
Special Article: Plastic Surgery
Austin J Surg. 2024; 11(6): 1343.
The Role of 3D Scanning in Evaluating Burn Progression
Bednarcíková L¹; Michalíková M¹; Ondrejová B¹; Štefanovic B¹; Lengyel P²*; Eliáš E²; Demcák T²
¹Department of Biomedical Engineering and Measurement, Faculty of Mechanical Engineering, Technical University of Košice, Slovakia
²Burns and Reconstructive Plast. Surg. Clinic AGEL Hospital Košice šaca, Slovak Republic
*Corresponding author: Peter Lengyel, PhD, Burns and Reconstructive Plastic Surgery Clinic AGEL Hospital Košice šaca, Lucna 57, 040 15 Košice, Slovak Republic. Tel: +421911500551 Email: peter.lengyel@nke.agel.sk
Received: November 21, 2024; Accepted: December 12, 2024; Published: December 19, 2024
Abstract
Effective treatment of burn scars requires accurate evaluation of burn severity and progression, and 3D scanning technologies may provide a valuable alternative to traditional clinical assessments. This article explores the use of 3D scanning to assess the size and extent of burns. It details the methodology of scanning burns and compares photographs of burn injuries with textured 3D scans. The study involved four patients with varying clinical histories, degrees of burns, and stages of healing. The results demonstrate that 3D scanning can aid in tailoring individual treatment plans based on healing progression and may also offer new opportunities for designing burn orthoses.
Keywords: 3D scanning; Burn identification; Burn analysis
Introduction
At present, burn treatment progress is typically documented through photographs to monitor the healing process, including its progression or regression. With the advancement and greater availability of innovative technologies, 3D scanners are becoming an alternative for this purpose. These devices can be used to capture the spatial morphology and surface texture of the human body.
3D scanning enables visualization of an object as a virtual 3D entity, which can be manipulated and observed from any angle, as if it were in the examiner's hands. This is achieved by the 3D scanner, which records the 3D coordinates of an object's surface, usually in the form of a dense cloud of points [1]. This data can then be processed, typically using specialized software, into a 3D surface model. The latest innovation in 3D scanning is the non-contact, portable, handheld optical scanner. This type of scanner was chosen for the study due to its ease of use and ability to be used in a clinical setting [2].
The aim of the study is to investigate the efficacy of 3D scanning in accurately determining the area and extent of burns, compare the results obtained from two different methods (photography and 3D scanning), and explore the potential of 3D scans in guiding individualized treatment strategies and modelling burn orthoses.
Materials and Methods
To obtain patient data, ethical approval was first secured, and informed consent was obtained from each patient. The study was approved by the Ethics Committee of AGEL Hospital Košicešaca (Slovakia) under the number 17-2023. In addition, a set of questionnaires was developed for doctors and nurses, which covered a wide range of information. The questionnaire for doctors included questions about the gender of the patient, the extent of the burn determined by traditional methods, the degree of the burn and the possible need for surgery. A separate questionnaire was prepared for the nurses, focused on information about bandages, methods of conservative treatment, and photo documentation was also obtained. This cooperation was established with the Department of Burns and Reconstructive Surgery in the AGEL Hospital Košice-šaca (Slovakia), which ensured access to relevant clinical data and created a solid basis for scientific research. Such a comprehensive approach to data collection and collaboration with the clinical environment provided an important framework for subsequent analysis and interpretation of results.
For each patient, detailed photographic documentation of the affected area was taken to capture the entire burned tissue area and approximately 2 cm of healthy tissue around the edge of the burn.
The Artec Eva (Artec 3D, Senningerberg, Luxembourg) 3D scanner was used to digitize the burned tissue. This scanner captures fine details with high surface accuracy (<0.1 mm) and creates a digital model that includes the object's texture. Burn scans were conducted at regular intervals during the healing process, except on the day of admission, or when the physician observed a significant change in the extent of the burn.
When scanning a burn patient, it is crucial to follow a proper methodological procedure. The patient should be informed of the need to stay as still as possible and minimize movement during the scan. Before the scanning begins, a thorough examination of the burns and areas of interest is necessary. If multiple areas are affected, the scanning process should be planned to minimize patient movement and ensure the most comfortable position during the procedure. If staff assistance is required, clear instructions should be provided to avoid the need for re-scanning and to reduce patient discomfort and the exposure time of the burned tissue.
