
Special Article: Gastrointestinal Surgery
Austin J Surg. 2023; 10(1): 1295.
Prognostic Significance of the Proximal Margin for Esophagogastric Junction Adenocarcinoma with Type II and III Tumors after Surgery
Yan Q1,2#, Zheng J1#, Lv Z1,2, Wang J1 and Li Y1,2*
1Department of General Surgery, Guangdong Provincial People’s Hospital (Guangdong Academy of Medical Sciences), Southern Medical University, Guangzhou 510080, China
2School of Medicine South China University of Technology, Guangzhou, 51000, China
*Corresponding author: Yong Li Department of General Surgery, Guangdong Provincial People’s Hospital (Guangdong Academy of Medical Sciences), Southern Medical University, Guangzhou 510080, China
Received: February 06, 2023; Accepted: March 21, 2023; Published: March 28, 2023
Abstract
Background: The incidence of Esophagogastric Junction Adenocarcinoma (EJA) has increased in recent years, with surgical resection the main choice of treatment. The optimal length of the proximal margin for EJA is still under debate, and the impact of EJA survival and recurrence remains unclear. The aim of the present study was to investigate the influence of the optimal length of the proximal margin on EJA.
Methods: From January 2011 to December 2015, 131 patients who had EJA with type II tumors were included and retrospectively analyzed. All patients underwent radical R0 resection. The proximal margin was measured promptly after resection, and the frozen-section pathological examination was negative for the margin.
Results: There were 3 cases of Siewert type I EJA (2.9%), 75 cases of Siewert type II EJA (57.9%), and 53 cases (40.1%) of Siewert type III EJA. The median number of lymph nodes examined was 19 (range: 1-41), and the median number of positive lymph nodes was 2 (range: 0–18). Sixty-three patients underwent total gastrectomy (48.1%), and 68 underwent proximal gastrectomy (51.9%). The median follow-up time was 57.3 months: (range 1.9–174.1); 34 patients (26%) relapsed and 74 (56.5%) died. The 5-year overall survival rate of type II tumor patients was 68.2%, and that of type III tumor patients was 38.5% (P=0.02). For patients with a proximal margin <2cm, the median recurrence time was 41.6 months, whereas it was for 42.8 months for patients with proximal margin >2cm (log–rank: 0.496). Our data analysis found that a proximal margin length of 2cm was a prognostic variable for type II and type III tumors.
Conclusions: There are a number of factors associated with recurrence and overall survival at 5 years for patients who have EJA with type II and type III tumors, and a proximal margin >2cm may indicate better prognosis.
Keywords: Proximal margin; Siewert type; Esophagogastric junction adenocarcinoma (EJA); Prognosis
Abbreviations: EJA: Esophagogastric Junction Adenocarcinoma; AJCC: American Joint Committee on Cancer; ROC: Receiver–Operator Curve
Backround
The incidence of Esophagogastric Junction Adenocarcinoma (EJA) has increased in recent years, particularly in Western and Asian countries [1-3]. According to data from Japan, the incidence of EJA has increased by 7.3% from the 1960s to the beginning of the 21st century [4]. A single-center registration study of gastric cancer in China found that the proportion of Esophageal–Gastric Junction (EGJ) cancer increased from 22.3% to 35.7% between 1988 and 2012 [5]. According to the Siewert classification, there are three types of EJA: Siewert type I is defined as tumors located 1–5cm above the esophagogastric junction, Siewert type II tumors are located at the upper l–2 cm below the esophagogastric junction, and Siewert type III tumors are located 2–5cm below the esophagogastric junction [6]. According to the AJCC Cancer Staging Manual, 8th edition, EJA is categorized and staged as esophageal cancer, as long as the tumor center is within 2cm of the junction, regardless of whether it invades the esophagus. If it is not within 2cm of the junction, the tumor is grouped and treated as stomach cancer, even if it has invaded the EGJ [7]. Currently, surgical resection is regarded as the cornerstone of curative treatment, although the introduction of neoadjuvant/adjuvant chemotherapy and radiotherapy and chemotherapy have been found to improve disease prognosis [8]. Due to the complexities of EJA tumor location, a consensus has yet to be reached on the best surgical strategy. The appropriate resection range of the esophagus and stomach, the scope and location of lymph node resection, and the best surgical method are still unclear [9].
