
Special Article: Cardiac Surgery
Austin J Surg. 2024; 11(2): 1326.
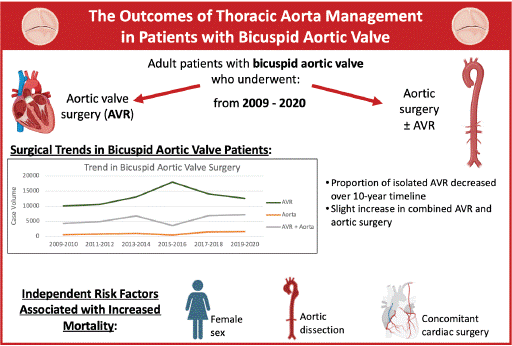
The Outcomes of Thoracic Aorta Management in Patients with Bicuspid Aortic Valve
Ahmed Alnajar, MD, MSPH¹*; Abdul Kabir Khan, BS¹; Kelley N Benck, BS¹; Ibrahim Khan²; Tawseef Dar, MD¹; Sameer A Hirji, MD, MPH³
¹Division of Cardiothoracic Surgery, DeWitt Daughtry Department of Surgery, University of Miami Miller School of Medicine, Miami, FL, USA
²Pomona College, Claremont, CA, USA
³Division of Cardiothoracic Surgery, Brigham and Women’s Hospital, Allston, MA, USA
*Corresponding author: Ahmed Alnajar, MD, MSPH Division of Cardiothoracic Surgery, DeWitt Daughtry Department of Surgery, University of Miami Miller School of Medicine, Miami, FL, USA. Email: alnajarmd@gmail.com
Received: July 10, 2024 Accepted: July 30, 2024 Published: August 06, 2024
Abstract
Objective: We aimed to evaluate recent trends and outcomes of thoracic aortic intervention in comparison to isolated Aortic Valve Surgery (AVR) in patients with a Bicuspid Aortic Valve (BAV).
Methods: Patients with BAV from the National Inpatient Sample (2009-2020) who underwent thoracic aortic and/or aortic valve surgery were identified. Covariates included age, sex, aortic aneurysm, dissection, concomitant comorbidities, concomitant surgeries, insurance, and hospital status. A sensitivity analysis was performed in patients without isolated aortic surgery, endocarditis, aortic dissection, or non-elective admission.
Results: There were 122,104 patients with BAV, of which 38,973 underwent direct aortic surgery with or without AVR while 83,129 underwent isolated AVR. The proportion of AVR decreased from 68% in 2009/2010 to 61% in 2019/2020, with a peak of 82% in 2015/2016. In aortic surgery patients, mortality risk increased by 86% (aOR:1.86, CI:1.29-2.69), with an independent increased risk of mortality for females by 35% (aOR:1.35, CI: 1.04-1.73), aortic dissection by 5-fold (aOR:5.10, CI:3.06-8.48), and concomitant cardiac surgery by 101% (aOR:2.01, CI:1.55-2.63). After excluding patients for the sensitivity analysis, female sex was no longer associated with higher mortality risk. In-hospital complications such as stroke, sternal wound complications, bleeding, cardiac arrest, and respiratory complications were all higher in direct aortic surgery patients.
Conclusion: Direct aortic surgery management for BAV has been increasingly advised. As expected, in-hospital mortality and complications were more frequent in aortic surgery. Continued effort to select on patients who would benefit from addressing aortopathy at the time of their index operation is important.
Central Message: In-hospital outcomes of aortic surgery for bicuspid aortic valve indicate the need for better decision making in prophylactic surgery.
Perspective Statement: The increased adoption of aortic surgery for bicuspid aortic valve does not result in favorable outcomes; however, these could be balanced by better long-term outcomes. While the current guidelines are not definitive regarding aortic size, understanding particular patients’ risk factors, characteristics, and possible complications can help surgeons decide the optimal treatment plan for their patients.
Central Picture legend: National trends of aortic valve, aortic surgery, or both over 12 years.
