
Research Article
Austin J Surg. 2024; 11(5): 1337.
Management of Esophageal Atresia with Right Aortic Arch: Is the Side of Approach Important?
Meye J¹; Louis D¹; Gerstner A¹; Maldonado C²; Breaud J¹; Freyssinet E¹; Lecompte JF¹*
¹Department of Pediatric Surgery, Hôpitaux pédiatriques de Nice – CHU Lenval, France
²Centre Hospitalier Régional Universitaire de Strasbourg, France
*Corresponding author: Lecompte JF, Service de Chirurgie Pédiatrique, Hôpitaux Pédiatriques de Nice CHU-Lenval, 57 Avenue de la Californie, 06200, Nice, France Tel: +33492030016 Email: jean-francois.lecompte@hpu.lenval.com
Received: September 26, 2024 Accepted: October 17, 2024 Published: October 24, 2024
Abstract
Background: Esophageal atresia is associated with a right aortic arch in 2 to 13% of cases. Despite previous studies, consensus on the optimal surgical approach remains lacking. This study aims to analyze the management of esophageal atresia with a right aortic arch in France over three decades, to define the most effective surgical strategy and identify associated complications.
Methods: We conducted a two-phase study. Firstly, we surveyed pediatric surgeons regarding their management preferences for esophageal atresia with and without right aortic arch. Secondly, retrospective data on patients treated over three decades were collected, analyzing surgical approaches and immediate postoperative outcomes.
Results: When dealing with a right aortic arch, 77% of the surveyed surgeons opted for a right approach, primarily by thoracoscopy. We present a cohort of 21 patients with esophageal atresia and right aortic arch. Six patients (28%) underwent surgery via a left approach, and 15 (72%) via right approach, with 7 patients managed by thoracoscopy as the primary approach. One patient underwent a two-stage repair with a change of side from right to left during the second time. There was no significant difference between right and left approaches in terms of postoperative complications. The incidence of chylothorax was 23%.
Conclusion: No superiority between left and right surgical approaches was observed. Video-assisted surgery emerges as a promising option. Additionally, we advocate for the systematic placement of a chest drain due to the notable risk of thoracic duct injury and subsequent chylothorax.
Keywords: Esophageal atresia; Right aortic arch; Chylothorax
Introduction
Esophageal atresia is a congenital malformation characterized by a discontinuity in the esophageal lumen, which may be associated with a communication between one or both pouches of the esophagus and the tracheobronchial tree. It is the most common congenital anomaly of the esophagus, affecting one in 2500 to 3000 newborns annually [1]. Half of the patients born with esophageal atresia also have another associated malformation [1,4]. The prevalence of cardiac malformations in esophageal atresia is reported to be 15 to 30% [5]. In 2.5 to 13% of cases, it is associated with a right aortic arch [7,20] resulting from persistence of the right fourth aortic arch instead of the left as the definitive aortic arch [13]. This anomaly poses the challenge of poor exposure of the esophagus during surgical repair of the atresia and may make the esophago-esophageal anastomosis difficult to perform. The earliest publication addressing the management of this rare malformation association is a case series published by Harrison et al. in 1977 [21]. The author described the main difficulties encountered, particularly exposure constraints greatly hindering the achievement of the esophago-esophageal anastomosis. Since then, several authors have explored this issue, but no consensus has been reached regarding the optimal surgical approach for these patients. The aim of this study is to analyze the management of patients with esophageal atresia and right aortic arch in France over the past thirty years to define the best surgical approach. The secondary objective is to identify specific complications related to the management of these patients.
Materials and Methods
Data Collection
Our study was conducted in 2 phases. Firstly, we distributed a questionnaire to pediatric surgeons who are members of the Thoracic Surgery Committee of the French Society of Pediatric Surgery to gather information on the management of esophageal atresia with right aortic arch (Figure 1) The questionnaire focused on their personal experience in esophageal atresia repair in general, including their preference between performing a thoracotomy or a thoracoscopy, and the advantages they found in a minimally invasive approach for this procedure. Other questions specifically addressed the management of esophageal atresia with right aortic arch, including the role of preoperative echocardiography and the best surgical approach according to them.