Before scanning, it is crucial to ensure that the wound is fully exposed, clean, and free of any remaining dressing material. This step is necessary for accurate diagnosis and assessment of the burn's extent. Additionally, the area around the patient must be properly prepared, as most 3D scanners suitable for scanning the human body require a working distance of 0.4 to 1.0 meters from the patient.
This requires that the space around the patient be clear of any obstacles that could interfere with the scanning process and cause loss of object tracking, which would necessitate re-scanning. The patient should be positioned in an area that allows movement around them from all sides without obstruction. However, the patient must also be placed close enough to ensure the 3D scanner can be connected to the computer via USB cable for data collection and processing.
During the 3D scanning process, it is important to ensure that both the burned and undamaged tissue are scanned adequately to accurately define the boundaries of the burned area. To precisely outline the burn’s edges, we selected a minimum margin of approximately 2 cm of healthy tissue around the burn's perimeter.
Results
A total of 18 patients with burns of various etiologies, stages, and extents have been monitored, and the case studies of four selected patients are presented in the current article.
Subject 1
A 64-year-old woman sustained burns from boiling water on her lower extremities, including the foot and shin, with a burn area of 5% (3% superficial and 2% moderate). She was initially treated in the emergency department on the same day and was then referred for outpatient care. However, due to the progression of the local condition, she was admitted acutely three days later with worsening signs on her right lower leg, including edema. Antibiotic and analgesic therapy were initiated in the hospital, and the patient was indicated for surgical intervention. Under spinal anesthesia, a necrosectomy and dermoepidermal burn grafting were performed. The postoperative period was uncomplicated, with regular dressings being applied. Upon discharge, the dermoepidermal grafts on her right lower leg were fully integrated with minimal scabbing, and the donor site was calm, in the healing stage. The patient was discharged to outpatient care on the 21st day after admission.
On the 11th day after the injury, photographs and scans of the affected area were taken (Figure 1). The photograph shows a burn on the dorsum of the right foot, deeper second-grade burns centrally, with third-grade burns extending to the back of the shin. On the periphery of the wound, gradual epithelialization is visible, while the center of the wound shows necrotic brownish tissue with a pale surrounding base. The 3D scan of the area clearly distinguishes the zone of epithelialization and the areas containing necrotic tissue.
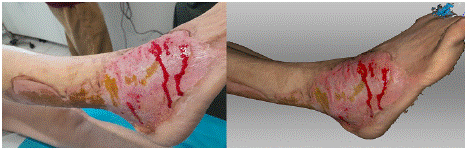
Figure 1: Photograph (left) and 3D scan (right) of the burn on the 11th day after its occurrence.
In the photograph taken on the 21st day after the injury, meshed dermoepidermal grafts in the healing stage are visible (Figure 2). On the periphery, a border of spontaneously reepithelialized tissue can be seen. Additionally, on the back of the shin, in the medial third, there is an area left for spontaneous healing. The scan shows a meshed dermoepidermal graft on the dorsum of the foot, corresponding to the extent visible in the photograph. In the area surrounding the graft on the dorsum of the foot, the tissue that has healed spontaneously is not optimally distinguishable.
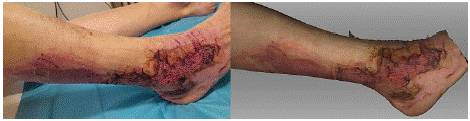
Figure 2: Photograph (left) and 3D scan (right) of the burn on the 21st day
after its occurrence, following the application of meshed dermoepidermal
skin grafts.
On the 26th day after the injury, the photograph shows a nearly completely healed dermoepidermal graft, with scabs present at its edges (Figure 3). A clearly defined area of the wound, which healed spontaneously, is visible next to the graft on the dorsum of the foot. On the back of the shin, a completely healed wound is visible. The scan shows the healed dermoepidermal graft in the same extent as seen in the photograph. The boundary of the graft, where scabs are present, is clearly defined, and beyond it, the peripheral area of the wound that healed spontaneously is visible. The back of the shin corresponds to the healed area.