Keywords: Adult cardiac; Aortic; Bicuspid; Mortality; Thoracic aorta; Congenital
Abbreviations: BAV: Bicuspid Aortic Valve; AVR: Aortic valve surgery (repair or replacement); ICD: International Classification of Disease used to classify diseases, injuries, and procedures; LOS: Length of stay; TAVR: Transcatheter aortic valve replacement; CI: Confident interval; MI: Myocardial infarction; CKD: Chronic kidney disease; CHF: Congestive heart failure; CAD: Coronary artery disease; OR: Odds Ratio; aOR: Adjusted odd ratios; FET: Frozen elephant trunks; CABG: Coronary artery bypass grafting; DM: Diabetes mellitus
Introduction
Bicuspid Aortic Valve (BAV) is the most common congenital heart abnormality and is frequently associated with aortopathy [1,2], which could lead to aneurysms, dissections, or ruptures [3]. In addition, people with BAV also have a higher rate of aortic growth than people with tricuspid aortic valves [4]. Therefore, while the decision to perform ascending aortic surgery carries significant hope for these patients, it also brings significant increased perioperative risk.
The guidelines of the American College of Cardiology (ACC) and the American Heart Association (AHA) recommend immediate thoracic aortic surgery in patients with aortopathy based on aortic dilatation size, which has led to a major shift in approaching patients with BAV [5]. Since surgical techniques have improved and the guidelines have been refined over the last two decades, we aimed to assess outcomes and trends in patients with BAV who undergo ascending aortic surgery or isolated Aortic Valve Surgery (AVR) or over 12 years, from 2009 through 2020.
Methods
Data Source
A retrospective analysis was conducted using discharge data from the Health Care Cost and Utilization Project's (HCUP) National Inpatient Sample (NIS). The NIS is the largest publicly available, all-payer, nationally representative hospital discharge database in the United States. The NIS dataset constitutes a 20% stratified sample of US hospitals. Data from the NIS can be used as an estimate of the total hospitalized population. This database has already been used to study aspects of patients’ hospitalization for BAV operations [5,7]. This study was considered exempt from institutional review board approval because the NIS deidentifies patient information.
Study Population
This study included adult (age 18+ years) patients with BAV who underwent aortic and/or AVR from January 1, 2009, to December 31, 2020. Patient characteristics and procedure details were identified using the International Classification of Diseases, Ninth and Tenth Revision (ICD-9 and ICD-10) codes. A summary of the relevant ICD codes is in Supplementary Table 1. Patient characteristics included age, sex, year of surgery, medical history of aneurysm, previous valve surgery, concomitant surgery, primary insurance type, teaching hospital status, and Elixhauser Score.
Characteristic
Overall
N = 122,1041AVR
N = 83,1291Aortic surgery
N = 38,9751p-value2
Age (Years)
59 (51, 67)
60 (52, 67)
58 (48, 65)
<0.001
Sex
<0.001
Female
32,094 (26%)
23,375 (28%)
8,719 (22%)
Male
89,994 (74%)
59,744 (72%)
30,251 (78%)
Unknown
15
10
5
Surgery Year
2015 (2012, 2018)
2015 (2012, 2018)
2015 (2012, 2018)
0.006
Weighted Elixhauser Score
7 (2, 13)
7 (2, 12)
8 (4, 13)
<0.001
HTN
>0.9
No
48,079 (39%)
32,723 (39%)
15,356 (39%)
Yes
74,025 (61%)
50,406 (61%)
23,619 (61%)
DM
<0.001
No
100,061 (82%)
66,188 (80%)
33,873 (87%)
Yes
22,043 (18%)
16,940 (20%)
5,102 (13%)
Previous MI
0.001
No
117,357 (96%)
79,654 (96%)
37,703 (97%)
Yes
4,747 (3.9%)
3,475 (4.2%)
1,272 (3.3%)
PAD
0.4
No
117,516 (96%)
79,953 (96%)
37,564 (96%)
Yes
4,587 (3.8%)
3,176 (3.8%)
1,411 (3.6%)
Endocarditis
<0.001
No
117,761 (96%)
79,473 (96%)
38,288 (98%)
Yes
4,342 (3.6%)
3,656 (4.4%)
687 (1.8%)
TIA
0.030
No
119,131 (98%)
80,975 (97%)
38,156 (98%)
Yes
2,972 (2.4%)
2,153 (2.6%)
819 (2.1%)
Stroke
0.001
No
115,682 (95%)
78,483 (94%)
37,199 (95%)
Yes
6,422 (5.3%)