The optimal length of the proximal margin for EJA is also still under debate, with only a limited number of studies published on this. In their study, Barbour et al. showed that the proximal margin length might be associated with patient survival in type II–IV tumors, but not in type I tumors, and they found that if the length of the proximal margin was >3.8cm, then the prognosis of type II+ tumor patients could be significantly improved [10]. However, Mine et al. found that, for patients who have EJA with type II or type III tumors, the length of the proximal margin exceeds 2cm, which seems to be satisfactory [11]. The proximal margin length is key for R0 and R1 resection status, and thus for survival outcome [12,13]. Therefore, it is crucial to determine a safe operation range when performing surgery.
Materials
We conducted a retrospective, observational study. Based on the classification of the AJCC Cancer Staging Manual, 8th edition, patients diagnosed with EJA type II and type III tumors, treated with surgery between January 2011 and December 2015, were included in the present study, except patients with gastric cancer and/or those undergoing neoadjuvant therapy. The inclusion criteria were: (i) patients undergoing radical surgery, including radical proximal ortotal gastrectomy; (ii) a negative confirmation of the proximal margin; and (iii) type II and type III tumors without distant metastasis. Patients with incomplete medical information, those undergoing neoadjuvant therapy, those with malignant tumors in other locations, and those who had previously had exploratory or tumor-reduction surgeries were excluded. Fresh specimens were cut longitudinally immediately after resection. The sample was then stretched to the maximum extent and fixed to a plate. The surgeon then measured and recorded the length of the proximal edge. The proximal margin was sent for frozen-section pathological examination to confirm whether the proximal margin length was sufficient. If insufficient, further resection was performed until there was a negative confirmation of the proximal margin. All surgical procedures and the extent of lymph node clearance conformed to the Japanese Gastric Cancer Treatment Guidelines (Japanese Gastric Cancer Association 2011). All procedures were conducted in accordance with the Declaration of Helsinki, as revisedin 2013 [14].
Data on age, sex, Siewert type, extent of surgery, tumor size, proximal margin, T stage, clinical stage, lymphatic–vascular invasion, neural invasion, differentiation status, total lymph nodes, lymph node metastasis, mediastinal lymph node dissection, Lauren type, human epidermal growth factor 2 status, adjuvant therapy, and relapse or recovery were collected. All patients underwent enhanced chest and abdominal computed tomography every 6 months after discharge to evaluate tumor recurrence and distant metastasis until October 2015. Follow-up was generally conducted through outpatient visits, email, and telephone interviews and follow-up data were updated until November 1, 2015. The follow-up rate, median follow-up time (months), and overall survival results were included in the study. The main reason that patients could not be followed up was because they declined outpatient visits or changed their telephone numbers and addresses.
All variables were analyzed using descriptive statistics. The results are presented as percentages, means, and dispersion measures. We used the unadjusted Kaplan–Meier method for visualization of the survival curves, and the log–rank test to compare survival curves using SPSS version 22.0. Logistic regression analysis was used for survival identified by univariate analysis were further assessed by multivariate analysis. The P-value was considered to be statistically significant at the 5% level. To better define the surgical margin, we use the Receiver–Operator Curve (ROC). Based on the Declaration of Helsinki and the general research health law [14]. Informed consent was not required for the present study, and patient confidentiality was assured. We present the following article in accordance with the STROBE reporting checklist.
