1Department of Radiology, Harvard Medical School, USA
2Department of Surgery, Harvard Medical School, USA
*Corresponding author: Tatsuo Kawai, Massachusetts General Hospital, White 521B, 55 Fruit St, Boston, MA 02114, Department of Surgery, Harvard Medical School, USA
Received: September 29, 2014; Accepted: November 26, 2014; Published: November 26, 2014
Citation: Irani Z, Romano L, Walker TG and Kawai T. HeRO - The Last Resort Measure - Single Center Series and Review of the Literature. Austin J Surg. 2014;1(9): 1041. ISSN: 2381-9030.
Despite recent improvements in the percentage of AV fistula in use among hemodialysis patients, more than 20% of patients remain dependent on a hemodialysis catheter for vascular access. Therefore, development of a novel device for the dialysis vascular access is critically important to improve the management of dialysis patients. The Hemodialysis Reliable Outflow (HeRO®; CryoLife, Kennesaw, GA) graft is a novel vascular access option that is ideally suited for catheter-dependent patients and those in whom previously functional fistulas and grafts have failed as a result of venous outflow stenoses and/ or occlusions. In this manuscript, we report our initial results of HeRO graft implantation with a review of the reported literature regarding this new vascular access device. HeRO graft placement may provide a last resort vascular access option other than tunneled catheter placement for hemodialysis patients in whom there are no longer traditional upper extremity options. However, careful monitoring for the development of central veins and/or right a trial thrombus is critically important after placement of the HeRO graft.
Keywords: Dialysis vascular access; Vascular access graft; HeRO graft
HeRO graft: Hemodialysis Reliable Outflow graft; AVF: Arteriovenous Fistula; ePTFE: Expanded Polytetrafluoroethylene; AVG: Arteriovenous Graft; UEAVG: Upper Extremity AVG; LEAVG: Lower Extremity AVG; BMI: Body Mass Index; SVC: Superior Vena Cava; TDC: Tunneled Dialysis Catheter
There are an increasing number of patients with end-stage renal disease in the United States, with nearly 400,000 patients being treated with some form of dialysis in 2009. After implementation of the Kidney Disease Outcomes Quality Initiative (KDOQI) recommendations [1], the percentage of patients who receive hemodialysis via an Arteriovenous Fistula (AVF) has increased from less than 32% in 2003 to nearly 60% in 2011. Conversely the number of patients in whom an Arteriovenous Graft (AVG) is used for hemodialysis has significantly dropped from almost 65 % ten years ago, to roughly 20% in 2009. However, over 20% of patients remain dependent on a hemodialysis catheter for vascular access [2]. In the majority of these patients there are no longer alternative options, as a result of multiple failures of vascular access surgeries.
The Hemodialysis Reliable Outflow (HeRO®; CryoLife, Kennesaw, GA) graft is a novel vascular access option that is ideally suited for catheter-dependent patients and those in whom previously functional fistulas and grafts have failed as a result of venous outflow stenoses and/or occlusions. The device consists of a 6 mm internal diameter and 7.4 mm outer diameter expanded Polytetrafluoroethylene (ePTFE) arterial graft component with PTFE beading to provide kink resistance that is placed subcutaneously and a 5 mm internal diameter 19 Fr (6.3 mm) external diameter braided nitinol reinforced silicone venous outflow component that is placed via a transjugular venous approach (Figure 1). The two components are subsequently joined together subcutaneously using a titanium connector that is part of the arterial component. Since the HeRO device does not have a venous anastomosis, which is a well-recognized cause of the majority of AVG failures [3,4], it provides an alternative to hemodialysis catheter placement in patients who have either inadequate outflow veins or occluded central veins. For patients in whom an Upper Extremity AVG (UEAVG) has either failed or is not an option, our clinical approach has been to place an AVG in the lower extremity (LEAVG). However, these grafts are susceptible to graft thrombosis due to venous outflow stricture. Limb ischemia is also often a serious complication in some patients such as diabetics, as there is a high prevalence of severe vasculopathy in this subpopulation. Given these concerns, we have explored placing a HeRO graft rather than a lower extremity AVG in patients who are no longer suitable for conventional upper extremity vascular access. Here we report our initial results of HeRO graft implantation with a review of the reported literature regarding this new vascular access device.