4,646 (5.6%)
1,776 (4.6%)
CKD
<0.001
No
111,275 (91%)
75,212 (90%)
36,063 (93%)
Yes
10,829 (8.9%)
7,917 (9.5%)
2,912 (7.5%)
CHF
<0.001
No
88,443 (72%)
58,447 (70%)
29,997 (77%)
Yes
33,660 (28%)
24,682 (30%)
8,978 (23%)
CAD
<0.001
No
92,964 (76%)
61,839 (74%)
31,125 (80%)
Yes
29,140 (24%)
21,290 (26%)
7,850 (20%)
Concomitant Surgery
<0.001
No concomitant CABG or valve
101,794 (83%)
68,114 (82%)
33,680 (86%)
+CABG
14,658 (12%)
10,752 (13%)
3,905 (10%)
+MVR
3,826 (3.1%)
3,024 (3.6%)
802 (2.1%)
+TVR
565 (0.5%)
421 (0.5%)
144 (0.4%)
Other
1,261 (1.0%)
817 (1.0%)
443 (1.1%)
Previous Valve Surgery
0.5
No
120,848 (99%)
82,300 (99%)
38,548 (99%)
Yes
1,256 (1.0%)
829 (1.0%)
427 (1.1%)
Teaching Hospital
<0.001
No
19,110 (16%)
14,009 (17%)
5,100 (13%)
Yes
102,994 (84%)
69,119 (83%)
33,874 (87%)
Elective Admission
<0.001
No
24,563 (20%)
17,602 (21%)
6,961 (18%)
Yes
97,288 (80%)
65,327 (79%)
31,960 (82%)
Unknown
253
199
53
Primary Insurance
<0.001
Medicare
38,905 (32%)
28,183 (34%)
10,722 (28%)
Medicaid/No insurance
12,740 (10%)
9,121 (11%)
3,619 (9.3%)
Private
66,533 (55%)
43,257 (52%)
23,277 (60%)
Other
3,739 (3.1%)
2,415 (2.9%)
1,324 (3.4%)
Unknown
186
153
34
1Median (IQR); n (%)
2Wilcoxon rank-sum test for complex survey samples; chi-squared test with Rao & Scott's second-order correction
*This could reveal information about cell size=10 (protected by HCUP).
AVR: Isolated aortic valve surgery; AVRe: Aortic valve repair; CABG: Coronary artery bypass grafting; CAD: Coronary artery disease; CHF: Congestive heart failure; CKD: Chronic kidney disease; DM: Diabetes mellitus; HTN: Hypertension; MVR: Mitral valve regurgitation; PAD: Peripheral arterial disease; TIA: Transient ischemic attack.
Table 1: Baseline characteristics.
Study Outcomes
The primary outcome of interest was in-hospital mortality. Secondary outcomes included perioperative complications (such as acute myocardial infarction, stroke, major bleeding, and acute kidney injury), discharge disposition, hospital Length of Stay (LOS), and hospitalization cost.
Analysis Methods
Using survey analysis methods, we generated weighted national estimates and variances that accounted for the clustering of outcomes within hospitals and sampling variation across strata (region and year) as recommended by AHRQ to describe patients' characteristics and outcomes (Supplementary analysis code) [8]. Observation weight was then incorporated into subsequent models. Descriptive statistics were presented as frequencies for categorical variables and medians (with an interquartile range) for continuous variables after normality assessment with histograms and QQ plots. Chi-square and Wilcoxon rank-sum tests for survey samples were used to compare groups. To determine which risk factors were associated with in-hospital mortality, observations were omitted if they were missing mortality status (unweighted n=6) or had transcatheter aortic valve replacement (TAVR, unweighted n=22), due to the controversial rule of earlier TAVR devices in patients with BAV [9]. Then, univariable (unadjusted) and multivariable (adjusted) logistic regression analyses were performed following multicollinearity assessment (with the variance inflation factor) and imputations (missing values of each variable before imputation were presented in a separate category in Tables 1 and 3). A sensitivity analysis for adjusted odds of mortality was performed in patients without isolated aortic surgery, endocarditis, dissection, or non-elective admission. The analysis was performed using R (4.2.2 [2022-10-31 ucrt], R Foundation for Statistical Computing, Vienna, Austria) with multiple packages, including `comorbidity`, `gtsummary`, and `survey` (Supplementary references). Significant associations were determined using a=0.05, and P values <0.05 were considered statistically significant. For reproducibility, the analysis code and output knitted from the R Markdown file can be accessed in the supplementary analysis code document.