Figure 1: Questionnaire sent to the members of the Thoracic Surgery Committee of the French Society of Pediatric Surgery.
Secondly, surgeons were asked to complete retrospective data collection forms concerning patients treated for this condition in their centers over the past 30 years. Information requested included gestational age and birth weight, presence of associated malformations, performance of preoperative echocardiography, surgical approach, immediate postoperative outcomes, and total length of hospital stay. We conducted a descriptive analysis of these data and then categorized the patients into 2 groups based on whether they underwent surgery via a right or left approach to conduct a comparative analysis of immediate postoperative complications.
Statistical Analysis
Comparisons between the two groups (right approach versus left approach) were conducted using a non-parametric Mann-Whitney (or Wilcoxon) test for unpaired samples due to the small sample size, which does not follow a normal distribution.
Percentage comparisons between the two groups (right approach versus left approach) were conducted using Fisher's exact test because the conditions for applying the Chi-square test were not met (expected cell counts < 5).
Survival analysis was represented by Kaplan-Meier curves, and comparison of survival curves between the two groups was performed using a Log Rank test.
Statistical analyses were performed using R Studio version 2023.06.0+421 for Macintosh (studio, 2023).
Results
Surgeons Response
Eighteen pediatric surgeons practicing in 16 different centers responded to the questionnaire (Table 1). Eight practitioners treated at least 10 esophageal atresia per year. Fourteen surgeons had previously managed more than 20 esophageal atresia in their careers. Ninety-five percent of surgeons routinely performed preoperative echocardiography. When asked about the purpose of preoperative echocardiography, 13 of the surveyed surgeons stated that it was to identify the laterality of the aorta and detect congenital heart diseases. Among the surgeons who performed preoperative echocardiography, four only looked for the presence of congenital heart diseases and not the laterality of the aorta in order to anticipate potential hemodynamic and ventilatory difficulties that could complicate general anesthesia management. Fifteen surgeons (83%) stated a preference for performing thoracoscopy as the primary approach for esophageal repair regardless of the position of the aorta. Among the surgeons practicing thoracoscopy in this indication, 10 (62%) claimed it was a better option than thoracotomy, citing better exposure leading to more precise dissection and long-term musculoskeletal benefits. In cases where a right aortic arch was diagnosed preoperatively, 14 of them (77%) chose a right approach, with 12 opting for thoracoscopy. Only four of them reported changing their approach in case of intraoperative discovery of a right aortic arch. When asked about the main difficulties encountered in the repair of esophageal atresia with a right aortic arch, practitioners mentioned a difficult dissection of structures, particularly the upper esophageal pouch, and the challenging achievement of the anastomosis under non-ideal conditions due to proximity to the thoracic aorta.
Performing anastomosis: 27%
Exposure difficulties: 22%
Dissection of the proximal pouch: 16%
Fistula ligation: 11%
Dissection of the distal pouch: 5%
Table 1: Main reported challenges for repair of esophageal atresia with right aortic arch.
Clinical Characteristics
We collected a total of 21 patient records managed for esophageal atresia with right aortic arch between 1990 and 2023. The cohort consisted of 66% male patients (Table 2). The median gestational age at birth was 37 weeks, and the median birth weight was 2520 grams. Seventy-one percent of patients had associated malformations other than a right aortic arch. These were mostly cardiovascular (47%), but also digestive (23%), musculoskeletal (28%), and genitourinary (23%).
Total
Right approach
Left approach
p value
Female
7
4
3
0.35
Male
14
11
3
0.44
Gestational age
3
3
0
0.2
28 - 31 weeks
Gestational age
4
4
0
0.72
32-36 weeks
Gestational age
13
8
5
0.15
> 37 weeks
Cardiac malformation
9
5
4
0.33
Other congenital malformation
10
8
2
0.63
Birth weight, median
2520
1890
2770
0.051
Table 2: Patients clinical characteristics.