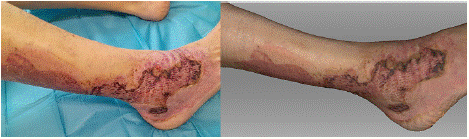
Figure 3: Photograph (left) and 3D scan (right) of the burn on the 26st day
after its occurrence, following the application of meshed dermoepidermal
skin grafts.
Subject 2
A 53-year-old man was transported by air ambulance after sustaining burns from an explosion of a gasoline canister. He suffered burns to the face, neck, chest, abdomen, back, upper extremities bilaterally, and lower extremities bilaterally on the ventral side of the body, covering a total of 51% Total Body Surface Area, with the following distribution: Grade I - 13%, Grade IIa - 23%, and Grade IIb - 15%. The patient was intubated upon arrival, with a nasogastric tube and a permanent urinary catheter, along with two peripheral venous accesses.
He was initially treated at the emergency department at the scene of the accident, where he was intubated prophylactically to allow for dressing changes under analgesia, with no thermal injury to the airways. Upon admission, he received a dressing change under analgesia, an arterial catheter was inserted into the left radial artery, a central venous catheter was placed into the right femoral vein, and he was subsequently hospitalized in the burn intensive care unit. An ENT examination was performed with no evidence of airway burns, and an eye examination revealed bilateral eyelid edema with purulent conjunctivitis but no corneal damage.
Preoperative internal examination showed no pathological findings. During hospitalization in the burn intensive care unit, the patient developed elevated blood pressure, prompting a followup examination by an internist, who recommended treatment. The patient was regularly dressed under analgesia, then in premedication. After stabilization and without further blood pressure increases, the patient was transferred to the general ward. The healing of the burn areas was favorable, and the patient was managed conservatively without the need for surgical intervention or systemic complications. Upon discharge, the burn areas were almost completely healed, with minimal residual defects left for spontaneous healing. The patient was discharged to outpatient care on the 16th day after the injury.
In the photograph taken on the 9th day after the injury, a superficial burn on the left knee is visible, affecting only the partial thickness of the dermis (Fig. 4). The wound shows red areas of tissue with a vital base and pale pink tissue between them, forming epithelial islands. On the 3D scan, this contrast between the unhealed wound and the epithelial islands is even more pronounced. Additionally, on the lateral side of the knee, a spontaneously reepithelialized portion of the wound is clearly visible, both in the photograph and on the scan.
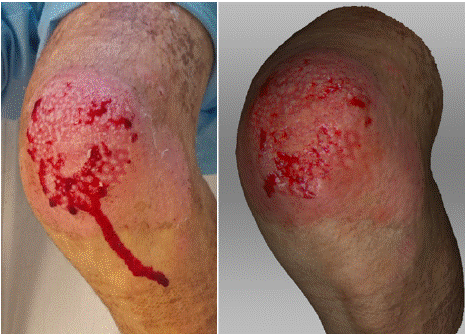
Figure 4: Photograph (left) and 3D scan (right) of the burn on the 9th day
after its occurrence.
On the 9th day after the injury, following wound treatment, the photograph shows a nearly completely healed wound, with visible epithelial areas and almost fully reepithelialized tissue (Figure 5). The 3D scan corresponds to the extent of the wound seen in the photograph, with epithelial areas also visible. However, at the mediodistal periphery, a slightly larger area is observed, which would be assessed from the scan as still unhealed.
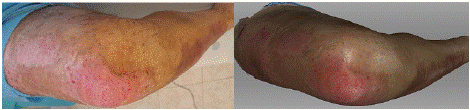
Figure 5: Photograph (left) and 3D scan (right) of the burn on the 9th day
after its occurrence, after treatment.
Subject 3
A 48-year-old man was admitted to the hospital for a burn on his left upper extremity, from the shoulder to the wrist, after being scalded with hot water. He was initially hospitalized at a local hospital and then transferred to a specialized burn center on the 10th day after the injury for burn therapy. The burn covered 6% of his body (3% superficial and 3% moderate to deep). Areas requiring surgical treatment were identified, and a necrosectomy and dermoepidermal grafting were performed. The postoperative course was uncomplicated, and the areas gradually healed completely. On the 10th day after the injury, the photograph clearly shows the area after necrosectomy and subsequent meshed skin grafts in the left shoulder and forearm, following a previous deep full-thickness burn (Figure 6). On the lateral side of the shoulder and the proximal third of the forearm, small areas of necrotic tissue and scabs are visible in addition to the grafts. The scan clearly shows the meshed dermoepidermal graft, with the necrotic tissue in brown color, distinguishable from the scabs, which appear slightly darker than in the photograph.
