
Special Article: Tumor Surgery
Austin J Surg. 2024; 11(1): 1317.
Sudden Disruptions in Motor Evoked Responses: Unraveling Complexities in Intradural Dorsal Meningioma Resection
Guarrera B¹*; Giarletta M²
¹Department of Neuroscience, Ospedale dell’Angelo-Mestre, Mestre, Italy
²Department of Neurosurgery, Sant’Andrea University Hospital, Roma, Italy
*Corresponding author: Brando Guarrera Department of Neuroscience, Ospedale dell’Angelo-Mestre, Mestre, Italy. Email: brandoguarrera@gmail.com
Received: February 09, 2024 Accepted: March 15, 2024 Published: March 22, 2024
Abstract
In the epoch of neuromonitoring advancements, the surgical resection of spinal intradural lesions is meticulously guided by Intraoperative Neurophysiological Monitoring (IONM), specifically targeting Motor Evoked Potentials (MEP). Within this context, we present an intriguing singular case involving a patient in their seventies undergoing surgical intervention for a sizable dorsal intradural juxtamedullary meningioma.
Throughout the surgical procedure, a sudden cessation of motor evoked potentials manifested bilaterally in the lower limbs, swiftly succeeded by a resurgence of normal responses confined exclusively to the right side, with a conspicuous absence on the left. Following the awakening phase, the patient exhibited a transient complete monoplegia of the left inferior limb persisting for 30 minutes, promptly resolving with a swift restoration of the overall neurological status.
Background
Spinal meningiomas stand out as the predominant spinal tumors in the adult population [1,2]. The majority of these meningiomas are benign, exhibiting no histopathological disparities between intracranial and intraspinal counterparts [3]. However, certain aggressive subtypes of spinal meningiomas correlate with more adverse surgical and functional outcomes [4]. Despite the proven efficacy of Intraoperative Neurophysiological Monitoring (IONM) in minimizing the risks of post-surgical neurological deterioration for spinal meningiomas [5], its clinical effectiveness in the context of intradural extramedullary tumors remains indeterminate. The monitoring scope for intramedullary lesions is clearly defined [6,7]. In a recent meta-analysis addressing complication avoidance in the resection of spinal meningiomas [8], IONM was incorporated in 4 out of 16 surgical series, with universal usage in only 1 [9]. Notwithstanding meticulous IONM application, unfortunate and unexpected events can manifest. For many cases, particularly in our patient's circumstance, unraveling a plausible explanation for these events remains a formidable medical challenge.
Case Presentation
Clinical History and Examination
We present a complex case involving a 70-year-old female who presented to our department with a nuanced medical history. The patient reported various forms of paresthesia in both feet, encompassing sensations of burning, tingling, and stinging that manifested a year prior. Over subsequent months, a progressive deterioration in gait coordination ensued, culminating in an inability to maintain an upright position. Additionally, the patient described a bar-like sensation of chest constriction, coupled with urinary urgency without incontinence. Upon neurological examination, manifestations included gait ataxia, spastic paraparesis, hyperreflexia of bilateral patellar tendons, and dysesthesia, predominantly affecting the right limb. Notably, no segmental motor deficit was identified, and the neurological dysfunction was classified as McCormack grade 3 [10]. To unravel the complexities of the presented case, an urgent spine contrast MRI was meticulously conducted. The imaging unveiled a substantial and solid intradural extramedullary lesion, characterized by marked contrast enhancement. Positioned at the fourth thoracic vertebra level, the lesion raised suspicions of a left dorsal-ventral-lateral meningioma. Occupying 5/6 of the spinal canal, the tumor elicited significant spinal cord compression with discernible displacement to the right side (Figure 1).
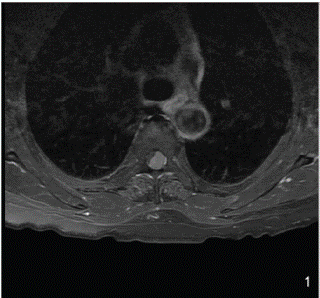
Figure 1:
Operative Procedure and Unanticipated Discoveries:
The operative intervention incorporated a comprehensive Intraoperative Neurophysiological Monitoring (IONM), encompassing:
- Transcranial Motor Evoked Potentials (Tc-MEP): Utilizing 5-7 stimuli administered through electrodes on the surface of the head (ranging from 90 mA to 200 mA), motor responses were elicited in bilateral tibialis anterior and abductor hallucis muscles. Additionally, motor responses were recorded in the right abductor pollicis brevis muscle to monitor the effects of anesthesia and blood pressure.
- Somatosensory Evoked Potentials (SSEP): Stimulation of the bilateral posterior tibial nerve facilitated the recording of transcranial cortical responses (P40 potentials).