Results
The sample was comprised of 24,705 encounters with a nationally weighted estimate of 122,104 patients. Of the sample, 68% (n=83,139) patients underwent isolated AVR and 32% (n=38,975) underwent aortic surgery with or without AVR. Isolated aortic surgery accounted for 4.6% of patients (n=5,614). From 2009 through 2020, there were changes in the proportions of aortic surgery throughout the years compared to isolated AVR (Figure 1), where isolated AVR decreased from 68% in 2009/2010 (n=10,695) to 61% in 2019/2020 (n= 13,760), with a peak of 82% in 2015/2016 (n=17,905). The corresponding rates of aortic surgery were 32% in 2009/2010 (n= 5,051), 39% in 2019/2020 (n= 8,775), and 18% in 2015/2016 (n= 3,880).
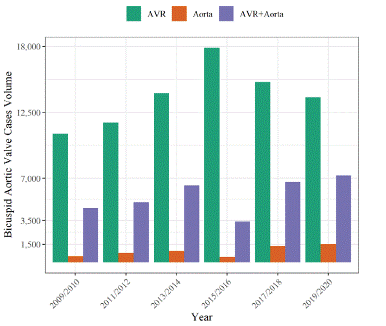
Figure 1: Temporal trends of aortic valve, aortic surgery, or both between 2009 and 2020.
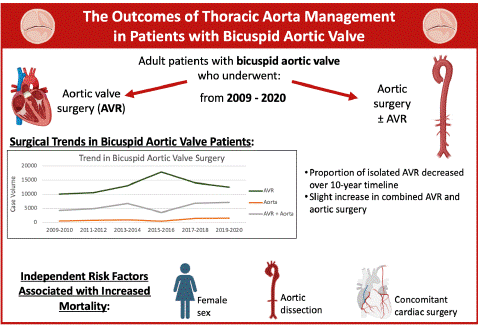
Figure 2: Graphical abstract (Created with BioRender.com).
Patient Characteristics
The median age of patients was 59 years (Interquartile Range [IQR] of 51 to 67) with a minority of patients being female (26%). Isolated AVR surgery was associated with significantly younger patients and a greater proportion of women compared to aortic surgery (p<0.001). Patients undergoing isolated AVR were likely to have a lower Elixhauser Comorbidities Index score than those undergoing aortic surgery (p<0.001). However, patients receiving isolated AVR were more likely to have a history of previous myocardial infarction (p<0.001), endocarditis (p<0.001), stroke (p<0.001), chronic kidney disease (p<0.001), congestive heart failure (p<0.001), or coronary artery disease (p<0.001) than patients undergoing aortic surgery. Most patients had an elective admission (80%), had surgery at a teaching hospital (84%), and had primary coverage through private insurance (55%). Patients undergoing isolated AVR were less likely to have been admitted electively (p<0.001), and less likely to have been admitted to a teaching hospital (p<0.001) compared to patients undergoing aortic surgery. Patient characteristics are summarized in Table 1.
Primary Outcome
The overall in-hospital mortality rate was 1.3% (n=1,609). After removing patients who had missing mortality status and TAVR, the sample was comprised of 24,677 encounters representing 121,964 patients with the same mortality rate. In the sensitivity analysis group, the sample of patients without isolated aortic surgery, urgent admission, aortic dissection, or endocarditis was comprised of 18,518 encounters representing 91,549 patients with a mortality rate of 0.9% (n=863).
In the unadjusted model, risk factors included aortic surgery (36% higher mortality risk than isolated AVR [OR:1.36, CI:1.08-1.71]), aortic dissection (higher mortality risk of tenfold than no dissection [OR: 10.0, CI:7.17-14.1]), endocarditis, concomitant surgery, Medicaid or no insurance, and higher Elixhauser score. Protective factors included aneurysms, teaching hospitals, private insurance, and elective admission. Surgery year was not significantly associated with mortality (Table 2A).