Management and Postoperative Outcomes
Laterality
In total, 6 patients underwent surgery via a left approach (Figure 2). 5 patients underwent thoracotomy, and one underwent thoracoscopy. One of these patients underwent a two-stage repair without changing sides during the second stage. All the patients had undergone preoperative echocardiography, which correctly diagnosed the right aortic arch.
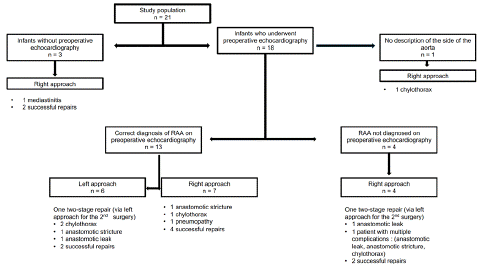
Figure 2: Flow chart.
Fifteen patients underwent surgery via a right approach, including 6 who underwent thoracoscopy as the primary approach. Among these 15 patients, 3 did not undergo preoperative echocardiography, but intraoperative discovery of the vascular anomaly did not lead to a change in the side of the approach. Twelve patients had undergone preoperative echocardiography, with a correct diagnosis of right aortic arch in 7 of them. In the remaining 5 patients, the anomaly was not diagnosed. In this latter group, one patient underwent a two-stage repair with a change in the side of the approach from right to left for the second stage.
Postoperative Complications
Data on postoperative complications were available for all patients. In total, 11 patients (52%), experienced postoperative complications (Table 3). Among them were one pneumonia, 2 cases of mediastinitis requiring antibiotics, 2 anastomotic leaks, and 3 symptomatic anastomotic strictures requiring endoscopic dilatations, 5 chylothoraxes (23%). One patient experienced multiple complications, including mediastinitis, chylothorax, and anastomotic stricture. The median length of hospital stay was 45 days.
Total
Right approach
Left Approach
p value
Total complications
11 (52%)
7
4
0.63
Hemorrhage
0
0
0
NA
Chylothorax
5 (23%)
3
2
0.59
Anastomotic stricture
3 (15%)
2
1
1
Anastomotic leak
2 (9%)
1
1
1
Infection
3 (15%)
3
0
0,52
Table 3: Comparison of postoperative complications between right approach and left approach.
Comparison between Left Approach and Right Approach
There was no significant difference regarding gender, gestational age, birth weight, and presence of malformations between patients operated via left approach and those operated via right approach. Survival analysis on the occurrence of postoperative complications also did not show a significant difference between the two groups of patients (Figure 3).
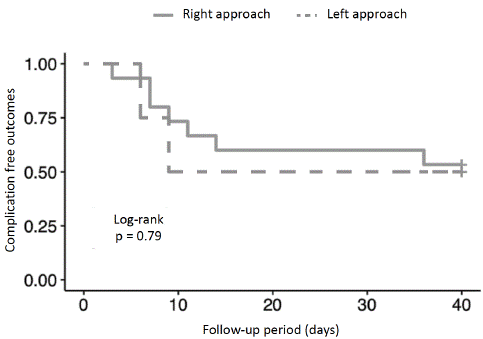
Figure 3: Survival analysis on the occurrence of postoperative complications.
Discussion
The development of the aorta begins in the third week of gestation with 6 pairs of aortic arches formed between the aortic sac and the dorsal aortas. Under the influence of neural crest cell migration and programmed apoptosis, these arches will appear and involute asynchronously. Observation of a right aortic arch occurs when the fourth right arch, destined to give rise to the proximal portion of the right subclavian artery, persists as the definitive aortic arch [11]. There are 2 main types of right aortic arches: type I, a mirror image of the left arch, represents 85% of cases; and type II, which may more often be responsible for symptoms of esophageal or tracheal compression [16]. In the absence of a right aortic arch, the surgical repair of esophageal atresia through the traditional right approach poses little exposure-related difficulties. The main obstacle is the presence of the azygos vein arch, which can be divided between 2 ligatures to improve esophageal visibility.