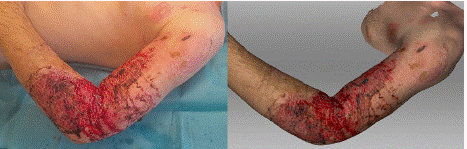
Figure 6: Photograph (left) and 3D scan (right) of the burn on the 10th day
after its occurrence, after the application of meshed dermoepidermal skin
grafts.
Subject 4
A 79-year-old woman was transported by air ambulance after initial treatment at the central surgical emergency department in her area. She sustained burns from hot syrup while cooking. The burn covered 11% of her body (4% superficial and 7% moderate to deep). The patient was admitted to the intensive care unit due to the risk of edema development. Wound care therapy, analgesic therapy, and fluid resuscitation were initiated. Respiratory burns were diagnostically excluded. After stabilization, the patient was transferred to a general ward, where wound care and antibiotic therapy continued. Deep burn areas were present on her right extremity and chest, which were indicated for surgical treatment—necrotomy and dermoepidermal grafting. The postoperative period was uncomplicated, with all grafts fully integrated and vital. The patient underwent intensive rehabilitation during hospitalization. At the time of transfer, a minimal residual defect was present on her right shoulder, left for healing with dressings. The patient was transferred to the surgical department in her area of residence for further treatment and wound care. She was discharged home on the 29th day after the injury.
In the photograph taken on the 4th day after the injury, a superficial burn is visible on the ventral surface of the right thigh, with a subcutaneous hematoma located distally (Figure 7). The scan clearly defines the contours of the burned area, both in terms of extent and depth, providing sufficient detail for objective assessment. The scan also shows a well-defined hematoma, with color and extent corresponding to the injury.
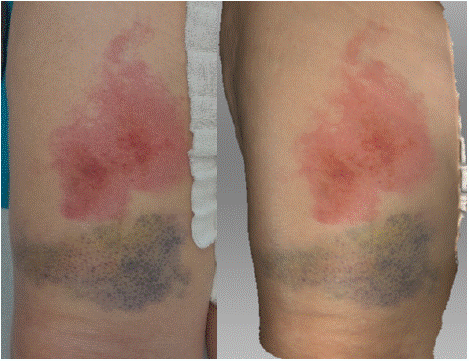
Figure 7: Photograph (left) and 3D scan (right) of the burn on the 4th day
after its occurrence.
On the 15th day post-injury, the photograph shows burns in the area of the right shoulder, upper arm, chest, and breast, which affect almost the full thickness of the skin and have a shiny appearance due to the formation of necrotic coverings (Figure 8). On the trunk to the right and on both breasts, there are clearly defined dry necroses of brown color. To the right of the trunk, peripheral to the deep burns, there is distinct pale pink tissue corresponding to the spontaneously healed portion of the wound. On the skin, there is a clearly defined brown necrosis with a whitish border observed on the right side of the chest and subsequently better defined developing necrotic coverings. The vitality of the wound base corresponds to the photograph. However, on the skin, the boundary between the already healed wound and the open wound is better defined. On the breast to the right in the upper medial quadrant extending into adjacent quadrants, there is a well-defined dry necrosis, with a surrounding area presenting a wound with a vital base, where the developing necrotic coverings are clearly visible in the corresponding extent as seen in the photograph.
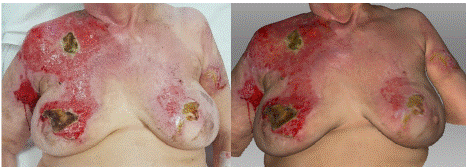
Figure 8: Photograph (left) and 3D scan (right) of the burn on the 15th day
after its occurrence.