Results
In total, 168 patients diagnosed with EJA without neoadjuvant chemotherapy were included in the present study, according to our admission and discharge criteria. Thirty-seven patients were excluded; 4 had undergone exploration or tumor-reduction surgery, 10 were diagnosed with T1 tumors according to the final pathological report, and 23 had incomplete information (Figure 1). Finally, 131 patients were included: 100 men (76.3%) and 31 women (23.7%, male-to-female ratio 3.22:1), with a median age of 64 years (range: 38–86). Three patients (2.9%) had Siewert type I tumors, 75 (57.3%) had Siewert type II tumors, and 53 (40.1%) had Siewert type III tumors. For Siewert type I tumors, the median tumor size was 4.5cm (range: 4–5.5cm); for Siewert type II tumors, the median tumor size was 4 cm (range: 1–10 cm); and for Siewert type III tumors, the median tumor size was6 cm (range: 2.5–10cm). All patients underwent open or laparoscopic surgery. Sixty-three (48.1%) patients underwent total gastrectomy, and 68 (51.9%) underwent subtotal gastrectomy. The median number of lymph nodes examined was 19 (range: 1–41), and the median number of positive lymph nodes was 2 (range: 0–18). The median delay time of adjuvant therapy was 8 weeks (range: 4–13 weeks). Chemotherapy regimens included XELOX, CapeOx, FOLFOX, and capecitabine. The patient clinical characteristics are shown in (Table 1).
Variables
Scale
No. of patients
sex ratio(M:F)
100:31
Age(year)
64(38-86)
Siewert type
I
3(2.3)
II
75(57.3)
III
53(40.4)
Resection
Total gastrectomy
63(48.1)
Subtotal gastrectomy
68(51.9)
Size(cm)
4.5(1-10)
Proximal margin(cm)
1.0(0.4-5.5)
Lauren type
intestinal
77(58.8)
diffuse
54(41.2)
Differentiation status
Poor
67(51.1)
Median-high
64(48.9)
Invasion
T2
22(16.8)
T3
109(83.2)
Total lymph nodes
19(1-47)
Positive lymph nodes
2(0-18)
Mediastinal lymph node dissection
Yes
53(49.5)
No
78(59.5)
Neural invasion
Yes
71(54.2)
No
60(45.8)
Lymphatic–vascular invasion
Yes
69(52.7)
No
62(47.3)
Adjuvant treatment
Yes
91(69.5)
No
40(30.5)
HER-2 stastus
Positive
19(14.5)
Negative
112(85.5)
Recurrence
Yes
34(26.0)
No
97(74.0)
Clinical stage
I
1(0.8)
II
29(22.1)
III
73(55.7)
IV
28(21.4)
Table 1: Population characteristics.
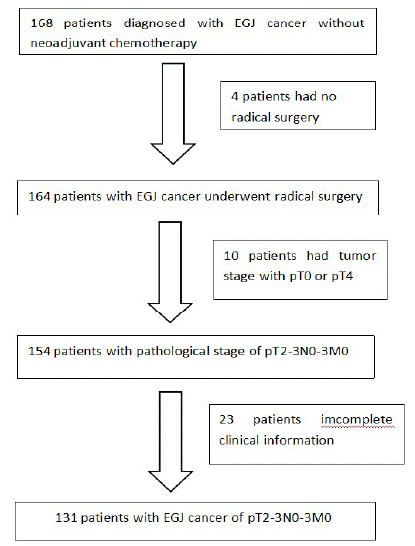
Figure 1: CONSORT diagram showing patient selection for the study according to inclusion criteria.
The median length of the proximal margin was 1cm (range: 0.5–2.5) in patients with Siewert type I tumors, 1cm (range: 0.4–5) in Siewert type II tumors, and 1.2cm (range: 0.4–5) in patients with Siewert type III tumors (Table 1).
The impact of the proximal margin length on overall survival was analyzed. A proximal margin length of 2cm was found to be a prognostic variable for patients with type II and type III tumors in both the univariate (Kaplan–Meier method, P = 0.02) and multivariable analyses (hazard ratio: 2.00, 95% confidence interval: 0.54–7.41, P =0.25, Cox method) (Tables 2 & 3).