The association of esophageal atresia with right aortic arch occurs in only 2.5 to 13% of cases of esophageal atresia [7,9,20,22–24]. We present in this study the largest cohort of patients managed for this malformation association. Among esophageal atresia patients, the reported rate of associated malformations in the literature varies between 48 and 71% [4,25,26]. Of the 21 patients included in our study, 71% had various types of congenital anomalies, particularly cardiovascular. Among the anomalies found, the most frequent were Tetralogy of Fallot and ventricular septal defect. These 2 malformations are frequently described in association with aortic arch anomalies [12].
Our literature search identified only 8 studies with patients managed for esophageal atresia with right aortic arch. Although there is not many publications addressing this topic in the literature, determining the laterality of the aorta remains a concern for many surgeons managing this condition because none of the aforementioned studies reach a consensus, and the management of this association has remained controversial over the years.
In an article published in 1977, Harrison et al. described a series of 7 patients managed for esophageal atresia and right aortic arch, 3 of whom required a change from a right to a left approach during the same operative time, and concluded on the need for a left approach if the anomaly is diagnosed preoperatively or intraoperatively [21]. In 1990, Stringel et al. were the first authors to conclude that right thoracotomy is not contraindicated in a study based on a series of 5 patients [27]. Bowkett et al., in a series of 15 patients published in 1999, recommended approaching patients from the left in case of preoperative diagnosis of right aortic arch or performing a left thoracotomy in the same operative time if discovered intraoperatively [9]. The same conclusion was found in a study of 12 patients published in 2000 by Babu et al., with the difference that the authors recommended delayed left thoracotomy in case of intraoperative discovery of the anomaly to better characterize the cardiovascular malformation before the second intervention [7]. Between 2006 and 2019, 4 publications focused on the management of these patients. Allen et al. simply recommended performing an anatomical assessment before the intervention [22], while Bicakci et al. and Lal et al. concluded that right thoracotomy is not contraindicated for these patients [20,24]. Finally, Wood et al. went further and recommended performing a right thoracotomy in this indication [23].
Even today, the question of the optimal approach for these patients is not consensual among practitioners. In their questionnaires regarding surgeons' attitudes towards managing esophageal atresia with right aortic arch, Aguilera et al. and Zani et al. showed that half of the surveyed surgeons preferred a left approach when the diagnosis of right aortic arch is made preoperatively, which differs from the responses obtained through our questionnaire where most surgeons preferred the traditional right approach. This preference is reflected in practice in our series, with a predominance of the right approach in patients whose aortic positional anomaly was diagnosed preoperatively.
Of the 7 patients in our series who were diagnosed preoperatively and operated on via a right approach, none had a change in approach to the left during surgery. We made the same observation for patients who did not undergo preoperative echocardiography and were approached from the right. This choice can be explained by the fact that the repair of esophageal atresia through the right approach is a highly standardized procedure, and even in cases of anatomical variation leading to difficulties, surgeons will be more comfortable performing the procedure under familiar conditions.
Furthermore, among the 8 publications on esophageal atresia with right aortic arch identified in the literature, only 3 were published after the first type III esophageal atresia repair by thoracoscopy [30], but none described the results of thoracoscopy in this indication. Our series differs from previous studies because it is the first to report such results. There was a total of 7 patients in the studied cohort operated on by minimally invasive surgery, including 6 by right thoracoscopy as a first-line approach without needing to change sides. There are several advantages to choosing a minimally invasive approach. First, thoracoscopy allows for a minimally invasive exploration of the hemithorax and thus helps determine whether conditions are favorable enough to perform the anastomosis on the chosen side. If the conditions are deemed unfavorable, a change of sides to the left will be more easily tolerated in the short and long term in terms of musculoskeletal aspects than a double thoracotomy [18]. Furthermore, video-assisted surgery provides better visualization of anatomical structures, which is a significant advantage for this intervention where the practitioner is required to perform an anastomosis near important vascular structures. The benefits of thoracoscopy seem to improve the issue of changing sides. This may explain why patients operated on by right thoracoscopy in our series were able to complete their anastomosis without the need to change sides and proceed to the left.
