the treatment of glioma. Perioperative considerations inform the surgical strategy and aid in maximal safe resection while identifying and preserving eloquent brain regions. In both low grade and high grade glioma, survival is improved when the surgeon is able optimize extent of resection. Significant improvements in glioma treatment are desperately needed to extend overall survival in this deadly disease.
References
- Gulati S, Jakola AS, Nerland US, Weber C, Solheim O. The risk of getting worse: surgically acquired deficits, perioperative complications, and functional outcomes after primary resection of glioblastoma. World Neurosurg. 2011; 76: 572-579.
- Chen HI, Attiah M, Baltuch G, Smith DH, Hamilton RH, Lucas TH. Harnessing Plasticity for the Treatment of Neurosurgical Disorders: An Overview. World Neurosurg. 2014.
- Sanes JN, Donoghue JP. Plasticity and primary motor cortex. Annu Rev Neurosci. 2000; 23: 393-415.
- Ojemann G, Ojemann J, Lettich E, Berger M. Cortical language localization in left, dominant hemisphere. An electrical stimulation mapping investigation in 117 patients. J Neurosurg. 1989; 71: 316-326.
- Sanai N, Mirzadeh Z, Berger MS. Functional outcome after language mapping for glioma resection. N Engl J Med. 2008; 358: 18-27.
- Lucas TH, McKhann GM, Ojemann GA. Functional separation of languages in the bilingual brain: a comparison of electrical stimulation language mapping in 25 bilingual patients and 117 monolingual control patients. J Neurosurg. 2004; 101: 449-457.
- Lucas TH, Drane DL, Dodrill CB, Ojemann GA. Language reorganization in aphasics: an electrical stimulation mapping investigation. Neurosurgery. 2008; 63: 487-497.
- Duffau H, Capelle L, Denvil D, Sichez N, Gatignol P, Taillandier L, et al. Usefulness of intraoperative electrical subcortical mapping during surgery for low-grade gliomas located within eloquent brain regions: functional results in a consecutive series of 103 patients. Journal of neurosurgery. 2003; 98:764-778.
- Sanai N, Berger MS. Intraoperative stimulation techniques for functional pathway preservation and glioma resection. Neurosurg Focus. 2010; 28: E1.
- Sarubbo S, Latini F, Panajia A, Candela C, Quatrale R, Milani P, et al. Awake surgery in low-grade gliomas harboring eloquent areas: 3-year mean follow-up. Neurological sciences. 2011; 32: 801-810.
- Spena G, Garbossa D, Panciani PP, Griva F, Fontanella MM. Purely subcortical tumors in eloquent areas: awake surgery and cortical and subcortical electrical stimulation (CSES) ensure safe and effective surgery. Clin Neurol Neurosurg. 2013; 115: 1595-1601.
- Conner CR, Ellmore TM, Pieters TA, DiSano MA, Tandon N. Variability of the relationship between electrophysiology and BOLD-fMRI across cortical regions in humans. J Neurosci. 2011; 31: 12855-12865.
- Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001; 412: 150-157.
- Mukamel R, Gelbard H, Arieli A, Hasson U, Fried I, Malach R. Coupling between neuronal firing, field potentials, and FMRI in human auditory cortex. Science. 2005; 309: 951-954.
- Giussani C, Roux FE, Ojemann J, Sganzerla EP, Pirillo D, Papagno C. Is preoperative functional magnetic resonance imaging reliable for language areas mapping in brain tumor surgery? Review of language functional magnetic resonance imaging and direct cortical stimulation correlation studies. Neurosurgery. 2010; 66: 113-120.
- Talacchi A, Turazzi S, Locatelli F, Sala F, Beltramello A, Alessandrini F, et al. Surgical treatment of high-grade gliomas in motor areas. The impact of different supportive technologies: a 171-patient series. J Neurooncol. 2010; 100: 417-426.
- Basser PJ. Inferring microstructural features and the physiological state of tissues from diffusion-weighted images. NMR Biomed. 1995; 8: 333-344.
- Catani M, Jones DK, Ffytche DH. Perisylvian language networks of the human brain. Ann Neurol. 2005; 57: 8-16.
- Berman JI, Berger MS, Mukherjee P, Henry RG. Diffusion-tensor imaging-guided tracking of fibers of the pyramidal tract combined with intraoperative cortical stimulation mapping in patients with gliomas. J Neurosurg. 2004; 101: 66-72.