Variables
Scale
Survived 57 (%)
No survived 74 (%)
P value
Recurrence 34 (%)
No recurrence 97 (%)
P value
Gender
Male
44 (77.2)
56 (75.7)
0.989
27 (79.4)
73 (75.3)
0.618
Female
13 (22.8)
18 (24.3)
7 (20.6)
24 (24.7)
Age
<65
24(42.1)
29 (39.2)
0.259
13(38.2)
40(41.2)
0.941
=65
33 (57.9)
45 (60.8)
21 (61.8)
57 (58.8)
Siewert type
I
2(3.5)
1 (1.4)
0.682
1(2.9)
2 (2.1)
0.769
II
30 (52.6)
45(60.8)
20 (58.8)
55 (56.7)
III
25 (43.9)
28 (37.8)
13 (38.2)
40 (41.2)
Resection
Total gastrectomy
30 (52.6)
33 (44.6)
0.409
15 (44.1)
48 (49.5)
0.606
Subtotal gastrectomy
27 (47.4)
41(55.4)
19 (55.9)
49 (50.5)
Size
<4 cm
27(47.4)
27 (36.5)
0.359
12 (35.3)
42 (43.3)
0.388
=4 cm
30 (52.6)
47(63.5)
22(64.7)
55 (56.7)
Proximal margin
<2.0cm
42(73.7)
67(90.5)
0.019
28(82.4)
81(83.5)
0.497
=2.0cm
15 (16.8)
7 (9.5)
6(17.6)
16(16.5)
Lauren type
intestinal
40 (70.2)
37 (50.0)
0.075
19 (55.9)
58 (59.8)
0.581
diffuse
17 (29.8)
37 (50.0)
15(44.1)
39 (40.2)
Differentiation status
Poor
21 (36.8)
46(62.2)
0.005
23(67.6)
44 (45.4)
0.010
Median-high
36(63.2)
28(37.8)
11(32.4)
53(54.6)
Invasion
T2
15 (16.8)
7 (9.5)
0.028
4 (11.8)
18(18.6)
0.194
T3
42 (73.7)
67 (90.5)
30 (88.2)
79(81.4)
Total lymph nodes
<16
24(42.1)
26(35.1)
0.803
14 (41.2)
36 (37.1)
0.865
=16
33(57.9)
48(64.9)
20 (58.8)
61 (62.9)
Positive lymph nodes
<5
44(77.2)
42(56.8)
0.013
21(61.8)
65(67.0)
0.159
=5
13 (22.8)
32 (43.2)
13 (38.2)
32(33.0)
Mediastinal Lymph node dissection
Yes
21(36.8)
32 (43.2)
0.622
19 (55.9)
34(35.1)
0.056
No
36(63.2)
42 (56.8)
15(44.1)
63(64.9)
Neural membrame
Yes
29 (50.9)
42 (56.8)
0.342
23 (67.6)
48 (49.5)
0.044
No
28(49.1)
32 (43.2)
11 (32.4)
49(50.5)
Vascular thrombus
Yes
27(47.4)
42 (56.8)
0.135
21(61.8)
49(50.5)
0.059
No
30 (52.6)
32 (43.2)
13 (38.2)
48 (49.5)
I
1 (1.8)
0(0.00)
0.012
0(0.0)
1 (1.0)
0.008
II
19 (33.3)
10(13.5)
4(11.8)
25 (25.8)
III
28(49.1)
45 60.8)
21 (61.8)
52 (53.6)
IV
9 (15.8)
19(25.7)
9(26.5)
19(19.6)
Adjuvant treatment
Yes
39 (68.4)
52 (70.3)
0.517
25(73.5)
66 (68.0)
0.480
No
18(31.6)
22 (29.7)
9(26.5)
31(32.0)
HER-2
positive
9 (15.8)
10 (13.5)
0.727
7 (20.6)
12(12.4)
0.392
negtive
48(84.2)
64 (86.5)
27 (79.4)
85 (87.6)
Recurrence
Yes
8 (14.0)
26(35.1)
0.001
N/A
N/A
No
49(86.0)
48 (64.9)
N/A
N/A
Table 2: Univariate analysis of potential risk factors for T2-3 AEJ cancers.
B
Hazard ratio(95% CI)
P
Proximal margin
-0.935
0.393(0.179-0.86)
0.019
Recurrence
0.717
2.048(1.254-3.343)
0.004
Differentiation status
-0.489
0.614(0.379-0.992)
0.046
Table 3: Cox regression of the factors associated with 5-OS.