Under general anesthesia, with the patient positioned in ventral decubitus, a midline skin incision was meticulously executed, followed by a T3-T5 laminectomy. Post-dural incision, the expansive meningioma manifested with a notably soft consistency. The procedural sequence involved devascularization from the internal dural surface, progressive reduction of tumor volume from the left to the right side, and ultimately, meticulous separation of the remaining meningioma portion without manipulation or traction of the spinal cord, adhering to an optimal dissection plan.
Upon complete meningioma resection, an unexpected loss of left lower limb MEPs (tibialis anterior and abductor hallucis muscles) and right anterior tibialis MEPs was noted. Intriguingly, a series of 5-7 stimuli, reaching up to 200 mA, failed to evoke any motor responses. Swift intervention through warm irrigation and the administration of 1 gram of Methylprednisolone led to the reappearance of right anterior tibialis MEPs (Figure 2). This electrical status remained consistent until the conclusion of the surgical procedure, with left lower limb MEPs re-emerging approximately 40 minutes later. Importantly, no variations in right abductor hallucis and right abductor pollicis brevis muscle MEPs, as well as bilateral posterior tibial nerve SSEPs, were recorded throughout the operation.
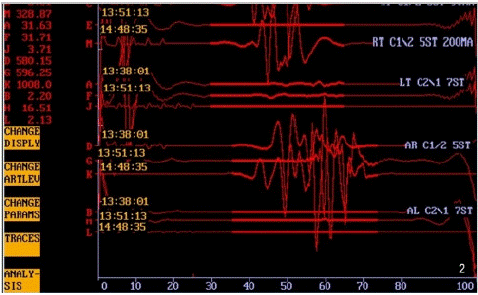
Figure 2:
Histological scrutiny validated the diagnosis of a WHO grade I meningioma.
Outcome and Follow-Up
Following the patient's emergence from anesthesia, the neurological assessment unveiled a transient state of complete monoplegia in the left lower limb, enduring for approximately 30 minutes. This was succeeded by a rapid and progressive recuperation of motor function, ultimately resulting in a subtle monoparesis graded at 4/5 on the MRC Muscle Power Scale. A postoperative spine contrast MRI, conducted 48 hours post-surgery, showcased a comprehensive re-expansion of the spinal cord following the successful total resection of the tumor, without any discernible complications. Upon a follow-up MRI six months later, the absence of tumor recurrence was confirmed, although the mild hemiparesis persisted without noteworthy alterations.
Discussion
It is common knowledge that a transient or permanent postoperative motor deficit could occur in case of loss of Motor Evoked Potentials (MEPs) during Intraoperative Neurophysiological Monitoring (IONM) in the resection of intramedullary lesions [11]. The D-wave analysis permits to evaluate of the direct activation of fast-conducting fibres in the cortico-spinal tract [12]; considering the surgery of intramedullary tumor, less or more than 50% on the decrease in its amplitude revests an important predictor value of neurological damage.
D-wave analysis as a predictive and preventive element of post-operative neurological damage is not clear in patients who underwent surgery as a treatment for an intradural extramedullary lesion, as in the case of our patient: only a few authors show how the application of D-wave could assist the resection of spinal meningiomas [13,14].
Considering the important displacement of the spinal cord of our patient, we prefer to not apply D-wave analysis; instead, MEPs recording was accurate and highly predictive.
The first atypical event consisted of the sudden loss of bilateral lower limbs MEPs after resection of meningioma. No surgical procedure was in progress at the time of the spinal cord electrical stupor recording, and no traumatic manipulation of the spinal cord was provided during the surgery: the dissection plane permitted a gentle dissection between the meningioma and the medial surface of the dorsal spinal cord.
The second atypical event was the extremally rapid clinical recovery of left lower limb motor deficit after awakening. Some patients showed a transient worsening in neurological deficits after intradural extramedullary tumor resection, typically secondary to vasogenic edema or as a result of dissection, with resolution after on average 6 months [15,16]. The neurological exam after awakening showed a left lower limb complete monoplegia persisting for about 30 minutes, followed by a rapid progressive recovery of the motor function until a faint monoparesis. The postoperative spine contrast MRI acquired 48 hours after the surgical procedure showed a total spinal cord re-expansion of the gross total resection of the tumor without complications.
We sustain 2 different theories to explain these atypical events:
• Reperfusion injury: the intradural juxtamedullary space, which became free as a consequence of the resection of the dorsal meningioma, permitted the re-expansion of the spinal cord in this cavity; it probably produced temporary oxidative stress as a consequence of the rapid revascularization of the compressed medullary segment. The clinical manifestation was a temporary medullary stupor. This theory appears less plausible because benign meningioma is a slow-growing tumor, and reperfusion injury is more frequent after resection of rapidly compressing causes.