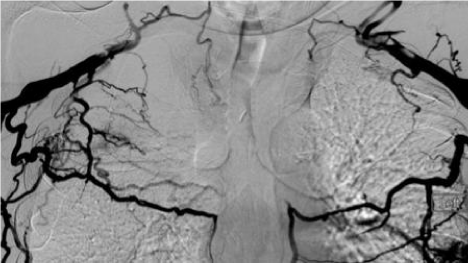
Twelve patients with end-stage renal disease underwent placement of the HeRO graft between 7/31/2012 and 8/27/2014. Most patients have a long history of ESRD with multiple failures of vascular access surgeries. Half of these patients (6/12) were also markedly obese with a Body Mass Index (BMI) higher than 40. In eight patients, HeRO placement was indicated because of bilateral subclavian and Superior Vena Cava (SVC) occlusion (Figure 2) and in four patients because of inadequate veins for AVF or AVG placement. The venous component of the HeRO graft was placed via the right internal jugular vein in six patients and via the left internal jugular vein in the other six patients. To access the internal jugular vein, a micro-access needle and 0.014” guidewire were used with ultrasound guidance. A transitional dilator was then introduced over the 0.014” guidewire using fluoroscopic guidance and then the guidewire was upsized to 0.035” diameter after removal of the inner dilator of the transitional dilator. The 0.035” guidewire then served as the working guidewire, over which all subsequent dilators, sheaths and catheters were introduced, using fluoroscopic guidance. Eight of 12 patients had bilateral central vein occlusion. In some instances, there was an indwelling hemodialysis catheter crossing the areas that were otherwise occluded and the HD catheter was removed after introducing a guidewire through one lumen of the HD catheter. That guidewire then served as the working guidewire during introduction of subsequent dilators, sheaths and catheters. In some cases the occlusion was reanalyzed using a combination of a hydrophilic guidewire and a hydrophilic catheter. After successfully advancing a guidewire through the occluded venous segment, balloon angioplasty of the occluded segment was performed in order to create a channel of sufficient diameter to allow introduction of subsequent dilators, sheaths and catheters.
During the HeRO graft placement procedure, a tunneled hemodialysis catheter was also placed via either the contra-lateral internal jugular vein or the common femoral vein so as to provide vascular access for continued hemodialysis until the HeRO graft became ready for use. The patients were followed at local dialysis centers with a close contact with the access coordinators.
There were three cases of failed HeRO placement. The first patient (Patient #1) with chronic hypotension developed thrombosis of the device occurred three months following placement. Patient #6 developed steal syndrome due to severe peripheral arterial disease. After banding, the graft was removed due to infection. The HeRO graft placed in Patient #9 migrated to the SVC. An attempt to advance the HeRO to the right atrium failed due to severe fibrotic central vein stenosis. The HeRO placed in the remaining nine patients functioned well for a period ranging from 3-18 months (Table 1).
According to the results of the randomized multicenter study for the HeRO vs. conventional AV graft reported by Nassar GM et al. [5], there was no statistical difference in graft patency rates between the HeRO (n=52) and conventional AV grafts (n=20). Twelve month primary and secondary patency rates were 34.8% and 67.6% in the HeRO and 30.6% and 58.4% in the control cohort (p=0.687 and 0.656, respectively). There was also no statistical difference in the rates of intervention (2.2/year in HeRO vs. 1.6/year in control). Adverse events included bleeding, which is directly attributed to an earlier HeRO graft generation in which a 22 French venous outflow component was used and which required an internal jugular venous cut-down. There was one HeRO-related death those occurred13 months post-implantation as a result of sepsis. Thromboembolic complications also occurred in two patients in the HeRO group: pulmonary embolism after thrombotic occlusion of the HeRO and cerebral infarction via a patent foramen ovale after a declot procedure. Wallace JR et al. reported a retrospective study of 19 patients who underwent placement of the HeRO [6]. The primary and secondary patency rates at 12 months were 11% and 32%, which were significantly inferior to those reported by Nassar et al. [5]. The reported adverse events included steal syndrome, which was observed in four female patients. Katzman HE implanted the HeRO graft in 36 access-challenged patients and compared results with those of patients in whom a Tunneled Dialysis Catheter (TDC) was used for hemodialysis access [7]. The HeRO related bacteremia rate was 0.70/1000 days, all of which was observed during the bridging period when a TDC was still indwelling before using the HeRO graft for hemodialysis. In contrast, bacteremia rates of the TDC have been reported as 2.3/1000 days and reduction of bacteremia rates appeared to be a critical advantage of the HeRO over the TDC [7]. The other controversial issue is the comparison of outcomes after HeRO placement with those of LEAVG. Until recently, our first choice of the vascular access in patients in whom upper extremity vascular access is no longer feasible had been LEAVG. In the Steerman SN et al. retrospective comparison of the HeRO with LEAVG [8], the secondary patency rate at 6 months was 77% for the HeRO and 83% for the LEAVG (p=0.14). The number of interventions required to maintain patency was 2.21/year in the HeRO group and 1.17/year in the LEAVG (p=0.003). As this study is a retrospective analysis, a large-scale prospective evaluation will be necessary to definitively demonstrate superiority of the LEAVG over HeRO. However, as LEAVG potentially increases the risk of distal limb ischemia, there have been some limitations in consistently using this access option especially in diabetic patients. In such patients, the HeRO may be a preferable option to the LEAVG. Although the HeRO is indicated for patients with central venous occlusion, the device itself can cause severe central vein (e.g., SVC) or right a trial occlusion. Pillai et al. reported a patient who developed esophageal varies as a result of SVC/right a trial occlusion caused by a HeRO [9]. Therefore, a careful monitoring of thrombus formation in the SVC and/or right atrium should be mandatory after HeRO placement. Finally, a cost analysis comparing Medicare billing prices of the HeRO, LEAVG and TDC in patients with central venous stenosis was performed by Dageforde et al. [10]. They reported that the HeRO is the least costly of these hemodialysis access options, with an average 1-year cost of $6521, versus $8477 with TDC and $9567 with LEAVG. The better financial results of the HeRO over LEAVG and TDC are attributed to lower costs both for access maintenance and treatment of infectious complications.