The length of the proximal edge in the surgical specimens and its relationship with recurrence and the 5-year overall survival rate, which is sorted by centimeters (from nearest to farthest), is shown in (Figures 1 & 2). In the univariate analysis, patients with poorly differentiated tumors (P = 0.005), late clinical stage (P = 0·008), and neural invasion (P = 0.044) were at higher risk of recurrence. Patients with higher pT category tumors (P = 0·028), morelymph node metastasis (P=0.013), poorly differentiated tumors (P=0.005), proximal margins of <2cm (P=0.019), late clinical stage (P=0.012), and postsurgical recurrence had significantly worse survival (Table 2).The median follow-up time was 57.3 months (range: 1.9–174.1); 34 patients (26%) relapsed and 74 patients (56.5%) died. The median overall survival time of patients with a tumor proximal margin of <2cm was 55.5 months, and that of patients with a proximal margin of >2cm was 68 months (log–rank: 0.019). The median recurrence time for patients with a proximal margin of <2cm was 41.6 months, whereas it was 42.8 months for patients with a proximal margin of >2cm (log–rank: 0.496) (Table 1).

Figure 2: The length of proximal margin in the surgical specimen and its relationship with 5-year overall survival rate, sorted by cm from nearest to farthest.
In the multivariate analysis, only tumor differentiation status was found to be the prognostic factor for recurrence. The variables related to the 5-year overall survival rate were tumor infiltration (type II vs. III), proximal margin, tumor differentiation status, and recurrence. However, in the ROC analysis, we did not find that a definitive margin showed better tumor outcomes (Tables 3 & 4).
B
Hazard ratio(95% CI)
P
Differentiation status
0.744
0.292
2.917
2.105(1.008-4.395)
0.048
Table 4: Cox regression of the factors associated with recurrence.
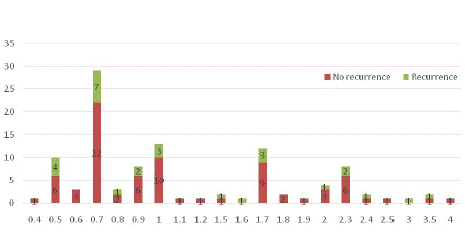
Figure 3: The length of proximal margin in the surgical specimen and its relationship with recurrence , sorted by cm from nearest to farthest.
Type II tumor patients had a 5-year overall survival of 68.2%, and type III tumor patients had a 5-year overall survival of 38.5% (P=0.02) (Figure 4). We observed an 18.2% recurrence in type II tumor patients, and a 27.5% recurrence in type III tumor patients (P=0.19) (Figure 5). Patients with type II and type III tumors had a 5-year overall survival of 43.5%. The recurrence rate was 26%; 11 patients had local–regional relapse (32.4%), 23 (67.6%) had distant metastasis, 5 had relapse at 1 distant site (21.7%), 10 had relapse at 2 distant sites (43.5%), 6 had relapse at 3 distant sites (26.1%), and 2 patients had relapse at 4 or more sites (17.4%). No patients died post surgery.
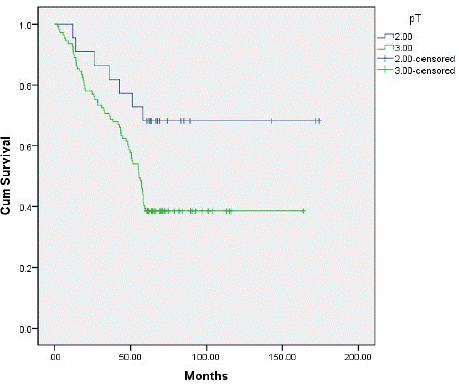
Figure 4: Illustration of the survival curves based on the T stage of] AEG patients. The 5-year OS rate for the T2 and T3 group was 68.2% and38.5%, respectively.
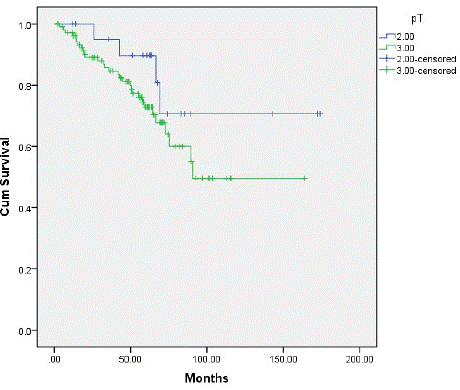
Figure 5: Illustration of the survival curves based on the T stage of the AEG patients. The 5-year recurrence rate for the T2 and T3 was 18.2% and 27.5%, respectively.