• Cavitational effect: the rapid stretching of axons related to rapid revascularization, at the same as cerebral concussion [17] but in this case as the results of the re-expansion of the spinal cord, could cause an altered membrane conductivity, dysfunction in glucose metabolism, and a mitochondrial metabolic blockade [18]; this explains an energetic interruption of nervous conduction. Giza et al. [19,20] showed how the mitochondrial metabolic dysfunction, determined by calcium sequestration into mitochondria, provokes low production of ATP and a dysfunction of sodium–potassium pump, triggering a consequences cytoplasmatic molecular cascade that exacerbates problems related to oxidative stress and the cellular energetic crisis.
To the best of our knowledge, considering the note limitations of IONM, no authors has ever described a case of loss of MEPs during the resection of intradural extramedullary lesion, corresponding in a single transient neurological deficit with a rapid resolution. In these cases, our experience suggests that if the neurological deficit cannot be explained by a macroscopical surgical injury, such as spinal cord resection or traumatic manipulation, they may be transient with even an almost complete recovery.
Learning Points/Take Home Messages
- IONM represents an indispensable guide in surgical treatment, especially in neurooncological spine surgery.
- Sudden IONM modifications without a clear explanation, are often sustained by a real clinical change.
All events recorded by IONM show different meanings: true positive or false positive have to be interpreted considering the level and the site of the lesion, and clinical results after surgery.
References
- Kshettry VR, Hsieh JK, Ostrom QT, Kruchko C, Benzel EC, Barnholtz-Sloan JS. Descriptive Epidemiology of Spinal Meningiomas in the United States. Spine (Phila Pa 1976). 2015; 40: E886-9.
- Ravindra VM, Schmidt MH: Management of spinal meningiomas. Neurosurg Clin N Am. 2015; 27: 195-205.
- Gottfried ON, Gluf W, Quinones-Hinojosa A, Kan P, Schmidt MH: Spinal meningiomas: surgical management and outcome. Neurosurg Focus. 2003; 14: e2.
- Schaller B: Spinal meningioma: relationship between histological subtypes and surgical outcome? J Neurooncol. 2005; 75: 157-61.
- King AT, Sharr MM, Gullan RW, Bartlett JR: Spinal meningiomas: a 20-year review. Br J Neurosurg. 1998; 12: 521-6.
- Kothbauer K, Deletis V, Epstein FJ: Intraoperative spinal cord monitoring for intramedullary surgery: an essential adjunct. Pediatr Neurosurg. 1997; 26: 247-54.
- Kothbauer KF: Intraoperative neurophysiologic monitoring for intramedullary spinal-cord tumor surgery. Neurophysiol Clin. 2007; 37: 407-14.
- Westwick HJ, Yuh SJ, Shamji MF: Complication avoidance in the resection of spinal meningiomas. World Neurosurg. 2015; 83: 627-34.
- Sandalcioglu IE1, Hunold A, Müller O, Bassiouni H, Stolke D, Asgari S: Spinal meningiomas: critical review of 131 surgically treated patients. Eur Spine J. 2008; 17: 1035-41.
- McCormick PC, Torres R, Post KD, Stein BM: Intramedullary ependymoma of the spinal cord. J Neurosurg. 1990; 72: 523-32.
- Kothbauer KF1, Deletis V, Epstein FJ: Motor-evoked potential monitoring for intramedullary spinal cord tumor surgery: correlation of clinical and neurophysiological data in a series of 100 consecutive procedures. Neurosurg Focus. 1998; 4: e1.
- Deletis V, Sala F: Intraoperative neurophysiological monitoring of the spinal cord during spinal cord and spine surgery: a review focus on the corticospinal tracts. Clin Neurophysiol. 2008; 119: 248-64.
- Korn A, Halevi D, Lidar Z, Biron T, Ekstein P, Constantini S: Intraoperative neurophysiological monitoring during resection of intradural extramedullary spinal cord tumors: experience with 100 cases. Acta Neurochir. 2015; 157: 819–830.
- Harel R, Schleifer D, Appel S, Attia M, Cohen ZR, Knoller N: Spinal intradural extramedullary tumors: the value of intraoperative neurophysiologic monitoring on surgical outcome. Neurosurg Rev. 2017; 40: 613-619.
- Roux FX, Nataf F, Pinaudeau M, et al: Intraspinal meningiomas: review of 54 cases with discussion of poor prognosis factors and modern therapeutic management. Surg Neurol. 1996; 46: 458–464.
- Klekamp J, Samii M: Surgical results for spinal meningiomas. Surg Neurol. 1999; 52: 552–562.
- Banks RE, Dominguez DC. Sports-related concussion: Neurometabolic aspects. Semin Speech Lang. 2019; 40: 333-343.
- Barkhoudarian G, Hovda DA, Giza CC: The Molecular Pathophysiology of Concussive Brain Injury - an Update. Phys Med Rehabil Clin N Am. 2016; 27: 373-93.
- Giza CC, Hovda DA. The Neurometabolic Cascade of Concussion. J Athl Train. 2001; 36: 228-235.
- Giza CC, Hovda DA: The new neurometabolic cascade of concussion. Neurosurgery. 2014; 75: S24-33.