In conclusion, HeRO graft placement may provide a last resort vascular access option other than tunneled catheter placement for hemodialysis patients in whom there are no longer traditional upper extremity options. However, careful monitoring for the development of SVC and/or right atrial thrombus is critically important after placement of HeRO graft. Longer-term evaluation in more patients will be necessary to conclude the real value of HeRO graft.
Patient Characteristics.
|
Age |
length of dialysis |
Reason of HeRO |
#of Previous surgeries |
BMI |
Side placed |
date of operation |
Outcome |
1 |
46 |
15 yrs |
Bilateral central vein occlusion |
>20 |
42 |
left |
7/31/2012 |
Failed due to low blood pressure |
2 |
45 |
5 yrs |
No veins |
>10 |
55 |
left |
3/11/2013 |
functioning, angioplasty X1 |
3 |
72 |
10 yrs |
Bilateral central vein occlusion |
>10 |
30 |
right |
10/1/2013 |
Functioning when pt expired on 3/25/14 |
4 |
52 |
2 yrs |
No veins |
>6 |
34 |
right |
2/4/2014 |
Functioning |
5 |
50 |
7 yrs |
Bilateral central vein occlusion |
>10 |
27 |
right |
4/17/2014 |
Functioning when transplanted 6/8/14 |
6 |
69 |
7 yrs |
Bilateral central vein occlusion |
>5 |
44 |
right |
5/12/2014 |
failed, removed due to steal syndrome and infection |
7 |
47 |
11 yrs |
No veins |
> 6 |
44 |
left |
6/17/2014 |
Functioning |
8 |
68 |
2 yrs |
Right complete occlusion, small veins |
>5 |
39 |
right |
8/6/2014 |
Functioning |
9 |
28 |
2 yrs |
Bilateral central vein occlusion |
3 |
53 |
left |
8/19/2014 |
Failed. Removed due to catheter migration |
10 |
76 |
7 mos |
Bilateral central vein occlusion |
1 |
32 |
right |
8/21/14 |
Functioning |
11 |
53 |
20 yrs |
Bilateral central vein occlusion |
>10 |
60 |
left |
8/25/2014 |
Functioning |
12 |
73 |
10 yrs |
Bilateral severe axillary vein stricture |
>12 |
22 |
left |
8/27/2014 |
Functioning |
Venogram showing Superior Vena Cava occlusion with numerous collaterals.
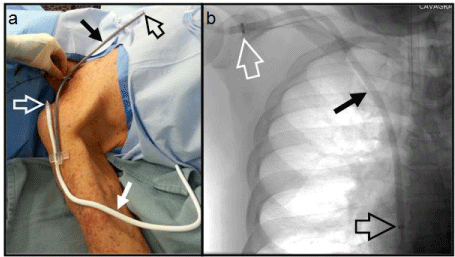
The components of HeRO graft. (a) OR photograph and (b) CXR. The arterial graft component (solid white arrow) is shown laid over the arm extending to shoulder. This will be cut to desired length and anastomosed to (in this instance) the brachial artery. The venous outflow component (solid black arrow) has the radio-opaque tip (open black arrow) positioned in the right atrium; the other end is cut to required length and joined to the arterial component near the shoulder via the titanium connector (open white arrow) attached to the graft. The hand is holding a guide-wire at the jugular puncture site; a peel-away sheath is advanced over the guide-wire into the central veins facilitating placement of the venous outflow component. All components are tunneled subcutaneously.