Discussion
An increase in EJA has been observed globally in recent years, particularly in Western and Asian countries. According to previous literature, the most common tumor types are types II and III [1-3,15,16]. Our retrospective study showed a slight increase in the prevalence of EJA in our hospital (data not shown) during the past 10 years, which is similar to findings reported in the literature. Our data revealed that patients with EJA had poorer survival outcomes than patients with distal gastric cancer because of the different tumor characteristics [17,18]. The optimal length of the proximal margin for EJA is still under debate, and the impact of EJA survival and recurrence remains unclear. Compared with subtotal esophagectomy, the proximal margin of patients undergoing extended gastrectomy should be shorter. Barbour et al. found that proximal margin length was a more significant prognostic factor in types II–IV tumors with N0–2 (P<0.01). However, the same cannot be said for patients with types II–IV N3 tumors (P=0.48). A proximal length of 3.8cm in resected specimens is considered an independent prognostic factor according to analyses limited to R0 or R1 resection. The proximal margin length was considered a prognostic factor between the esophagectomy group (5cm) and the gastrectomy group (2cm) and influenced the survival of patients with Siewert type I tumors [10]. Mine et al. found that, for patients with EJA with types II–IV tumors, a proximal margin length >2cm seemed to be associated with better survival (P=0.008). Type IV tumor patients are more likely to require neoadjuvant therapy. Thus, different surgical strategies can influence the proximal margin length. Barbour et al. performed used esophagectomy (69.7%), whereas Mine et al. exclusively used transhiatal extended gastrectomy [10,11]. Our research mainly focused on the effect of the proximal margin length on patients with EJA type II and III tumors, and we found that a gross proximal margin of >2cm was an independent prognostic factor for patients with EJA type II and III tumors undergoing radical surgery. Gross proximal margin lengths of 1.5, 2.5, 3, and 3.5cm had no statistically significant impact on survival. Patients with poor differentiation status seemed more prone to relapse and had a worse prognosis, according to our analysis, which was similar to previous literature [10,11,17,18]. However, Feng et al. and Ohe et al. found that a sufficient proximal margin was not an absolute factor related to survival and recurrence, and in the case of R0 resection, the distance between the free margin and tumor did not affect prognosis [19,20]. Squires et al. also demonstrated there are other pathological factors that affect survival other than the proximal margin [21].
We observed a 26% (34/131) recurrence rate, and tumor differentiation status was found to be the only significant prognostic factor of recurrence. Most recurrences were distant (23/34, 67.6%), and local–regional recurrences were relatively lower (11/34, 32.4%). According to Patrão et al., tumor differentiation status, pT stage were the strongest prognostic factors for poor outcome and relapse [22]. In their study, the relapse rate was 61% (108/177), with only 9 (8.3%) isolated cases of local–regional relapse with symptoms, whereas the majority of cases (99/108, 91.7%) presented with distant metastasis. In their study, Suh et al. had a recurrence rate of 30% after excluding type I cancers; distant metastasis was found to be more prevalent (14%), and only 4.1–0.6% of patients had local–regional recurrence [23]. This could be due to only suspicious clinical or laboratory findings undergoing more advanced imaging examinations, such as computed tomography, magnetic resonance imaging, and gastrointestinal endoscopy, and thus many asymptomatic local recurrences are missed due to a lack of timely imaging examinations. Therefore, we may be underestimated and cannot reliably describe the overall local recurrence rate. Almost all recurrences are only diagnosed when there are symptoms, which could be at a relatively late stage, and could explain why the incidence of isolated local metastases was relatively lower. These results suggest that effective systemic treatment is important, and to a large extent, represents the unmet needs of EJA. If we consider that our study only covers type II and type III tumor patients during the 5-year follow-up period, our data are different from those reported in the literature to some extent.