- Coenen VA, Krings T, Mayfrank L, Polin RS, Reinges MH, Thron A, et al. Three-dimensional visualization of the pyramidal tract in a neuronavigation system during brain tumor surgery: first experiences and technical note. Neurosurgery. 2001; 49: 86-92.
- Kamada K, Todo T, Masutani Y, Aoki S, Ino K, Takano T, et al. Combined use of tractography-integrated functional neuronavigation and direct fiber stimulation. J Neurosurg. 2005; 102: 664-672.
- Nimsky C, Ganslandt O, Hastreiter P, Wang R, Benner T, Sorensen AG, et al. Preoperative and intraoperative diffusion tensor imaging-based fiber tracking in glioma surgery. Neurosurgery. 2005; 56: 130-137.
- Wu JS, Zhou LF, Tang WJ, Mao Y, Hu J, Song YY, et al. Clinical evaluation and follow-up outcome of diffusion tensor imaging-based functional neuronavigation: a prospective, controlled study in patients with gliomas involving pyramidal tracts. Neurosurgery. 2007; 61: 935-948.
- Castellano A, Bello L, Michelozzi C, Gallucci M, Fava E, Iadanza A, et al. Role of diffusion tensor magnetic resonance tractography in predicting the extent of resection in glioma surgery. Neuro Oncol. 2012; 14: 192-202.
- Cavaliere R, Lopes MB, Schiff D. Low-grade gliomas: an update on pathology and therapy. Lancet Neurol. 2005; 4: 760-770.
- Ajlan A, Recht L. Supratentorial low-grade diffuse astrocytoma: medical management. Semin Oncol. 2014; 41: 446-457.
- Jakola AS, Myrmel KS, Kloster R, Torp SH, Lindal S, Unsgard G, et al. Comparison of a strategy favoring early surgical resection vs a strategy favoring watchful waiting in low-grade gliomas. JAMA. 2012; 308: 1881-1888.
- Recht LD, Lew R, Smith TW. Suspected low-grade glioma: is deferring treatment safe? Ann Neurol. 1992; 31: 431-436.
- Reijneveld JC, Sitskoorn MM, Klein M, Nuyen J, Taphoorn MJ. Cognitive status and quality of life in patients with suspected versus proven low-grade gliomas. Neurology. 2001; 56: 618-623.
- Potts MB, Smith JS, Molinaro AM, Berger MS. Natural history and surgical management of incidentally discovered low-grade gliomas. J Neurosurg. 2012; 116: 365-372.
- Capelle L, Fontaine D, Mandonnet E, Taillandier L, Golmard JL, Bauchet L, et al. Spontaneous and therapeutic prognostic factors in adult hemispheric World Health Organization Grade II gliomas: a series of 1097 cases: clinical article. Journal of neurosurgery. 2013b; 118: 1157-1168.
- Pallud J, Fontaine D, Duffau H, Mandonnet E, Sanai N, Taillandier L, et al. Natural history of incidental World Health Organization grade II gliomas. Ann Neurol. 2010; 68: 727-733.
- Sanai N, Berger MS. Glioma extent of resection and its impact on patient outcome. Neurosurgery. 2008; 62: 753-764.
- Capelle L, Fontaine D, Mandonnet E, Taillandier L, Golmard JL, Bauchet L, et al. Spontaneous and therapeutic prognostic factors in adult hemispheric World Health Organization Grade II gliomas: a series of 1097 cases. J Neurosurg. 2013a; 118:1157-1168.
- Ius T, Isola M, Budai R, Pauletto G, Tomasino B, Fadiga L, et al. Low-grade glioma surgery in eloquent areas: volumetric analysis of extent of resection and its impact on overall survival. A single-institution experience in 190 patients: clinical article. J Neurosurg. 2012; 117: 1039-1052.
- Yordanova YN, Moritz-Gasser S, Duffau H. Awake surgery for WHO Grade II gliomas within "noneloquent" areas in the left dominant hemisphere: toward a "supratotal" resection. Clinical article. J Neurosurg. 2011; 115: 232-239.
- Pallud J, Varlet P, Devaux B, Geha S, Badoual M, Deroulers C, et al. Diffuse low-grade oligodendrogliomas extend beyond MRI-defined abnormalities. Neurology. 2010; 74: 1724-1731.