Overall, N =121,964
Characteristic
OR1
95% CI1
p-value
Surgery
AVR
–
–
Aortic surgery
1.36
1.08, 1.71
0.008
Age (Years)
1.01
1.00, 1.02
0.14
Sex (female)
1.17
0.93, 1.49
0.2
Primary Insurance
Medicare
–
–
Medicaid/No insurance
1.54
1.14, 2.08
0.005
Private
0.59
0.46, 0.75
<0.001
Other
0.66
0.32, 1.36
0.3
Surgery Year
1.02
0.99, 1.05
0.2
Elective Admission
0.35
0.28, 0.43
<0.001
Endocarditis
3.02
2.09, 4.37
<0.001
Dissection
10.0
7.17, 14.1
<0.001
Aneurysm
0.57
0.44, 0.73
<0.001
Concomitant Surgery
2.69
2.15, 3.38
<0.001
Previous Valve Surgery
1.53
0.62, 3.74
0.4
Teaching Hospital
0.71
0.55, 0.93
0.014
Weighted Elixhauser Score
1.13
1.12, 1.14
<0.001
1OR: Odds Ratio; CI: Confidence Interval; AVR: Isolated aortic valve surgery
Table 2: A) Univariable (unadjusted) logistic regression model assessing mortality risk.
In the final adjusted model (Table 2B), aortic surgery was associated 86% increased risk in mortality (aOR:1.86, CI:1.29-2.69), significantly more than isolated AVR. Excluding patients with isolated aortic surgery, non-elective admission, endocarditis, and aortic dissection (sensitivity analysis group) yielded a higher mortality risk of 115% (OR: 2.15, CI: 1.26-3.66). Patients with an aortic dissection had a fivefold increased mortality risk (aOR:5.10, CI: 3.06-8.48). Concomitant surgery had increased risk with a twofold increased mortality risk (aOR:2.01, CI: 1.55-2.63). Other factors associated with mortality included female sex, with a 35% greater risk of in-hospital mortality (aOR:1.35, CI: 1.04-1.73), but this risk was not significant in the sensitivity analysis group (aOR:1.19, CI: 0.83-1.71). Primary insurance coverage types were not significantly associated with in-hospital mortality. The status of the admitting hospital as a teaching hospital was associated with a significant decrease in mortality by 34% of all patients, both the main analysis and sensitivity analysis groups (aOR:0.66, CI: 0.48-0.89). Elective admission did not have a significant impact on in-hospital mortality (aOR:0.95, CI: 0.71-1.26).
Multivariable module
N = 121,964Sensitivity analysis*
N = 91,549Characteristic
OR1
95% CI1
p-value
OR1
95% CI1
p-value
Surgery
AVR
–
–
–
–
Aortic surgery
1.86
1.29, 2.69
<0.001
2.15
1.26, 3.66
0.005
Age (Years)
1.00
0.98, 1.01
0.6
1.00
0.98, 1.02
0.7
Sex (female)
1.35
1.04, 1.73
0.022
1.19
0.83, 1.71
0.4
Primary Insurance
Medicare
–
–
–
–
Medicaid/No insurance
1.38
0.94, 2.04
0.10
1.32
0.70, 2.48
0.4
Private
0.84
0.61, 1.14
0.3
0.78
0.52, 1.17
0.2
Other
0.79
0.37, 1.68
0.5
0.78
0.28, 2.22
0.6
Surgery Year
1.01
0.98, 1.05
0.5
1.01
0.96, 1.06
0.7
Elective Admission
0.95
0.71, 1.26
0.7
X
X
X
Endocarditis
1.45
0.92, 2.27
0.11
X
X
X
Dissection
5.10
3.06, 8.48
<0.001
X
X
X
Aneurysm
0.47
0.32, 0.69
<0.001
0.46
0.27, 0.79
0.005
Concomitant Surgery
2.01
1.55, 2.63
<0.001
1.93
1.34, 2.76
<0.001
Previous Valve Surgery
1.12
0.41, 3.05
0.8
1.75
0.55, 5.56
0.3
Teaching Hospital
0.66
0.48, 0.89
0.007
0.48
0.33, 0.71
<0.001
Weighted Elixhauser Score
1.12
1.10, 1.13
<0.001
1.14
1.12, 1.16
<0.001
1OR = Odds Ratio, CI = Confidence Interval
*Patients without Isolated Aortic/Urgent/Dissection/Endocarditis
AVR: Isolated aortic valve surgery
Table 2: B) Multivariable (adjusted) logistic regression model assessing mortality risk.