Our specimens were cut longitudinally and lymph nodes were removed for pathological examination, stretched to the maximum extent, and placed on plates. The total length of the proximal edge was determined by vision and touch, and was measured and recorded by the surgeon. If insufficient and additional distal esophagus was removed, we measured the total length of the proximal edge. Because of shrinkage of the specimens, these measured lengths did not true reflect the corresponding in situ lengths before the conclusion of the operation. In 1986, Siu et al. found that esophageal specimens shrunk to approximately half their length after resection, and the upper margin was reduced to a greater extent than the lower edge (44% vs 54% of in situ length, respectively) after resection and before fxation [18]. Thus, based on their findings, a proximal margin of 2 cm would be 4cm, and the cited minimal proximal margin ranging between 2 and 5cm would be between 4 and 10cm in situ.
Limitations
The present retrospective study had several limitations. First, we did not have accurate measurements of the proximal margin length due to shrinkage of the esophagus after resection, and due to the difference between observers; therefore, the lack of a centralized examination of pathological specimens may have led to deviation in the results. Second, the sample size used in the present study was small and was limited to a single institution; thus, more prospective studies are needed to verify our findings in the Chinese population. Finally, we did not evaluate the risk factors for the positive proximal margin, nor did we evaluate the effect of neo adjuvant therapy on the state of the proximal margin after resection.
Conclusion
There are a number of factors associated with recurrence and overall survival at 5years for patients who have EJA with type II and type III tumors, and a proximal margin of >2cm may indicate a better prognosis.
Funding
“Standing Young Medical Talents" supporting research funds, KJ012019439. Natural Science Foundation of Guangdong Province (Project 2 020A1515010573), and Accurate Prediction of gIST Risk Grading Based on CT Imaging omics.
Data Availability Statement
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Contributions
Conception and design: Y Li, Q Yan; (II) Administrative support: The Ethics Committee of Guangdong Provincial People’s Hospital;(III) Provision of study materials or patients: Q Yan, W Hu, J Zheng; (IV) Collection and assembly of data:Q Yan, W Hu, J Zheng,Z Lv;J Wang(V) Data analysis and interpretation: Q Yan, W Hu, J Zheng; (VI) Manuscript writing: All authors; (VII) Final approval of manuscript: All authors.
Ethics Declarations
Ethics approval and consent to participate
The Institutional Review Board approved this retrospective study and waived the need for written informed consent.
Statement
It has been presented as "PREPRINT" in Prognostic Significance of the Proximal Margin for Esophagogastric Junction Adenocarcinoma With Type II and III Tumors After Surgery according to the following link: https://www.researchsquare.com/article/rs-121091/v1.
References
- Devesa SS, Blot WJ, Fraumeni JF Jr. Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer. 1998; 83: 2049-53.
- Bollschweiler E, Wolfgarten E, Gutschow C. H patterns in the incidence of esophageal and gastric carcinoma in the United States. he lower marginollow Cancer. 2001; 92: 549.
- Steevens J, Botterweck AA, Dirx MJ, van den Brandt PA, Schouten LJ. Trends in incidence of oesophageal and stomach cancer subtypes in Europe. Eur J Gastroenterol Hepatol. 2010; 22: 669-78.
- Kusano C, Gotoda T, Khor CJ, Katai CJ, Kato H, et al. Changing trends in the proportion of adenocarcinoma of the esophagogastric junction in a large tertiary referral center in Japan.eJ Gastroenterol Hepatol. 2008; 23: 1662-5.
- Liu K, Yang K, Zhang W, Chen X, Chen X, et al. Changes of Esophagogastric Junctional Adenocarcinoma and Gastroesophageal Reflux Disease Among Surgical Patients During 1988-2012: A Single-institution, High-volume Experience in China. Ann Surg. 2016; 263: 88-95.
- Maric R, Cheng KK. Classification of adenocarcinoma of the oesophagogastric junction. Br J Surg. 1999; 86: 1098-9.
- Rice TW, Ishwaran H, Ferguson MK, Blackstone EH, Goldstraw P. Cancer of the esophagus and esophagogastric junction: an eigth edition staging primer. J Thorac Oncol. 2017; 12: 36-42.