In terms of complications, there was no significant difference between the right and left approaches in our series. The complication rate of 52% and their types are similar to the data found in the literature, with a majority of anastomotic strictures [19,26,31–33]. However, the incidence of chylothorax found was 23%, with a similar distribution between patients operated on by the right and left approaches. The thoracic duct originates from the cisterna chyli at the level of the 2nd lumbar vertebra intra-abdominally and enters the thorax via the aortic hiatus. It then ascends extrapleurally along the right anterolateral aspect of the vertebral bodies between the azygos vein on the right, the aorta on the left, and the esophagus posteriorly [34]. Between the 5th and 7th thoracic vertebrae, the thoracic duct crosses the aorta posteriorly to then run along the left side of the esophagus. This anatomical position, as well as that of its afferent branches, is likely altered in cases of right aortic arch, explaining the high rate of chylothorax found in our series.
Chylothorax is a complication rarely described in studies on postoperative outcomes of esophageal atresia repairs, but it can significantly prolong hospitalization [22,24]. Three case reports about children who developed chylothorax after esophageal atresia repair (not associated with right aortic arch) discuss treatment options such as argon laser coagulation, thoracic duct embolization, or biological glue [35–37]. In our series, all chylothoraxes were treated conservatively. However, considering that the latest European recommendations do not advocate leaving a thoracic drain in place after esophageal atresia repair [38], it seems appropriate to recommend systematic placement of a thoracic drain in cases of esophageal atresia repair associated with right aortic arch to detect any potential chylothorax as early as possible.
However, this study has several limitations. Firstly, data collection was retrospective and non-exhaustive, as we did not use a registry of esophageal atresia cases. Secondly, the small number of patients included did not allow us to perform analyses with sufficient statistical power or subgroup analyses. Further studies, prospective and involving a larger number of patients, are therefore necessary to validate our conclusions.
Conclusion
We draw three main lessons from this work. Firstly, preoperative echocardiographic evaluation remains important but may be inadequate in diagnosing a right aortic arch. Secondly, the left approach does not appear to be superior to the right approach in the repair of esophageal atresia with right aortic arch in terms of complications. Despite recommendations to perform a left approach in this indication if right aortic arch is diagnosed preoperatively, we did not demonstrate its superiority, and surgeons who performed a right approach were able to successfully complete the procedure without changing their strategy. Minimally invasive surgery appears to be a viable alternative, allowing for better exposure, and if exposure difficulties arise, a contralateral approach will be better tolerated in terms of musculoskeletal aspects. This modality therefore improves the issue of the approach side in this indication. Lastly, we recommend the systematic placement of a thoracic drain in these patients due to the risk of thoracic duct injury and chylothorax.
References
- Depaepe A, Dolk H, Lechat MF. The epidemiology of tracheo-oesophageal fistula and oesophageal atresia in Europe. EUROCAT Working Group. Arch Dis Child. 1993; 68: 743–8.
- Spitz L. Oesophageal atresia. Orphanet J Rare Dis. 2007; 2: 24.
- Lirussi Borgnon J, Sapin E. Anomalies congénitales de l’oesophage. EMC - Pédiatrie - Maladies infectieuses. 2011; 6: 1–20.
- Chittmittrapap S, Spitz L, Kiely EM, Brereton RJ. Oesophageal atresia and associated anomalies. Arch Dis Child 1989; 64: 364–8.
- Protocole national de diagnostic et de soins (PNDS): atrésie de l’oesophage. Perfectionnement en Pédiatrie 2019; 2: 98–115.
- Spitz L, Kiely EM, Morecroft JA, Drake DP. Oesophageal atresia: At-risk groups for the 1990s. Journal of Pediatric Surgery. 1994; 29: 723–5.