Characteristic
Overall
N = 122,1041AVR
N = 83,1291Aortic surgery
N = 38,9751p-value
In-Hospital Mortality
0.0092
No
120,465 (99%)
82,125 (99%)
38,340 (98%)
Yes
1,609 (1.3%)
984 (1.2%)
625 (1.6%)
Unknown
30
20
10
Stroke
0.0092
No
121,258 (99%)
82,634 (99%)
38,624 (99%)
Yes
846 (0.7%)
495 (0.6%)
351 (0.9%)
Wound Complications
0.0492
No
121,869 (100%)
82,999 (100%)
38,870 (100%)
Yes
235 (0.2%)
130 (0.2%)
105 (0.3%)
Valve Complications
0.72
No
121,044 (99%)
82,394 (99%)
38,650 (99%)
Yes
1,060 (0.9%)
735 (0.9%)
325 (0.8%)
Bleeding Complications
0.0232
No
63,155 (52%)
43,516 (52%)
19,639 (50%)
Yes
58,949 (48%)
39,613 (48%)
19,336 (50%)
Transfusion for Bleeding
0.42
No
97,767 (80%)
66,706 (80%)
31,061 (80%)
Yes
24,337 (20%)
16,423 (20%)
7,914 (20%)
Cardiac Arrest
<0.0012
No
117,410 (96%)
80,359 (97%)
37,051 (95%)
Yes
4,693 (3.8%)
2,770 (3.3%)
1,924 (4.9%)
Acute Renal Failure
0.52
No
107,709 (88%)
73,412 (88%)
34,297 (88%)
Yes
14,395 (12%)
9,717 (12%)
4,678 (12%)
Respiratory Complications
0.0022
No
109,170 (89%)
74,713 (90%)
34,457 (88%)
Yes
12,934 (11%)
8,416 (10%)
4,518 (12%)
Respiratory Complications Type
0.0092
None
109,170 (89%)
74,713 (90%)
34,457 (88%)
Other
5,905 (4.8%)
3,774 (4.5%)
2,131 (5.5%)
Pneumonia
18 (<0.1%)
9 (<0.1%)
9 (<0.1%)
Pneumothorax
2,697 (2.2%)
1,803 (2.2%)
893 (2.3%)
Respiratory failure
4,314 (3.5%)
2,829 (3.4%)
1,485 (3.8%)
Length of Stay
6.0 (5.0, 9.0)
6.0 (5.0, 9.0)
6.0 (5.0, 9.0)
0.0023
Unknown
5
5
0
Prolonged Stay >10d
0.22
No
101,274 (83%)
68,762 (83%)
32,511 (83%)
Yes
20,825 (17%)
14,361 (17%)
6,464 (17%)
Unknown
5
5
0
Discharge Disposition
<0.0012
No Transfer
111,155 (91%)
75,270 (91%)
35,885 (92%)
Transfer to Other Facility
10,948 (9.0%)
7,858 (9.5%)
3,090 (7.9%)
Cost (USD)
$41,121 (31,974, 55,791)
$39,712 (31,087, 53,429)
$44,355 (34,236, 60,547)
<0.0013
Unknown
3,075
1,982
1,093
Total Cost (2009-2020)
$5,908,365,943
$3,862,861,700
$2,045,504,243
<0.0013
Unknown
3,075
1,982
1,093
1n (%); Median (IQR)
2chi-squared test with Rao & Scott's second-order correction
3Wilcoxon rank-sum test for complex survey samples
AVR: Isolated aortic valve surgery
Table 3: In-hospital outcomes, length of stay, cost, and discharge disposition.
Secondary Outcomes
Perioperative complications: The most common complication was bleeding (48%), followed by acute perioperative renal failure (12%), and respiratory complications (11%). Patients undergoing AVR were significantly less likely to experience complications including bleeding (p<0.022), sternal wound complications (p<0.049), cardiac arrest (p<0.001), permanent stroke (p<0.010), or respiratory complications (p<0.002). Acute perioperative renal failure, valve complications, and necessitating transfusion were not significantly different across the two groups.
Index hospitalization stay and cost: The median hospital LOS was 6 days (IQR 5-9), with 17% of patients had a total hospital time > 10 days (no difference between isolated AVR and aortic surgery groups).
The total cost of hospitalization over 12-year period was almost 6 billion dollars ($5,905,956,673). The median cost was $41,121 (IQR 31,974-55,791). Patients undergoing isolated AVR had significantly less hospitalization cost by $4,643 with a median cost of $39,710 compared to aortic surgery patients with a median cost of $44,355 (p<0.001).
Discharge disposition: Transfer to another facility accounted for 9% of discharges disposition. Patients who had isolated AVR were more likely to be discharged to another facility (9.5%) compared to patients who underwent aortic surgery (7.9%).