- van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, Henegouwen MIB, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer.aN Engl J Med. 2012; 366: 2074-84.
- Mariette C, Piessen G, Briez N, Gronnier C, Triboulet JP, et al. Oesophagogastric junction adenocarcinoma: which therapeutic approach?. Lancet Oncol. 2011; 12: 296-305.
- Barbour AP, Rizk NP, Gonen M, Tang L, Bains MS, et al. Adenocarcinoma of the gastroesophageal junction: influence of esophageal resection margin and operative approach on outcome.oAnn Surg. 2007; 246: 1-8.
- Mine S, Sano T, Hiki N, Yamada K, Kosuga T, et al. Proximal margin length with transhiatal gastrectomy for Siewert type II and III adenocarcinomas of the oesophagogastric junction. Br J Surg. 2013; 100: 1050-4.
- Mattioli S, Di Simone MP, Ferruzzi L, D’Ovidio F, Pilotti V, et al. Surgical therapy for adenocarcinoma of the cardia: modalities of recurrence and extension of resection. Dis Esophagus. 2001; 14: 104-109.
- Mariette C, Castel B, Balon JM, Seuningen IV, Triboulet JP. Extent of oesophageal resection for adenocarcinoma of the oesophagogastric junction. Eur J Surg Oncol. 2003; 29: 588-93.
- Hasegawa S, Yoshikawa T. Adenocarcinoma of the esophagogastric junction: incidence, characteristics, and treatment strategies. Gastric Cancer. 2010; 13: 63-73.
- Zhang LH, Huang Q. Changes in incidences of gastric cardiac gastroesophageal junctional and sub-cardiac carcinomas in Nanjing of China: 20-year retrospective study from a single tertiary medical center. N Am J Med Sci. 2009; 2: 35–38.
- Dexter SP, Sue-Ling H, McMahon MJ, Quirke P, Mapstone N, et al. Circumferential resection margin involvement: an independent predictor of survival following surgery for oesophageal cancer. Gut. 2001; 48: 667-670.
- Shen JG, Cheong JH, Hyung WJ, Kim J, Choi SH, et al. Influence of a microscopic positive proximal margin in the treatment of gastric adenocarcinoma of the cardia.pWorld J Gastroenterol. 2006; 12: 3883-3886.
- Siu KF, Cheung HC, Wong J. Shrinkage of the esophagus after resection for carcinoma. Ann Surg. 1986; 203: 173-176.
- Feng F, Tian Y, Xu G, Liu S, Liu Z, et al. The length of proximal margin does not influence the prognosis of Siewert type II/III adenocarcinoma of esophagogastric junction after transhiatal curative gastrectomy. Springerplus. 2016; 5: 588.
- Ohe H, Lee WY, Hong SW, Chang YG, Lee B. Prognostic value of the distance of proximal resection margin in patients who have undergone curative gastric cancer surgery. World J Surg Oncol. 2014; 12: 296.
- Squires MH 3rd, Kooby DA, Pawlik TM, Weber SM, Poultsides G, et al. Utility of the proximal margin frozen section for resection of gastric adenocarcinoma: a 7-Institution Study of the US Gastric Cancer Collaborative. Ann Surg Oncol. 2014; 21: 4202-10.
- Patr AS, Papaxoinis G, Kordatou Z, Weaver JM, Holt VO, et al. Prognostic significance of positive circumferential resection margin post neoadjuvant chemotherapy in patients with esophageal or gastro-esophageal junction adenocarcinoma. Eur J Surg Oncol. 2019; 45: 439-445.
- Suh YS, Lee KG, Oh SY, Kong SH, Lee HJ, et al.Recurrence Pattern and Lymph Node Metastasis of Adenocarcinoma at the Esophagogastric Junction. Ann Surg Oncol. 2017; 24: 3631-3639.
- Yan Q, Hu W, Zheng J, Lv Z, Wang J, et al. Prognostic Significance of the Proximal Margin for Esophagogastric Junction Adenocarcinoma With Type II and III Tumors After Surgery. Research Square.