- Babu R, Pierro A, Spitz L, Drake DP, Kiely EM. The Management of Oesophageal Atresia in Neonates with Right-Sided Aortic Arch. J Pediatr Surg. 2000; 35: 56-8.
- Parolini F, Armellini A, Boroni G, Bagolan P, Alberti D. The management of newborns with esophageal atresia and right aortic arch: A systematic review or still unsolved problem. Journal of Pediatric Surgery. 2016; 51: 304–9.
- Bowkett B, Beasley SW, Myers NA. The frequency, significance, and management of a right aortic arch in association with esophageal atresia. Pediatr Surg Int. 1999; 15: 28-31.
- Liechty JD, Shields TW, Anson BJ. Variations pertaining to the aortic arches and their branches; with comments on surgically important types. Q Bull Northwest Univ Med Sch. 1957; 31: 136–43.
- Murillo H, Lane MJ, Punn R, Fleischmann D, Restrepo CS. Imaging of the Aorta: Embryology and Anatomy. Seminars in Ultrasound, CT and MRI. 2012; 33: 169–90.
- Hastreiter AR, D’Cruz IA, Cantez T, Namin EP, Licata R. Right-sided aorta. I. Occurrence of right aortic arch in various types of congenital heart disease. II. Right aortic arch, right descending aorta, and associated anomalies. Br Heart J. 1966; 28: 722–39.
- Hanneman K, Newman B, Chan F. Congenital Variants and Anomalies of the Aortic Arch. Radio Graphics. 2017; 37: 32–51.
- Hsu K-C, Hsieh C, Chen M, Tsai HD. Right aortic arch with aberrant left subclavian artery-prenatal diagnosis and evaluation of postnatal outcomes: Report of three cases. Taiwanese Journal of Obstetrics & Gynecology. 2011; 50: 353–8.
- Felson B, Palayew MJ. The two types of right aortic arch. Radiology. 1963; 81: 745–59.
- Myers PO. L’Arc Aortique: embryologie, anatomie & variantes anatomiques pour le clinicien. Université de Genève. 2009.
- Kanne JP, Godwin JD. Right aortic arch and its variants. Journal of Cardiovascular Computed Tomography. 2010; 4: 293–300.
- Bastard F, Bonnard A, Rousseau V, Delorme B, Schmitt F, Podevin G, et al. Thoracic skeletal anomalies following surgical treatment of esophageal atresia. Lessons from a national cohort. Journal of Pediatric Surgery. 2018; 53: 605–9.
- Lal DR, Gadepalli SK, Downard CD, Ostile DJ, Minneci PC, Swedler EM, et al. Challenging surgical dogma in the management of proximal esophageal atresia with distal tracheoesophageal fistula: Outcomes from the Midwest Pediatric Surgery Consortium. Journal of Pediatric Surgery. 2018; 53: 1267–72.
- Bicakci U, Tander B, Ariturk E, Rizalar R, Ayyildiz SH, Bernay F. The right-sided aortic arch in children with esophageal atresia and tracheo-esophageal fistula: a repair through the right thoracotomy. Pediatr Surg Int. 2009; 25: 423–5.
- Harrison MR, Hanson BA, Mahour GH, Takahashi M, Weitzman JJ. The significance of right aortic arch in repair of esophageal atresia and tracheoesophageal fistula. Journal of Pediatric Surgery. 1977; 12: 861–9.
- Allen SR, Ignacio R, Falcone RA, Warner BW, Brown RL, Garcia VF, et al. The effect of a right-sided aortic arch on outcome in children with esophageal atresia and tracheoesophageal fistula. Journal of Pediatric Surgery. 2006; 41: 479–83.
- Wood JA, Carachi R. The Right-sided Aortic Arch in Children with Oesophageal Atresia and Tracheo-oesophageal Fistula. Eur J Pediatr Surg. 2012; 22: 003–7.
- Lal DR, Gadepalli SK, Downard CD, Minneci PC, Knezevich M, Chelius TH, et al. Infants with esophageal atresia and right aortic arch: Characteristics and outcomes from the Midwest Pediatric Surgery Consortium. Journal of Pediatric Surgery. 2019; 54: 688–92.