Discussion
From 2009 through 2020, there was a small (1.6%) in-hospital mortality rate for aortic surgery in patients with BAV; however, this surgery increased patients’ likelihood of mortality by 86%. Excluding patients with urgent admission, subacute bacterial endocarditis, and aortic dissection from the analysis (sensitivity analysis group) did not affect the observed trend of greatly increased in-hospital mortality. Surgery year was not associated with mortality. Patients undergoing aortic dissection had the greatest mortality, increasing their risk fivefold. Female patients had 35% more risk of mortality than males, but this significant risk did not hold in the sensitivity analysis group.
In contrast to the previously reported article from the same database (1998-2009) in an earlier report [5], there is no marked increase of aortic surgery during this era (2009-2020) as surgical guideline became standard practice. However, the mortality rate of 1.6% in this era was within close range of the 1.8% reported` previously. The 2010 ACC/AHA guidelines for the management of thoracic aortic disease suggested aortic intervention was indicated for an aortic diameter of 4 to 5 cm for BAV, independent of aortic valve function [10]. A decade later, the 2020 ACC/AHA guidelines show that most of the recommendations for aortic surgery in patients with BAV are not considered Class 1 (strong) recommendations [11]. In asymptomatic or symptomatic patients with BAV and a diameter of the aortic sinuses or ascending aorta >5.5 cm, replacing the aortic sinuses or ascending aorta is considered a strong recommendation. However, for patients with BAV who have smaller aortic diameters and other risk factors, further recommendations from the guidelines are considered Class 2a (moderate) or Class 2b (weak). Recommendations for isolated Aortic Valve Replacement (AVR) or repair in patients with BAV are considered Class 2b. Additionally, for patients with BAV who are undergoing aortic valve replacement, concomitant replacement of the ascending aorta is considered reasonable when the aortic diameter is >4.5 cm.
Regardless of the controversy of aortic cut-off size for optimal intervention, surgeons who are considering intervention on patients who do not meet the strong recommendation criteria should be aware of the mortality and morbidity associated with isolated and concomitant surgery and the means to produce better outcomes. Previous studies have suggested that thoracic surgeons should decide whether to perform ascending aortic surgery in patients with BAV using an individualized, patient-centered approach which considers risk factors and patient characteristics in addition to the developing and sometimes conflicting guidelines [20].
In this era, some technological advances have been developed for managing thoracic aorta despite the complex anatomy and challenging pathology. The minimally invasive thoracotomy-based approach results in favorable 30-day mortality rates to conventional sternotomy-based surgery, with decreased ICU and hospital LOS [13]. This approach could also be feasible for descending aortic aneurysms [12]. While sternal wound complications were accounted for <1% in this study for all patients, a sternotomy-sparing approach would be expected to further reduce or eliminate sternotomy-related complications. However, these mini-thoracotomy approaches were not considered for analysis in this study because they are not yet regularly adopted practice captured by NIS databases. These advanced techniques could be an armamentarium for high-volume, academic, surgical centers. Better outcomes could be related to the volume of the surgical center and surgeon-specific expertise. For example, mortality and stroke in high-volume centers were 0.25% and 0.75%, [3] compared to 1.8% and 0.9% in this national cohort analysis. This may indicate the need for optimizing proper referral and timely intervention.
Early referral for female patients, in particular, is necessary. In this analysis, only 26% of patients were females, with a fewer proportion undergoing aortic surgery (27%) than isolated AVR (73%) but have shown 35% more mortality risk after accounting for the surgery performed. Sex differences in mortality have been previously reported in ascending aortic cases [13,14]. Female patients have a 3-fold increased risk of aortic dissection or rupture compared to men. Additionally, aortic aneurysm growth is significantly faster in women compared to men as a result of greater aortic stiffness [18]. The outcomes for female patients during thoracic aortic surgery are significantly worse with higher rates of in-hospital mortality (11%) and stroke (8%) when compared to men [19]. Given the increased risk of aneurysm growth/dissection and increased mortality risk, further investigation into improving outcomes for female patients is warranted.
Limitations
The HCUP-NIS is a retrospective database of discharge records, making it susceptible to errors in ICD coding. The possibility of selection bias and the lack of data granularity, due to the administrative nature of this database and inconsistent coding practices among institutions may have resulted in over- or underestimations of events, although robust quality control measures were in place to minimize these discrepancies. In addition, the lack of data granularity and long-term follow-up information does not allow us to assess important long-term follow-up outcomes. Data on hospital charges reflect only inpatient charges without accounting for any associated outpatient costs related to the diagnoses and procedures studied.