In the photograph taken on the 22nd day post-injury, the patient is after surgery, during which dermoepidermal meshed skin grafts were transplanted to the right arm, chest, and both breasts (Figure 9). The grafts in the photograph appear vital, while the surrounding area on healthy skin is contaminated due to dried blood clots. The skin shows vital dermoepidermal grafts with a well-defined texture from the meshing, which correspond in extent and vitality to the photograph. Additionally, the skin contamination due to dried blood clots, which match the color seen in the photograph, is also visible.
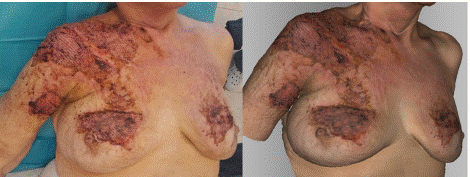
Figure 9: Photograph (left) and 3D scan (right) of the burn on the 22nd day
after its occurrence, after the application of meshed dermoepidermal skin
grafts.
Discussion
The application of 3D scanning in the analysis and identification of burn tissue marks a significant advancement in burn medicine. This innovative method offers several advantages over traditional techniques, especially regarding speed, accuracy, and the thoroughness of data collection [3,4]. One of the primary benefits of 3D scanning is its ability to quickly and accurately evaluate burned areas within minutes, enabling efficient data acquisition without causing patients the discomfort typically associated with conventional assessment methods. Furthermore, the digital nature of scan results allows for easy storage and analysis, enabling healthcare professionals to access and evaluate patient information without exposing the wounds, thereby reducing the risk of infection.
Additionally, the visualization of skin texture provided by 3D scans facilitates the comparison of color changes throughout the healing process, offering valuable insights into treatment progress and efficacy [5,6].
However, the study has some limitations that should be considered when interpreting the results. Notably, the small sample size of four patients with five burns restricts the generalizability of the findings and may not reflect the full spectrum of burn types and healing responses in a wider patient population. Moreover, the use of a single type of 3D scanner may limit the applicability of the results, as variations among scanners could impact accuracy and usability. These limitations indicate that future research should involve larger and more diverse samples, investigate various scanning technologies, and extend the follow-up period to better assess the long-term benefits and generalizability of 3D scanning in burn management.
Conclusion
Comprehensive and accurate information regarding the surface, structure, and texture of human tissue is essential for treating patients with burn injuries. 3D scanning allows for the creation of 3D models of burned areas and enables the tracking of their progression over time, providing critical data for evaluating the effectiveness of various treatment methods.
Future research should focus on broadening the clinical applications of 3D scanning in managing burn tissue. This could include examining its effectiveness in monitoring the healing process, guiding surgical procedures, and evaluating treatment outcomes over time. Moreover, investigating the potential for 3D scans to personalize and fabricate patient-specific burn orthoses would be beneficial in enhancing treatment outcomes and improving patient comfort.
Author Statements
Acknowledgement
This work was supported by the Slovak Research and Development Agency under the contract No. APVV-22-0340.
References
- Paneva M, Panev P, Stoimenov N, Gyoshev S. Methodology for 3D Scanning of Objects. WSEAS transactions on applied and theoretical mechanics. 2023; 18: 216–20.
- Stefanovic B, Ondrejova B, Bednarcikova L, Toth T, Zivcak J. Comparison of Optical Handheld 3D Scanners Suitable for Prosthetic and Orthotic Applications. 3DBODY.TECH 2022 13th International Conference and Exhibition on 3D Body Scanning a Processing Technologies; Lugano, Switzerland. 2022.
- Bharadia SK, Gabriel V. Comparison of Clinical Estimation and Stereophotogrammic Instrumented Imaging of Burn Scar Height and Volume. European Burn Journal. 2024; 5: 38–48.
- Furferi R, Governi L, Pinzauti E, Profili A, Puggelli L, Volpe Y. A computational method for the investigation of burn scars topology based on 3D optical scan. Computers in Biology and Medicine. 2022; 149: 105945.
- Parvizi D, Giretzlehner M, Wurzer P, Klein LD, Shoham Y, Bohanon FJ, et al. BurnCase 3D software validation study: Burn size measurement accuracy and inter-rater reliability. Burns. 2016; 42: 329–35.
- Stefanovic B, Bibiana Ondrejova, Bednarcikova L, Michalikova M, Jozef Zivcak. Technological process of design and production of facial burn mask. Acta Tecnología. 2023; 9: 109–14.