- Leighton C, Fisher B, Bauman G, Depiero S, Stitt L, MacDonald D, et al. Supratentorial low-grade glioma in adults: an analysis of prognostic factors and timing of radiation. J Clin Oncol. 1997; 15: 1294-1301.
- Nakamura M, Konishi N, Tsunoda S, Nakase H, Tsuzuki T, Aoki H, et al. Analysis of prognostic and survival factors related to treatment of low-grade astrocytomas in adults. Oncology. 2000; 58: 108-116.
- Philippon JH, Clemenceau SH, Fauchon FH, Foncin JF. Supratentorial low-grade astrocytomas in adults. Neurosurgery. 1993; 32: 554-559.
- Rajan B, Pickuth D, Ashley S, Traish D, Monro P, Elyan S, et al. The management of histologically unverified presumed cerebral gliomas with radiotherapy. Int J Radiat Oncol Biol Phys. 1994; 28: 405-413.
- Shaw E, Arusell R, Scheithauer B, O'Fallon J, O'Neill B, Dinapoli R, et al. Prospective randomized trial of low- versus high-dose radiation therapy in adults with supratentorial low-grade glioma: initial report of a North Central Cancer Treatment Group/Radiation Therapy Oncology Group/Eastern Cooperative Oncology Group study. J Clin Oncol. 2002; 20: 2267-2276.
- Yeh SA, Ho JT, Lui CC, Huang YJ, Hsiung CY, Huang EY. Treatment outcomes and prognostic factors in patients with supratentorial low-grade gliomas. Br J Radiol. 2005; 78: 230-235.
- Duffau H, Mandonnet E. The "onco-functional balance" in surgery for diffuse low-grade glioma: integrating the extent of resection with quality of life. Acta Neurochir (Wien). 2013; 155: 951-957.
- Weller M, Cloughesy T, Perry JR, Wick W. Standards of care for treatment of recurrent glioblastoma--are we there yet? Neuro Oncol. 2013; 15: 4-27.
- Huse JT, Phillips HS, Brennan CW. Molecular subclassification of diffuse gliomas: seeing order in the chaos. Glia. 2011; 59: 1190-1199.
- Riemenschneider MJ, Jeuken JW, Wesseling P, Reifenberger G. Molecular diagnostics of gliomas: state of the art. Acta Neuropathol. 2010; 120: 567-584.
- Wang Y, Jiang T. Understanding high grade glioma: molecular mechanism, therapy and comprehensive management. Cancer Lett. 2013; 331: 139-146.
- Stiles CD, Rowitch DH. Glioma stem cells: a midterm exam. Neuron. 2008; 58: 832-846.
- Stiles CD, Rowitch DH. Glioma stem cells: a midterm exam. Neuron. 2008; 58: 832-846. Stopschinski BE, Beier CP, Beier D. Glioblastoma cancer stem cells--from concept to clinical application. Cancer Lett. 2013; 338: 32-40.
- Vuorinen V, Hinkka S, Färkkilä M, Jääskeläinen J. Debulking or biopsy of malignant glioma in elderly people - a randomised study. Acta Neurochir (Wien). 2003; 145: 5-10.
- Lacroix M, Abi-Said D, Fourney DR, Gokaslan ZL, Shi W, DeMonte F, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg. 2001; 95: 190-198.
- Sanai N, Polley MY, McDermott MW, Parsa AT, Berger MS. An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg. 2011; 115: 3-8.
- Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ, et al. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. 2006; 7: 392-401.
- Stummer W, Reulen HJ, Meinel T, Pichlmeier U, Schumacher W, Tonn JC, et al. Extent of resection and survival in glioblastoma multiforme: identification of and adjustment for bias. Neurosurgery. 2008; 62: 564-576.
- McGirt MJ, Chaichana KL, Gathinji M, Attenello FJ, Than K, Olivi A, et al. Independent association of extent of resection with survival in patients with malignant brain astrocytoma. J Neurosurg. 2009; 110: 156-162.
- Bloch O, Han SJ, Cha S, Sun MZ, Aghi MK, McDermott MW, et al. Impact of extent of resection for recurrent glioblastoma on overall survival: clinical article. J Neurosurg. 2012; 117: 1032-1038.