Conclusion
Concomitant aortic surgery at the time of AVR demonstrated increased utilization over the 12-year study period. As expected, in-hospital mortality and complications are more frequent in the concomitant group. While follow-up data are needed for insights into post-discharge outcomes and more subtle functional effects, consideration of the risks and benefits of direct aortic intervention should be focused on patients who would benefit from addressing aortopathy at the time of their index operation.
Author Statements
Acknowledgment
The authors thank April Mann for writing support through the University of Miami CTSI.
Disclosures
The authors declare no conflict of interest related to this work.
Funding
No funding was received for this work.
IRB Statement
The study did not involve living human subjects or accessing identifiable information or identifiable biospecimens. For this reason, our human Subject Research Office (HSRO), determined that this study does not require Institutional Review Board (IRB) review, approval, or oversight.
References
- Borger MA, Fedak PWM, Stephens EH, Gleason TG, Girdauskas E, Ikonomidis JS, et al. The American Association for Thoracic Surgery consensus guidelines on bicuspid aortic valve-related aortopathy: Executive summary. J Thorac Cardiovasc Surg. 2018; 156: 473-480.
- Masri A, Svensson LG, Griffin BP, Desai MY. Contemporary natural history of bicuspid aortic valve disease: a systematic review. Heart. 2017; 103: 1323-1330.
- Wojnarski CM, Svensson LG, Roselli EE, Idrees JJ, Lowry AM, Ehrlinger J, et al. Aortic Dissection in Patients with Bicuspid Aortic Valve-Associated Aneurysms. Ann Thorac Surg. 2015; 100: 1666-1673; discussion 1673-1664.
- Davies RR, Kaple RK, Mandapati D, Gallo A, Botta DM, Elefteriades JA, et al. Natural history of ascending aortic aneurysms in the setting of an unreplaced bicuspid aortic valve. Ann Thorac Surg. 2007; 83: 1338-1344.
- Opotowsky AR, Perlstein T, Landzberg MJ, Colan SD, O’Gara PT, Body SC, et al. A shifting approach to management of the thoracic aorta in bicuspid aortic valve. The Journal of Thoracic and Cardiovascular Surgery. 2013; 146: 339-346.
- Elbadawi A, Mahmoud AA, Mahmoud K, Elgendy IY, Omer MA, Elsherbeny A, et al. Temporal Trends and Outcomes of Elective Thoracic Aortic Repair and Acute Aortic Syndromes in Bicuspid Aortic Valves: Insights from a National Database. Cardiol Ther. 2021; 10: 531-545.
- Elbadawi A, Saad M, Elgendy IY, Barssoum K, Omer MA, Soliman A, et al. Temporal Trends and Outcomes of Transcatheter Versus Surgical Aortic Valve Replacement for Bicuspid Aortic Valve Stenosis. JACC: Cardiovascular Interventions. 2019; 12: 1811-1822.
- HCUP Calculating Standard Errors.
- Forrest JK, Ramlawi B, Deeb GM, Zahr F, Song HK, Kleiman NS, et al. Transcatheter Aortic Valve Replacement in Low-risk Patients with Bicuspid Aortic Valve Stenosis. JAMA Cardiology. 2021; 6: 50-57.
- Hiratzka LF, Bakris GL, Beckman JA, Bersin RM, Carr VF, Casey DE, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with Thoracic Aortic Disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation. 2010; 121: e266-369.
- Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP, Gentile F, et al. 2020 ACC/AHA Guideline for the Management of Patients with Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021; 143: e35-e71.
- Alnajar A, Lamelas J. Commentary: The mini-thoracotomy approach for descending aorta: Small, simple and safe! JTCVS Techniques. 2021; 8: 31-32.
- Boczar KE, Cheung K, Boodhwani M, Beauchesne L, Dennie C, Nagpal S, et al. Sex Differences in Thoracic Aortic Aneurysm Growth. Hypertension. 2019; 73: 190-196.
- Chung J, Stevens L-M, Ouzounian M, El-Hamamsy I, Bouhout I, Dagenais F, et al. Sex-Related Differences in Patients Undergoing Thoracic Aortic Surgery. Circulation. 2019; 139: 1177-1184.