- Agurto-Ramírez A, García-Villodre L, Ruiz-Palacio A, Arribas-Diaz B, Barrachina-Bonet L, Paramo-Rodriguz L, et al. Oesophageal Atresia: Prevalence in the Valencian Region (Spain) and Associated Anomalies. Int J Environ Res Public Health. 2023; 20: 4042.
- Chang EY, Chang HK, Han SJ, Choi SH, Hwang EH, Oh JT. Clinical characteristics and treatment of esophageal atresia: a single institutional experience. J Korean Surg Soc. 2012; 83: 43–9.
- Stringel G, Coln D, Guertin L. Esophageal atresia and right aortic arch: Right or left thoracotomy?. Pediatr Surg Int. 1990; 5: 103-105.
- Aguilera-Pujabet M, Gahete JAM, Guillén G, Martin-Gimenez MP, Lloret J, Lopez M, et al. Management of neonates with right-sided aortic arch and esophageal atresia: International survey on IPEG AND ESPES members´ experience. Journal of Pediatric Surgery. 2018; 53: 1923–7.
- Zani A, Eaton S, Hoellwarth ME, Puri P, Tovar J, Fasching G, et al. International Survey on the Management of Esophageal Atresia. Eur J Pediatr Surg. 2014; 24: 003–8.
- Rothenberg SS. Thoracoscopic Repair of a Tracheoesophageal Fistula in a Newborn Infant. Pediatric Endosurgery & Innovative Techniques. 2000; 4: 289–94.
- Dingemann C, Dietrich J, Zeidler J, Blaser J, Gosemann JH, Ure BM, et al. Early complications after esophageal atresia repair: analysis of a German health insurance database covering a population of 8 million: Complications after esophageal atresia repair. Dis Esophagus. 2016; 29: 780–6.
- Michaud L, Guimber D, Sfeir R, Rakza T, Bajja H, Bonnevalle M, et al. Sténose anastomotique après traitement chirurgical de l'atrésie de l'oesophage : fréquence, facteurs de risque et efficacité des dilatations oesophagiennes. Archives de Pédiatrie. 2001; 8: 268–74.
- Sfeir R, Rousseau V, Bonnard A, Loplace C, Drumez E, Gottrand F, et al. Risk Factors of Early Mortality and Morbidity in Esophageal Atresia with Distal Tracheoesophageal Fistula: A Population-Based Cohort Study. The Journal of Pediatrics. 2021; 234: 99-105.e1.
- Griffith Pearson F, Jean Deslauriers, Robert J Ginsberg, Clement A Hiebert, Martin F McKneally, Harold C Urschel. Anatomy of the thoracic duct and chylothorax. Thoracic surgery, Toronto: Churchill Livingstone. 1995.
- Rifai N, Sfeir R, Rakza T, Alameh J, Besson R, Lequien P, et al. Successful management of severe chylothorax with argon plasma fulguration and fibrin glue in a premature infant. Eur J Pediatr Surg. 2003; 13: 324–6.
- Dhua AK, Ratan SK, Aggarwal SK. Chylothorax after Primary Repair of Esophageal Atresia with Tracheo-esophageal Fistula: Successful Management by Biological Fibrin Glue. APSP J Case Rep. 2012; 3: 16.
- Chick JFB, Gemmete JJ, Cline M, Srinivasa RN. Successful Thoracic Duct Embolization for Treatment of an Iatrogenic Left Chylothorax in a Neonate after Repair of a Tracheoesophageal Fistula and Esophageal Atresia. J Vasc Interv Radiol. 2017; 28: 1325–7.
- Dingemann C, Eaton S, Aksnes G, et al. ERNICA Consensus Conference on the Management of Patients with Esophageal Atresia and Tracheoesophageal Fistula: Diagnostics, Preoperative, Operative, and Postoperative Management. Eur J Pediatr Surg. 2020; 30: 326–36.